Abstract
Aim
We investigated whether the ongoing COVID‐19 pandemic was associated with the occurrence of Kawasaki disease or with multi‐inflammatory syndrome in children (MIS‐C).
Methods
This national Finnish register‐based study was based on laboratory‐confirmed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections, MIS‐C and Kawasaki disease cases. We performed a time series analysis on the occurrence of Kawasaki disease in 2016–2020.
Results
In 2020, there were 5170 laboratory‐confirmed COVID‐19 cases in children under 18 years of age and five fulfilled the MIS‐C case definition. The occurrence of MIS‐C was 0.97 per 1000 (95% confidence interval: 0.31‐2.26) laboratory‐confirmed SARS‐CoV‐2 infections in children. Our time series analysis showed that Kawasaki disease cases decreased during the COVID‐19 pandemic. The seasonally adjusted incidence rate ratio was 0.49 (95% confidence interval: 0.32‐0.74) when it was compared to pre‐pandemic levels. This coincided with a reduced occurrence of respiratory infections, due to social distancing in the population.
Conclusion
This nationwide register‐based study found that MIS‐C was a rare complication of the SARS‐CoV‐2 infection. The occurrence of Kawasaki disease and respiratory infections decreased during the pandemic. This suggests that transmissible microbes may play an important role in Kawasaki disease and social distancing may have a protective effect.
Keywords: epidemiology, Kawasaki disease, multi‐inflammatory syndrome in children, social distancing, transmissible microbes
Abbreviations
- MIS‐C
multisystem inflammatory syndrome in children
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Keynotes.
We used Finnish national registers to study the epidemiology of multi‐inflammatory syndrome in children (MIS‐C) and Kawasaki disease in relation to the COVID‐19 pandemic.
MIS‐C was a rare complication of confirmed severe acute respiratory syndrome coronavirus 2, and the occurrence of Kawasaki disease decreased during the pandemic.
This suggests that other transmissible microbes play an important role in Kawasaki disease and social distancing may have a protective effect.
1. INTRODUCTION
Since the global emergence of COVID‐19, several centres have reported clusters of critically ill children with an inflammatory syndrome that appeared similar to Kawasaki disease. This is now commonly known as multisystem inflammatory syndrome in children (MIS‐C). 1 , 2 Surveillance data of childhood COVID‐19 cases have indicated that children have lower rates of hospitalisation than adults. However, MIS‐C has emerged as a novel clinical entity with a broad clinical spectrum, including life‐threatening cardiogenic shock. 3 , 4
Several studies have reported typical symptoms of MIS‐C in patient clusters. 1 , 5 , 6 , 7 , 8 , 9 However, there have been limited epidemiological data about the estimated proportion of children who develop MIS‐C after mild, but symptomatic, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections. Furthermore, the overall incidence rate ratios of Kawasaki disease or Kawasaki disease–like diagnoses have rarely been compared before and during the COVID‐19 pandemic using a time series analysis. 10 , 11
The aim of this register‐based, nationwide study was to report the occurrence of MIS‐C during the COVID‐19 pandemic in 2020. Furthermore, we wanted to compare the relative changes in the occurrence of Kawasaki disease before and during the COVID‐19 pandemic using a time series analysis.
2. PATIENTS AND METHODS
2.1. Study design
We conducted a register‐based, nationwide study of the epidemiology of MIS‐C in Finland in 2020 and of Kawasaki disease in 2016‐2020, before and during the COVID‐19 pandemic. Since August 2020, Finland has strongly recommended free SARS‐CoV‐2 polymerase chain reaction testing for all children with symptoms that suggest COVID‐19, even if they are mild. On 16 March 2020, the Finnish Government announced a state of emergency, which included temporarily closing restaurants, food and beverage services and primary and secondary schools until 13 May. Preschools and day care centres were not closed. After the two‐month closure period, schools remained mostly open for the rest of 2020.
The study was covered by the institutional research permit of the Finnish Institute for Health and Welfare, who performed the analyses. According to national legislation, informed consent was not required for this study, because it was performed with register‐based data and no patients were contacted. Other authors used and analysed the summarised register‐based data.
2.2. National registers and data retrieval
We used the Finnish Care Register for Health Care to identify children under 18 years of age with MIS‐C from 1 January to 31 December 2020 and children with Kawasaki disease from 1 January 2016 to 31 December 2020. Finland’s Care Register for Health Care, maintained by the Finnish Institute for Health and Welfare, includes data on the diagnoses of patients who were discharged from hospitals or who visited outpatient clinics or family doctors at primary healthcare centres. We retrieved data for all patients with MIS‐C aged 0–18 using the International Classification of Diseases, Tenth Revision (ICD‐10) diagnosis code M35.8 and for patients with COVID‐19 using the ICD‐10 code U07.2. Information on patients with Kawasaki disease was retrieved using the ICD‐10 code M30.3. We contacted all five university hospitals in Finland with paediatric intensive care units to double‐check the MIS‐C cases during the study period. This confirmed that all the cases had been recorded in the registers. We calculated the annual population‐based incidence rate of Kawasaki disease from 2016 to 2020 using official government population data from Statistics Finland. The number of children with a laboratory‐confirmed SARS‐CoV‐2 infection was retrieved from the National Infectious Diseases Register. All clinical microbiology laboratories in Finland are required to report all positive cases of SARS‐CoV‐2 to this Register. We also used the Register to demonstrate the impact of social distancing on the occurrence of other infections during the COVID‐19 pandemic. To do this, we retrieved the numbers of influenza A, respiratory syncytial virus and Mycoplasma pneumoniae–positive respiratory samples, as well as positive Streptococcus pneumoniae blood cultures. Anonymised, summarised data from this Register are freely available to the public in Finland. The number of other respiratory infections was present in children and adolescents aged 0‐19 years, because of the pre‐defined age structure of the Register’s public database.
2.3. Case definition
We defined MIS‐C as follows: the patient had a registry entry in the Finnish Care Register for Health Care for MIS‐C or fulfilled the case definition of MIS‐C provided by The American Centers for Disease Control and Prevention. 12 They had also been discharged from, or died, in any of the five Finnish university hospitals with paediatric intensive care wards. Kawasaki disease was defined as a patient with an entry for Kawasaki disease in the same Finnish Register. The child’s first visit for Kawasaki disease was identified, and subsequent, or duplicate, visits were excluded.
2.4. Statistical analysis
We calculated the annual incidence rates, and 95% confidence intervals (95% CIs), for both Kawasaki disease and laboratory‐confirmed SARS‐CoV‐2 cases by dividing the total numbers by children aged 0‐18 in Finland in 2020, according to Statistics Finland. The MIS‐C incidence rates, and 95% CIs, were calculated per 1000 laboratory‐confirmed SARS‐CoV‐2 cases for the three‐week periods from 1 January 2020 to 31 December 2020. This enabled us to examine the impact of the COVID‐19 epidemic on the occurrence of Kawasaki disease.
We also performed an interrupted time series, which is a quasi‐experimental design that evaluates changes on longitudinal data in relation to an intervention point. In this case, the intervention point was the start of the pandemic, including the need for social distancing. 11 The outcome was the quarterly number of Kawasaki disease cases each year from 2016 to 2020. We chose the date 16 March as the cut‐off point for our pre‐pandemic and pandemic data, as that is when Finland declared a state of emergency. Our time series included an intercept time variable, which was 20 three‐month periods, and a period variable. Seasonality was adjusted using a Fourier term, and over‐dispersion of data was controlled by scaled parameters with Pearson’s chi‐square test. The goodness of fit of model was evaluated by the Hosmer‐Lemeshow chi‐square test (p=0.448). Autocorrelation was graphically examined, but it was not detected after the data were adjusted for seasonality. 11
We used the most feasible program for each statistical analysis, because all the statistical analyses were not available in one program. The differences between the two proportions were tested with a standard normal deviate test available in StatsDirect statistical software 3 (StatsDirect Ltd). The data management and calculation of basic statistics were conducted using SPSS, version 27 (IBM Corp), and the 95% CI for rates and proportion differences were calculated using StatsDirect statistical software 3 (StatsDirect Ltd). ITS analysis was performed using Stata 16 (StataCorp LLC), and the incidence rates were created using OriginPro 2020 (OriginLab Corporation).
3. RESULTS
From 1 January 2020 to 31 December 2020, there were 5,170 laboratory‐confirmed SARS‐CoV‐2 infections in children under 18 years of age (Figure 1A). The first confirmed paediatric cases were reported on 24 February 2020. In Finland, schools were closed from 16 March 2020 to 13 May 2020, but these did not include preschool children or those in day care. Since August 2020, active polymerase chain reaction SARS‐CoV‐2 testing has been strongly recommended for all Finnish children with infectious symptoms that suggested COVID‐19, as part of the national strategy.
FIGURE 1.
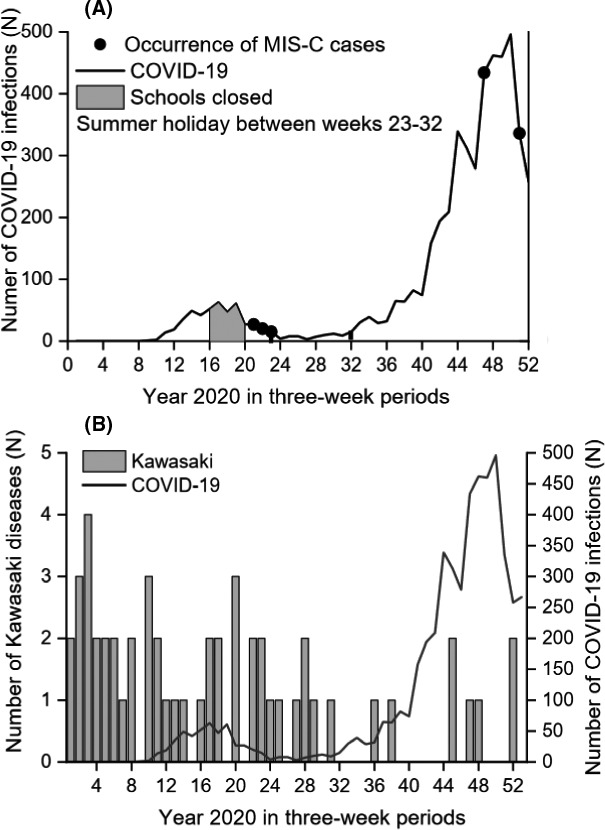
(A) Laboratory‐confirmed COVID‐19 infections in children and adolescents (<18years of age) in Finland in 2020. Active laboratory diagnostics for all children, even with mild respiratory symptoms, has been strongly recommended since August 2020, that is weeks 33‐34 onwards. (B) The occurrence of COVID‐19 and Kawasaki disease in children in Finland in 2020
The incidence of SARS‐CoV‐2 under 18 years of age was 470 per 100,000 (95% CI: 457‐483). Five paediatric patients fulfilled the case definition of MIS‐C from 1 March to 31 December 2020. There were no deaths. The annual population‐based incidence of MIS‐C was 0.45 per 100,000 in children under 18 years (95% CI: 0.15‐1.06). The occurrence of MIS‐C was 0.97 per 1,000 (95% CI: 0.31‐2.26) when the rate was calculated against all laboratory‐confirmed SARS‐CoV‐2 infections in that age group.
The annual population‐based incidence rate of Kawasaki disease in children was 4.6 per 100,000 (95% CI: 3.5‐6.1) in 2020 (Figure 1B). When we analysed the quarterly time periods from 2016 to 2020, the incidence ranged from 2.18 (95% CI: 1.40‐3.25) in January to March 2018 to 0.55 (95% CI: 0.20‐1.19) in both July to September 2020 and October to December 2020 (Table 1). Our time series analysis showed that the occurrence of Kawasaki disease decreased during the COVID‐19 pandemic (Figure 2). The incidence rate ratio was 0.49 (95% CI: 0.29‐0.72) when it was compared to the number of cases before the COVID‐19 pandemic. Data from the National Infectious Disease Registry showed that the occurrence of influenza A, the respiratory syncytial virus, Mycoplasma pneumoniae and Streptococcus pneumoniae markedly decreased in children and adolescents aged 0‐19 after March 2020 (Figure 3).
TABLE 1.
The population‐based incidence of Kawasaki disease among children in Finland from 2016 to 2020. The COVID‐19 pandemic began in March 2020
| Quarter | Year | ||||
|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | |
| Incidence (95% CI) | Incidence (95% CI) | Incidence (95% CI) | Incidence (95% CI) | Incidence (95% CI) | |
| Q1 | 1.50 (0.88 to 2.41) | 1.69 (1.02 to 2.63) | 2.15 (1.38 to 3.19) | 1.90 (1.17 to 2.90) | 2.18 (1.40 to 3.25) |
| Q2 | 1.86 (1.49 to 2.84) | 1.06 (0.06 to 1.86) | 1.79 (1.09 to 2.76) | 1.26 (0.69 to 2.12) | 1.36 (0.76 to 2.25) |
| Q3 | 1.50 (0.88 to 2.41) | 1.51 (0.88 to 2.42) | 1.25 (0.68 to 2.10) | 1.99 (1.24 to 3.01) | 0.55 (0.20 to 1.19) |
| Q4 | 1.06 (0.55 to 1.85) | 1.15 (0.61 to 1.97) | 1.43 (0.81 to 2.33) | 1.44 (0.83 to 2.35) | 0.55 (0.20 to 1.19) |
Q1 indicates January‐March, Q2 indicates April‐June, Q3 indicates July‐September, and Q4 indicates October‐December.
FIGURE 2.
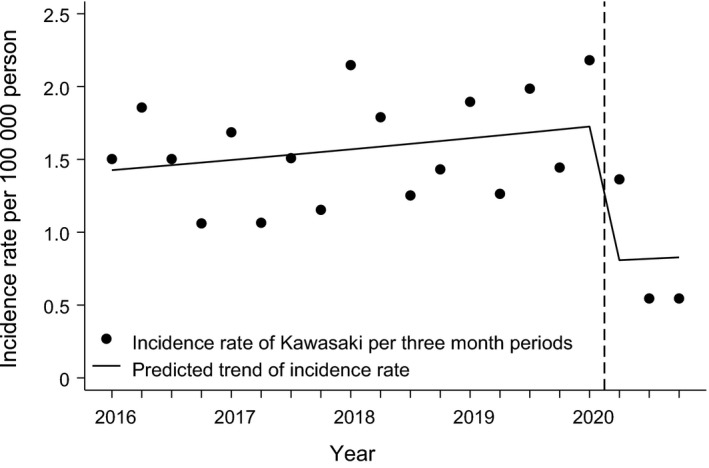
Time series analysis of the occurrence of Kawasaki disease among children in Finland from 2016 to 2020. The seasonally adjusted incidence rate ratio (IRR) was 0.46 (95% CI: 0.29 to 0.72) compared to the incidence rate before the COVID‐19 pandemic. The start of the global pandemic in March 2020 is indicated with a vertical dash line
FIGURE 3.
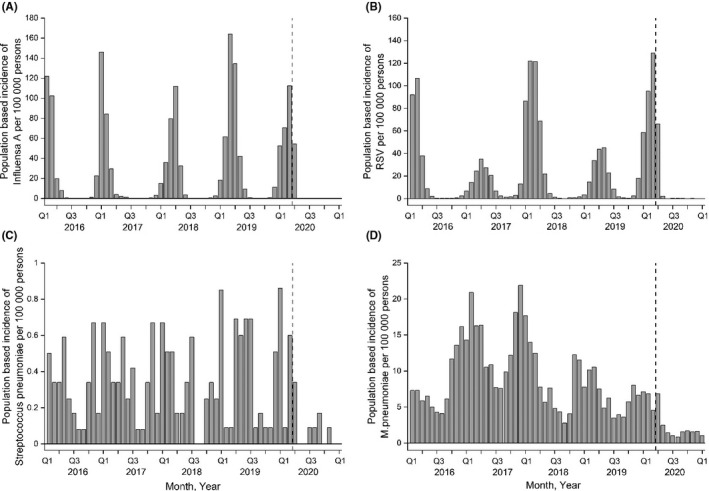
The occurrence of common respiratory viral infections (influenza A and RSV) (A‐B), blood‐culture positive Streptococcus pneumoniae infections (C) and Mycoplasma pneumoniae (D) in 0‐ to 19‐year‐old children and adolescents in Finland in 2016‐2020. Data from the public National Infectious Diseases Register are presented. The beginning of COVID‐19 pandemic is indicated with a vertical dash line. The 0 level has deliberately been elevated to show the absence of infections more clearly
4. DISCUSSION
This nationwide register‐based study showed that MIS‐C was a rare complication of the SARS‐CoV‐2 infection in children. The occurrence of Kawasaki disease markedly decreased during the COVID‐19 pandemic, coinciding with a reduced number of respiratory infections in children. This was probably due to social distancing. 13 , 14 The observed reduction suggests that transmissible microbes play an important role in the pathogenesis of Kawasaki disease.
MIS‐C is a novel, serious and life‐threatening disease associated with COVID‐19 in children. 1 , 4 , 6 , 8 There have been limited data on the proportion of children developing MIS‐C after being infected with SARS‐CoV‐2. Most previous studies have presented the symptoms in selected patient series or clear temporal patient clusters during the peak of the pandemic. 1 , 6 , 7 , 15 The data in our present study suggest that MIS‐C is an infrequent complication of COVID‐19, occurring in less than 0.1% of children in the population with laboratory‐confirmed SARS‐CoV‐2. Despite this, our study may still markedly overestimate the proportion of children developing MIS‐C after COVID‐19, because not all children, especially those with asymptomatic infections, were tested for SARS‐CoV‐2. According to the Centers for Disease Control and Prevention, there were 3,185 patients and 36 deaths that met the case definition of MIS‐C in the United States from mid‐May 2020, when reporting began, to 29 March 2021. Data from a surge in cases in New York showed that the incidence of SARS‐CoV‐2 infections in those under 21 years of age was 322 per 100,000 and the incidence of MIS‐C was two per 100,000 in the same age group. 7 A large cohort study from the United States reported similar results, namely that the cumulative MIS‐C incidence per 100,000 people under 21 years was 2.1 and varied from 0.2 to 6.3 by state. 16 In the present study, the annual population‐based incidence rate of MIS‐C was 0.3 per 100,000, which was lower than the rate reported in the United States. This may be explained by the different study populations and COVID‐19 epidemiology situation in Finland. 17 , 18
Some MIS‐C patients have met the diagnostic criteria for Kawasaki disease or Kawasaki‐like disease. 7 , 8 For instance, Ouldali et al. 10 reported a rapid fivefold increase in the incidence of Kawasaki disease in a French cohort during a period when SARS‐CoV‐2 was the only widely circulating virus. That is why we decided to perform a time series analysis of Kawasaki disease in children before and during the COVID‐19 pandemic. We found that the incidence rate of Kawasaki disease markedly decreased after the pandemic began. Furthermore, the reduced occurrence of Kawasaki disease coincided with the reduced respiratory infections in children and in adolescents in the same population. This was in line with several studies that reported that viral respiratory infections had decreased by 60%–70% in the population due to social distancing. 13 , 14 , 19 , 20 Thus, the reduced transmission of microbes other than SARS‐CoV‐2 may explain the observed decreased incidence of Kawasaki disease in the present study. Our finding was in line with a study from six hospitals in Japan, which showed a possible 27% reduction in Kawasaki disease, with an incidence rate ratio of 0.73 (95% CI: 0.48‐1.10) during the COVID‐19 pandemic. 21 The aetiopathogenesis of Kawasaki disease has been extensively studied but is not fully understood. It appears to result from an interplay between genetic susceptibility and an infectious trigger, followed by an abnormal immune response. Ouldali et al. reported the emergence of Kawasaki disease in France during the COVID‐19 pandemic 10 and noted that the study population was multi‐ethnic. The present study did not report the patients' ethnicity, as that is not registered in medical records or national registers in Finland. Most Finnish residents are Northern European, but it is still possible that differences in ethnicity and genetic susceptibility may have influenced the occurrence of Kawasaki disease in our study population.
It has been suggested that respiratory viruses, such as influenza, the respiratory syncytial virus and enteroviruses, may trigger Kawasaki disease in genetically predisposed children. 22 , 23 Esper et al. carried out a small case‐control study and reported that a novel human coronavirus called New Haven coronavirus was detected more frequently in the respiratory secretions of patients with Kawasaki disease than in control patients. However, this finding has not been replicated in other studies. 24 The idea that respiratory viruses are the main triggers of Kawasaki disease is supported by findings from its clear seasonal cycle, which is similar to that of respiratory infections, but the data are insufficient to prove its involvement with coronaviruses in general. 22 , 25 , 26
The present study had several strengths. This was a large nationwide study that used comprehensive data from high‐quality national registers that also covered the periods before the COVID‐19 pandemic. In addition, we performed a time series analysis, which has rarely been used to compare Kawasaki disease–like diagnoses before, and during, the COVID‐19 pandemic. Since August 2020, Finland has strongly recommended free, national and easily available testing for all children with symptoms, no matter how mild they are. This means that we were able to estimate the total number of SARS‐CoV‐2 infections in the study population better than most previous reports of MIS‐C patient clusters during the COVID‐19 pandemic. In addition to the register‐based data, we retrieved all MIS‐C cases directly from the five university hospitals with paediatric intensive care units in Finland to ensure they had all been included in that data. We also present the changes in the epidemiology of common respiratory infections in the study population.
Our register‐based study had some limitations. These included the lack of a specific ICD‐10 code for MIS‐C from the start of the COVID‐19 emergency, which may have affected the accuracy of the register‐based data. Finally, the low number of MIS‐C cases did not allow us to carry out analyses to compare the characteristics of MIS‐C and Kawasaki disease patients.
5. CONCLUSION
This nationwide register‐based study showed that MIS‐C was a rare complication of the SARS‐CoV‐2 infection in children. The occurrence of Kawasaki disease decreased during the COVID‐19 study period and coincided with a reduced number of respiratory infections due to social distancing. This finding suggests that transmissible microbes play an important role in the pathogenesis of Kawasaki disease.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
Koskela U, Helve O, Sarvikivi E, et al. Multi‐inflammatory syndrome and Kawasaki disease in children during the COVID‐19 pandemic: A nationwide register‐based study and time series analysis. Acta Paediatr. 2021;110:3063–3068. 10.1111/apa.16051
REFERENCES
- 1. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS‐C) compared with severe acute COVID‐19. JAMA. 2021;325(11):1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS‐CoV‐2 pandemic. Circulation. 2020;142:429–436. [DOI] [PubMed] [Google Scholar]
- 5. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bautista‐Rodriguez C, Sanchez‐de‐Toledo J, Clark BC, et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021;147(2):e2020024554. [DOI] [PubMed] [Google Scholar]
- 9. DeBiasi RL, Song X, Delaney M, et al. Severe coronavirus disease‐2019 in children and young adults in the Washington, DC. Metropolitan Region. J Pediatr. 2020;223:199–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ouldali N, Pouletty M, Mariani P, et al. Emergence of Kawasaki disease related to SARS‐CoV‐2 infection in an epicentre of the French COVID‐19 epidemic: a time‐series analysis. Lancet Child Adolesc Health. 2020;4:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for disease control and prevention. Emergency preparedness and response: Multisystem Inflammatory Syndrome in Children (MIS‐C) Associated with Coronavirus Disease 2019 (COVID‐19). https://emergency.cdc.gov/han/2020/han00432.asp. Accessed April 29, 2021.
- 13. Kuitunen I, Haapanen M, Artama M, Renko M. Closing Finnish schools and day care centres had a greater impact on primary care than secondary care emergency department visits. Acta Paediatr. 2021;110:937–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuitunen I, Artama M, Makela L, Backman K, Heiskanen‐Kosma T, Renko M. Effect of social distancing due to the COVID‐19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 2020;39:e423–e427. [DOI] [PubMed] [Google Scholar]
- 15. Toubiana J, Poirault C, Corsia A, et al. Kawasaki‐like multisystem inflammatory syndrome in children during the covid‐19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belay ED, Abrams J, Oster ME, et al. Trends in Geographic and Temporal Distribution of US Children With Multisystem Inflammatory Syndrome During the COVID‐19 Pandemic. JAMA Pediatr. 2021;6:e210630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. THL . Situation update on coronavirus in Finland. https://THL.fi/en/web/infectious‐diseases‐and‐vaccinations/what‐s‐new/coronavirus‐covid‐19‐latest‐updates/situation‐update‐on‐coronavirus. Accessed May 6, 2021.
- 18. Coronavirus COVID‐19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html. Accessed May 6, 2021.
- 19. Haapanen M, Renko M, Artama M, Kuitunen I. The impact of the lockdown and the re‐opening of schools and day cares on the epidemiology of SARS‐CoV‐2 and other respiratory infections in children ‐ A nationwide register study in Finland. EClinicalMedicine. 2021;34:100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shulman S, Geevarghese B, Kim KY, Rowley A. The Impact of Social Distancing for COVID‐19 Upon Diagnosis of Kawasaki Disease. J Pediatric Infect Dis Soc. 2021;23:piab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hara T, Furuno K, Yamamura K, et al. Assessment of pediatric admissions for Kawasaki disease or infectious disease during the COVID‐19 State of Emergency in Japan. JAMA Netw Open. 2021;4:e214475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitano N, Suzuki H, Takeuchi T. Patient age and the seasonal pattern of onset of Kawasaki's disease. N Engl J Med. 2018;378:2048–2049. [DOI] [PubMed] [Google Scholar]
- 23. Ae R, Makino N, Kosami K, Kuwabara M, Matsubara Y, Nakamura Y. Epidemiology, treatments, and cardiac complications in patients with Kawasaki disease: the nationwide survey in Japan, 2017–2018. J Pediatr. 2020;225:23–29.e2. [DOI] [PubMed] [Google Scholar]
- 24. Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burns JC, Cayan DR, Tong G, et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burns JC, Herzog L, Fabri O, et al. Seasonality of Kawasaki disease: a global perspective. PLoS ONE. 2013;8:e74529. [DOI] [PMC free article] [PubMed] [Google Scholar]


