Abstract
Background
As more people become vaccinated against the SARS‐CoV‐2 virus, reports of delayed cutaneous hypersensitivity reactions are beginning to emerge.
Methods
In this IRB‐approved retrospective case series, biopsy specimens of potential cutaneous adverse reactions from the Pfizer‐BioNTech or Moderna mRNA vaccine were identified and reviewed. Clinical information was obtained through the requisition form, referring clinician, or medical chart review.
Results
Twelve cases were included. Histopathological features from two injection‐site reactions showed a mixed‐cell infiltrate with eosinophils and a spongiotic dermatitis with eosinophils. Three biopsy specimens came from generalized eruptions that showed interface changes consistent with an exanthematous drug reaction. Three biopsy specimens revealed a predominantly spongiotic pattern, consistent with eczematous dermatitis. Small‐vessel vascular injury was seen in two specimens, which were diagnosed as urticarial vasculitis and leukocytoclastic vasculitis, respectively. There were two cases of new‐onset bullous pemphigoid supported by histopathological examination and direct immunofluorescence studies. Eosinophils were seen in 10 cases.
Conclusions
Dermatopathologists should be aware of potential cutaneous adverse reactions to mRNA‐based COVID‐19 vaccines. Histopathological patterns include mixed‐cell infiltrates, epidermal spongiosis, and interface changes. Eosinophils are a common finding but are not always present. Direct immunofluorescence studies may be helpful for immune‐mediated cutaneous presentations such as vasculitis or bullous pemphigoid.
Keywords: COVID‐19, cutaneous adverse reaction, vaccine
1. INTRODUCTION
The rapid development of two US Food and Drug Administration (FDA)‐approved mRNA vaccines against the SARS‐CoV‐2 virus, while hailed as a scientific breakthrough, has also raised concerns over adverse allergic events. 1 Immediate cutaneous allergic reactions occurring within minutes to hours after vaccine administration have included a diffuse erythematous rash, urticaria, and angioedema. 2 As a growing percentage of the population becomes vaccinated, a variety of delayed cutaneous reactions are also beginning to be reported. 3 Here, we describe the clinical and histopathologic features of delayed cutaneous adverse reactions from 12 patients after receiving an mRNA COVID‐19 vaccine.
2. MATERIALS AND METHODS
We conducted a retrospective case series of patients who underwent skin biopsy procedure by their dermatologist for cutaneous eruptions after receiving either the Pfizer‐BioNTech or Moderna mRNA vaccine against the SARS‐CoV‐2 virus between January 1 and May 31, 2021. Patients were seen in either a private practice or academic setting. Skin biopsies were submitted to a dermatopathology laboratory at a single urban academic center for microscopic examination and histopathologic diagnosis. Twenty‐three potential cases were identified from our pathology data management system using the search terms “vaccine,” “vaccination,” “Pfizer,” or “Moderna.” Cases were included if sufficient clinical and vaccine‐related information was available in our hospital‐based electronic medical record (seven cases) or provided by the referring dermatologist on the requisition form (five cases). This study was approved by our Institutional Review Board.
3. RESULTS
Twelve patients were identified to probably have a delayed cutaneous hypersensitivity reaction to the mRNA COVID vaccine based on clinical, temporal, and histopathological findings. Six patients were men and six were women, with an age range of 29 to 95 years (median age: 73.5 years). Five patients received the Pfizer‐BioNTech vaccine, and seven patients received the Moderna vaccine. Seven reactions initially occurred following the first vaccine dose, and five reactions occurred following the second vaccine dose. Latency ranged from several hours following the first dose to 2 weeks after the second dose. Vaccine administered, timing of reaction, clinical and histologic findings, and subsequent clinical course for each patient are summarized in Table 1. Patient demographics as well as available medical history, medications, and allergies are summarized in Table S1.
TABLE 1.
Patient demographics, clinical presentation, and histopathologic features
| Case | Age, Sex | Vaccine | Clinical presentation | Onset to rash | Histopathological features | Diagnosis | Clinical course and treatment |
|---|---|---|---|---|---|---|---|
| 1 | 71, F | Moderna | Multiple red and indurated plaques at injection site | 1 wk after first dose | Perivascular and interstitial mixed‐cell infiltrate with eosinophils and focal interface changes | Injection‐site reaction (“COVID arm”) | Unknown |
| 2 | 95, M | Pfizer‐BioNTech | Pruritic hyperpigmented and lichenified plaque around injection site | After second dose | Epidermal hyperplasia with spongiosis and eosinophil‐rich infiltrate | Injection‐site reaction (“COVID arm”) | Topical corticosteroids and antihistamines |
| 3 | 63, M | Moderna | Generalized, pruritic, morbilliform and PR‐like | 4 d after first dose with progression after second dose | Perivascular lymphocytic infiltrate with vacuolar interface changes | Drug eruption | Topical corticosteroids and antihistamines |
| 4 | 82, F | Pfizer‐BioNTech | Generalized eruption on trunk and extremities | 1 wk after second dose | Mixed‐cell infiltrate with eosinophils, epidermal spongiosis, and vacuolar interface changes | Drug eruption | Topical corticosteroids |
| 5 | 71, F | Moderna | Generalized psoriasiform eruption | 1‐2 d after second dose | Perivascular mixed‐cell infiltrate with eosinophils, papillary dermal edema, and vacuolar interface changes | Drug eruption | Improvement with topical corticosteroids |
| 6 | 77, M | Pfizer‐BioNTech | Widespread eczematous eruption | 10 d after first dose with progression after second dose | Epidermal hyperplasia with spongiosis and lymphohistiocytic infiltrate with eosinophils | Eczema | Improvement with prednisone |
| 7 | 29, F | Moderna | Small papules and patches with collarettes of scale | 1 wk after first dose | Mild epidermal hyperplasia with spongiosis and mounds of parakeratosis | Pityriasis‐rosea‐like eruption | Improvement with topical corticosteroid |
| 8 | 65, M | Moderna | Eczematous patches on the trunk and extremities |
Initially following COVID‐19 infection. Recurred after both first and second vaccine dose |
Epidermal hyperplasia with spongiosis and lymphohistiocytic infiltrate with eosinophils | Eczema | Improvement with topical corticosteroids and antihistamines |
| 9 | 35, F | Moderna | Erythematous and edematous plaques | Day of first dose | Sparse interstitial neutrophilic infiltrate with early fibrin deposition within vessel walls | Urticarial vasculitis | Improvement with antihistamines, methylprednisolone, and dapsone |
| 10 | 83, F | Pfizer‐BioNTech | Palpable purpuric papules | 1 wk after second dose | Perivascular and interstitial neutrophilic infiltrate with leukocytoclasia and fibrin deposition within vessel walls | Leukocytoclastic vasculitis | Improvement with oral antibiotics and topical corticosteroids |
| 11 | 76, M | Pfizer‐BioNTech | Clustered and tense bullae | 3 wk after first dose | Subepidermal bulla and a superficial interstitial infiltrate with eosinophils | Bullous pemphigoid | Some improvement with prednisone, topical corticosteroids, doxycycline, niacinamide, and antihistamines |
| 12 | 84, M | Moderna | Widespread erythematous papules and plaques, some with vesiculation | 2 wk after second dose | Intraepidermal spongiotic vesicles with eosinophilic spongiosis | Bullous pemphigoid | Improvement with oral prednisone and topical corticosteroids |
Two patients developed localized injection‐site reactions. Patient 1 was a 71‐year‐old woman with multiple pruritic, red and indurated plaques at the injection site approximately 1 week after receiving her first Moderna vaccine. A skin biopsy revealed a superficial and mid‐perivascular infiltrate comprised of lymphocytes and eosinophils with focal vacuolar alteration at the dermal‐epidermal junction (Figure 1A,B). Patient 2 was a 95‐year‐old man with a history of lichen simplex chronicus who developed a pruritic hyperpigmented and lichenified plaque around the injection site after his second dose of the Pfizer‐BioNTech vaccine. Skin biopsy revealed a hyperplastic and spongiotic epidermis with an eosinophil‐rich infiltrate (Figure 1C,D).
FIGURE 1.
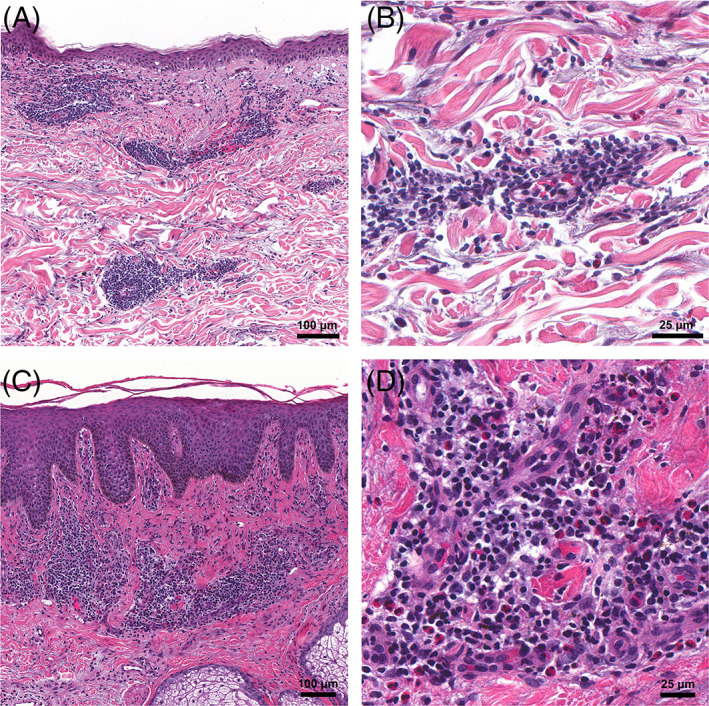
Histopathological findings from injection‐site reactions. A, Patient 1: Superficial perivascular and interstitial mixed‐cell infiltrate with eosinophils and focal interface changes; ×100 original magnification. B, ×400 original magnification. C, Patient 2: Epidermal hyperplasia with spongiosis; ×100 original magnification. D, Numerous eosinophils are seen within the infiltrate; ×400 original magnification
Three patients developed generalized eruptions that showed interface changes on histopathological examination. Patient 3 was a 63‐year‐old man with a history of inflammatory bowel disease on mesalamine, vedolizumab, and prednisone who developed a diffuse, scaly erythematous eruption with morbilliform and pityriasis‐rosea‐like features approximately 4 days after his first dose of the Moderna vaccine (Figure 2A). The lesions resolved spontaneously after approximately 10 days, but recurred 1 week after his second vaccine dose. On histopathological examination, a lymphocyte‐mediated vacuolar interface dermatitis with necrotic keratinocytes was present. Although eosinophils were not seen (Figure 2B), an exanthematous drug reaction was favored in light of the clinical history. Patient 4 was an 82‐year‐old woman with no prior dermatologic history or recent medication changes who developed a generalized eruption of pink plaques on the trunk and extremities approximately 1 week following her second Pfizer‐BioNTech vaccine. Notable findings from her skin biopsy include epidermal spongiosis, vacuolar interface, and a mixed‐cell infiltrate with eosinophils (Figure 2C), consistent with a drug reaction. Patient 5 was a 71‐year‐old woman who developed a generalized psoriasiform eruption 1 to 2 days after her second dose of the Moderna vaccine. Her skin biopsy showed a perivascular mixed‐cell infiltrate with eosinophils, papillary dermal edema, and vacuolar interface changes (Figure 2D), also diagnosed as a drug reaction.
FIGURE 2.
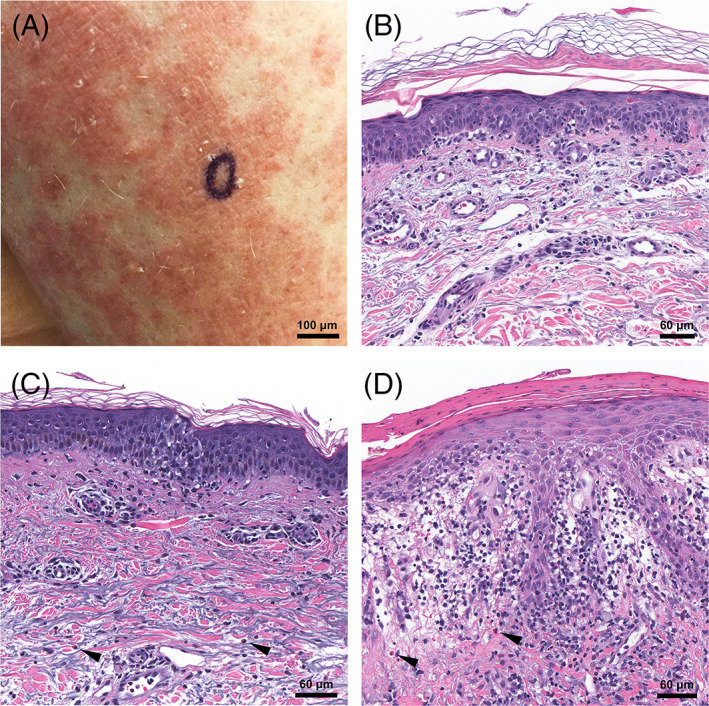
Exanthematous drug‐like reactions. A, Patient 3: Generalized morbilliform eruption with scale. B, Perivascular lymphocytic infiltrate with vacuolar interface changes and necrotic epidermal keratinocytes; ×400 original magnification. C, Patient 4: Mixed‐cell infiltrate with eosinophils (arrows), vacuolar interface changes, and mild epidermal spongiosis; ×400 original magnification. D, Patient 5: Vacuolar interface changes with papillary dermal edema and a mixed‐cell infiltrate with eosinophils (arrows); ×400 original magnification
Three patients had skin biopsies that showed a spongiotic process. Eosinophils were easily identified in two of the biopsy specimens. Patient 6 was a 77‐year‐old man with a history of well‐controlled eczema who developed a flare of his disease 10 days after his first Pfizer‐BioNTech vaccine. His rash significantly progressed to involve 50% body surface area (BSA) after his second vaccine dose. A skin biopsy showed epidermal hyperplasia with spongiosis and a perivascular lymphohistiocytic infiltrate with scattered eosinophils (Figure 3A). Patient 7 was a 29‐year‐old woman with a history of acne vulgaris and herpes simplex virus infection on suppressive valacyclovir who presented with pruritic pityriasis rosea‐like eruption 1 week after her first Moderna vaccine. She denied any new exposures or medication changes. Papules and patches with collarettes of scale were distributed along her chest, abdomen, and back. She underwent a skin biopsy 2 weeks after her second vaccine dose for persistence of the rash. Mild epidermal spongiosis with mounds of parakeratosis were seen. Because of the superficial nature of the biopsy, characterization of the dermal infiltrate was limited and eosinophils were not identified (Figure 3B). Patient 8 was a 65‐year‐old man with no dermatologic history or recent medication changes who initially developed erythematous scaly patches around the ankles after COVID‐19 infection. He subsequently developed a recurrence of similar lesions in a more widespread distribution after his first Moderna vaccine, which resolved over the course of 2 weeks without treatment. The lesions recurred following his second vaccine dose, at which time a biopsy specimen revealed epidermal hyperplasia with spongiosis and a perivascular lymphohistiocytic infiltrate with eosinophils (Figure 3C).
FIGURE 3.
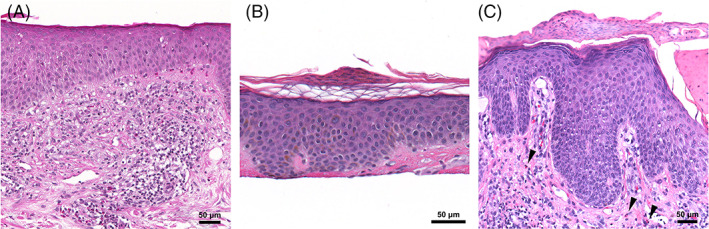
Eczematous reactions. A, Patient 6: Epidermal hyperplasia, spongiosis, and a eosinophil‐rich infiltrate; ×200 original magnification. B, Patient 7: Pityriasis‐rosea‐like eruption characterized by mild epidermal spongiosis and mounds of parakeratosis; ×400 original magnification. C, Patient 8: Epidermal hyperplasia, spongiosis, and parakeratotic scale‐crust in addition to a lymphohistiocytic infiltrate with eosinophils (arrows); ×400 original magnification
Vasculitic injury was seen in skin biopsies from two patients. Patient 9 was a 35‐year‐old woman with a history of acne vulgaris and allergic rhinitis who developed urticarial lesions within 24 hours of her first Moderna vaccine. She continued to develop erythematous and edematous plaques on her trunk and extremities for 5 weeks, at which time a skin biopsy was performed. Histopathologic examination revealed a sparse interstitial infiltrate with neutrophils and eosinophils, mild leukocytoclasia, and few extravasated erythrocytes (Figure 4A). Neutrophils were also seen within vessel walls where there were hints of fibrin deposition (Figure 4B). A diagnosis of urticarial vasculitis was made. The patient's cutaneous symptoms showed some improvement with antihistamines, methylprednisolone, and dapsone. Patient 10 was an 83‐year‐old woman with no prior dermatologic or autoimmune history or recent medication changes who developed bilateral palpable purpuric lesions with erythema and edema of her lower extremities approximately 5 days after her second Pfizer‐BioNTech vaccine dose. She presented to the emergency department and was found to have elevated levels of C‐reactive protein, erythrocyte sedimentation rate, and rheumatoid factor along with hypocomplementemia and detection of cryoglobulin. She had no other signs of systemic disease. A skin biopsy showed a predominantly neutrophilic perivascular infiltrate with marked leukocytoclasia and erythrocyte extravasation (Figure 4C). A specimen submitted for DIF showed deposition of fibrinogen around superficial blood vessels, consistent with a diagnosis of leukocytoclastic vasculitis. Her lesions improved with oral antibiotics and topical corticosteroids.
FIGURE 4.
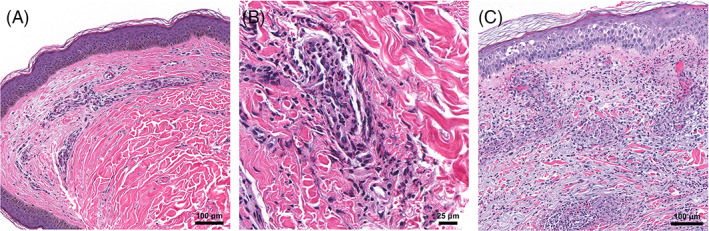
Vasculitic reactions. A, Patient 9: Sparse perivascular and interstitial infiltrate consistent with urticarial vasculitis; ×100 original magnification. B, Neutrophils and early fibrin deposition are seen within vessel walls; ×400 original magnification. C, Patient 10: Leukocytoclastic vasculitis with a dense perivascular and interstitial neutrophilic infiltrate, fibrin within vessels walls, and extravasated erythrocytes; ×100 original magnification
Two patients were diagnosed with new‐onset bullous pemphigoid following their vaccination. Patient 11 was a 76‐year‐old man with a history of psoriasis and no recent new medications who developed clustered tense bullae on his lower extremity approximately 3 weeks following his first Pfizer‐BioNTech vaccine (Figure 5A). Histopathological examination showed a subepidermal separation with numerous eosinophils within the blister cavity (Figure 5B). Direct immunofluorescence (DIF) studies showed linear C3 and IgG deposition along the dermal‐epidermal junction (Figure 5C) as well as strong C3 linear deposition along the basement membrane zone of eccrine glands. A separate specimen sent for salt‐split direct immunofluorescence studies revealed linear IgG localizing to the roof of the blister, confirming the diagnosis of bullous pemphigoid (BP). The patient's eruption dramatically worsened after his second vaccine dose and was subsequently started on a prednisone taper, topical corticosteroids, doxycycline, and niacinamide with some improvement. Patient 12 was an 84‐year‐old man with a past medical history of childhood atopic dermatitis and no recent medication changes who developed a widespread eruption of erythematous papules and plaques, some with vesiculation (Figure 5D), 2 weeks after his second Moderna vaccine. Histopathological examination showed intraepidermal spongiotic vesicles and eosinophilic spongiosis (Figure 5E). DIF studies showed strong linear deposition of C3 (Figure 5F) and weak linear deposition of IgG along the dermoepidermal junction, confirming a diagnosis of bullous pemphigoid.
FIGURE 5.
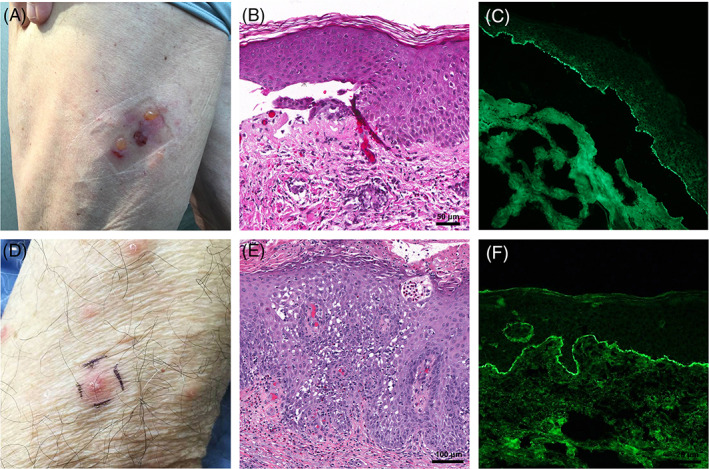
Bullous pemphigoid. A, Patient 11: Clustered, tense bullae on an erythematous base. B, Subepidermal separation with superficial infiltrate containing eosinophils; ×200 original magnification. C, Direct immunofluorescence showing linear deposition of C3 along the basement membrane zone from lesional tissue; ×200 original magnification. D, Patient 12: Scattered erythematous papulovesicles. E, Dense eosinophilic infiltrate with eosinophilic spongiosis and an intraepidermal vesicle; ×200 original magnification. F, Direct immunofluorescence showing linear deposition of C3 along the basement membrane zone from perilesional skin; ×200 original magnification
The majority of patients (7 out of 12) received conservative treatment with topical corticosteroids and/or antihistamines. Patients 6, 11, and 12 received oral prednisone for their eczema and bullous pemphigoid, respectively. Patient 11 additionally required treatment with oral doxycycline and niacinamide. Patient 9, who developed urticarial vasculitis, required initiation of dapsone along with anti‐histamines to suppress her lesions. At least 10 patients completed their vaccination series. Follow‐up information for patient 1 could not be obtained; and patient 9 declined her second vaccine dose because of persistent symptoms.
4. DISCUSSION
The BNT162b2 vaccine by Pfizer‐BioNTech and mRNA‐1273 vaccine by Moderna utilize mRNA encoded for the SARS‐CoV‐2 full‐length spike protein that is encapsulated in lipid nanoparticle for delivery. 4 , 5 The lipid shell is further stabilized by polyethylene glycol (PEG), a common polymer additive that has been proposed to be a potential antigenic source in hypersensitivity reactions. 6 In clinical trial data, delayed injection‐site reactions (ISRs), such as erythema, induration, and tenderness, were reported in 0.8% of trial participants after their first Moderna vaccine. 5 More recently, a transient and localized erythematous eruption around the injection site referred to as “COVID arm” has been reported to occur between 7 and 10 days after the first dose of the Moderna vaccine. 7 , 8 , 9 Patient 1 in our case series presented with a similar reaction after receiving the Moderna vaccine. The histopathologic features showed a mixed‐cell infiltrate with eosinophils, consistent with a dermal hypersensitivity reaction. ISRs can also occur with the Pfizer vaccine, albeit much more rarely. 3 We also report an ISR following the Pfizer vaccine with histopathological findings of an eczematous process.
The majority of the patients (patients 3‐8) in our series presented with generalized eruptions with various clinical appearances. These included morbilliform, pityriasiform, psoriasiform, and eczematous morphologies. These findings corroborate prior case reports of pityriasiform eruptions following administration of the Pfizer vaccine, 10 , 11 as well as a recent registry‐based study that described a variety of delayed reactions to COVID‐19 mRNA vaccines including morbilliform, pityriasiform, psoriasiform, and eczematous eruptions. 3 On histopathological examination, three of our cases showed a vacuolar interface pattern consistent with an exanthematous drug reaction while the other three cases revealed a spongiotic pattern. Eosinophils were a prominent feature in all but two cases. Compelling temporal associations were documented for four patients (patients 4, 6, 8, and 11), all of whom developed a cutaneous eruption after their first vaccine dose with recurrence or worsening of their rash after the second vaccine.
Another interesting observation from our case series is that vaccination may worsen pre‐existing dermatologic conditions, as in our patient 6 who developed a recurrence and subsequent flare of his eczema. Similar reports of reactivation following COVID‐19 vaccination include radiation recall skin reactions, 12 lichen planus occurring after 7 years of inactivity, 13 as well as flares of herpes simplex virus infection, atopic dermatitis, psoriasis, and urticarial vasculitis. 3 Interestingly, the majority of these reactions, including our patient, occurred in patients receiving the Pfizer‐BioNTech vaccine.
We also report more severe immune‐mediated events in our case series in conjunction with histopathological confirmation. In the case of patient 9, the development of urticaria within 24 hours of vaccination provided a strong temporal association. However, the persistence of these lesions 5 weeks later led to a biopsy, which showed evidence of vascular injury. Reactivation of known urticarial vasculitis has been reported in one patient following an unspecified mRNA vaccine 3 ; however, there have been no reports to date of de novo urticarial vasculitis following COVID‐19 vaccination. Patient 10 presented as leukocytoclastic vasculitis (LCV) with laboratory abnormalities including low complement levels, elevated rheumatoid factor, and detection of cryoglobulin. She otherwise had no other signs of systemic inflammation and no prior history of an autoimmune condition. Development of vasculitis was previously reported in three patients after receiving the Moderna or Pfizer vaccines; however, the presence of laboratory abnormalities including cryoglobulins was not reported. 3 Both vasculitis and cryoglobulinemia have been reported following influenza 14 and pneumococcal 15 vaccinations, so it is possible that the patient's constellation of symptoms may be an immune complex‐mediated sequelae of the COVID‐19 vaccine.
Lastly, we report two cases of new‐onset bullous pemphigoid. Patient 11 developed localized blisters 3 weeks after his first vaccine dose that rapidly progressed after receiving his second dose. Patient 12 developed lesions 2 weeks after the second vaccine dose. The longer latency period may reflect the period of antibody production. While the onset may be coincidental, postvaccination BP has been reported following vaccinations for herpes zoster, 16 diphtheria tetanus, 17 influenza, 18 among others. It is still unclear if this phenomenon represents non‐specific immune‐mediated activation or the development of antigen mimicry.
5. CONCLUSIONS
While a true association between these cutaneous adverse reactions and the COVID‐19 vaccine cannot be determined from this case series or from currently published clinical trial data, data registries to report vaccine‐related adverse events are available to physicians. 19 Dermatopathologists should be aware of the histopathological features of potential cutaneous adverse reactions to mRNA‐based COVID‐19 vaccines. In many cases, eosinophils are a prominent histopathological finding, although are not always identified. Other common histopathological features include epidermal spongiosis, vacuolar interface changes, and mixed‐cell infiltrates. For immune‐mediated cutaneous presentations, direct immunofluorescence studies may be helpful for diagnosis.
Supporting information
Supplemental Table 1. Patient Dermatologic, Medical, and Medication History
ACKNOWLEDGMENT
We thank Dr Mark Halsey and Dr Jo‐Ann Latkowski for providing clinical information and photographs.
Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID‐19 vaccination. J Cutan Pathol. 2022;49(1):34-41. 10.1111/cup.14104
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Meo S, Bukhari I, Akram J, Meo A, Klonoff D. COVID‐19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663‐1679. 10.26355/eurrev_202102_24877 [DOI] [PubMed] [Google Scholar]
- 2. Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer‐BioNTech COVID‐19 vaccine. JAMA. 2021;325(8):780‐781. 10.1001/jama.2021.0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46‐55. 10.1016/j.jaad.2021.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/nejmoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. 10.1056/nejmoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klimek L, Novak N, Hamelmann E, et al. Severe allergic reactions after COVID‐19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA: position statement of the German Allergy Societies: Medical Association of German Allergologists (AeDA), German Society for Allergology and Clinical Immunology (DGAKI) and Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J Int. 2021;30(2):51‐55. 10.1007/s40629-020-00160-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez‐Nieto D, Hammerle J, Fernandez‐Escribano M, et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. ‘COVID‐arm’: a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35(7):e425‐e427. 10.1111/jdv.17250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wei N, Fishman M, Wattenberg D, Gordon M, Lebwohl M. “COVID arm”: a reaction to the Moderna vaccine. JAAD Case Rep. 2021;10:92‐95. 10.1016/j.jdcr.2021.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID‐19 vaccine: a case series. JAMA Dermatol. 2021;157(6):716‐720. 10.1001/jamadermatol.2021.1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carballido Vázquez A, Morgado B. Pityriasis rosea‐like eruption after Pfizer‐BioNTech COVID‐19 vaccination. Br J Dermatol. 2021. [Online ahead of print]. 10.1111/bjd.20143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cyrenne B, Al‐Mohammedi F, DeKoven J, Alhusayen R. Pityriasis rosea‐like eruptions following vaccination with BNT162b2 mRNA COVID‐19 vaccine. J Eur Acad Dermatol Venereol. 2021. [Online ahead of print]. 10.1111/jdv.17342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soyfer V, Gutfeld O, Shamai S, Schlocker A, Mermisky O. COVID‐19 vaccine‐induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys. 2021;110(4):957‐961. 10.1016/j.ijrobp.2021.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiltun I, Sarriugarte J, Martínez‐de‐Espronceda I, et al. Lichen planus arising after COVID‐19 vaccination. J Eur Acad Dermatol Venereol. 2021;35(7):e414‐e415. 10.1111/jdv.17221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lohse A, Michel F, Auge B, Toussirot E, Wendling D. Vascular purpura and cryoglobulinemia after influenza vaccination. Case‐report and literature review. Rev Rhum Engl Ed. 1999;66(6):359‐360. [PubMed] [Google Scholar]
- 15. Eid S, Callen JP. Type II mixed cryoglobulinemia following influenza and pneumococcal vaccine administration. JAAD Case Rep. 2019;5(11):960‐962. 10.1016/j.jdcr.2019.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bell H, Kamal N, Wong U. Blistering autoimmune skin reaction following SHINGRIX vaccination in an ulcerative colitis patient: case report and literature review. Vaccine. 2020;38(47):7455‐7457. 10.1016/j.vaccine.2020.09.073 [DOI] [PubMed] [Google Scholar]
- 17. Sezin T, Egozi E, Hillou W, Avitan‐Hersh E, Bergman R. Anti‐laminin‐332 mucous membrane pemphigoid developing after a diphtheria tetanus vaccination. JAMA Dermatol. 2013;149(7):858‐862. 10.1001/jamadermatol.2013.741 [DOI] [PubMed] [Google Scholar]
- 18. Downs A, Lear J, Bower C, Kennedy C. Does influenza vaccination induce bullous pemphigoid? A report of four cases. Br J Dermatol. 1998;138(2):363. 10.1046/j.1365-2133.1998.02097.x [DOI] [PubMed] [Google Scholar]
- 19. Rice SM, Ferree SD, Mesinkovska NA, Kourosh AS. The art of prevention: COVID‐19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7(2):209‐212. 10.1016/j.ijwd.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Patient Dermatologic, Medical, and Medication History
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


