Dear Editor,
A 62‐year‐old woman with metastatic melanoma presented with shortness of breath 4 days after her fourth cycle of combination checkpoint inhibitor therapy (CPI) (nivolumab and ipilimumab) having previously received 12 months of treatment with adjuvant nivolumab 14 months earlier. Subsequent investigations confirmed CPI‐related myocarditis. She also described new onset of symptoms consistent with Raynaud disease (RD). Her medical history included recurrent migraines for which she took propranolol. She was admitted to hospital and received two doses of intravenous methylprednisolone 500 mg and was commenced on a reducing course or oral prednisolone 1 mg/kg, with lansoprazole and co‐trimoxazole prophylaxis. Blood tests initially revealed negative results for antinuclear antibody (ANA), lupus anticoagulant and anticardiolipin antibodies with normal levels of complement and rheumatoid factor. However, repeat blood tests 6 weeks later revealed a positive ANA and a very mildly positive extractable nuclear antigen (anti‐SSA52/Ro autoantibody). Assessment by the rheumatology team did not identify any underlying connective tissue disease and concluded that the RD was likely to be secondary to the CPI.
The patient was discharged, but 2 weeks later she was readmitted with pyrexia, a grade 3 skin rash (70% involvement) and worsening fatigue. The features were consistent with a drug eruption, thought most likely to be secondary to co‐trimoxazole, which she had been on for a week. She had also received one dose of co‐amoxiclav locally for presumed infection, but the skin eruption was already present at this stage. She remained on prednisolone 50 mg daily. A skin biopsy was taken, and histopathological examination revealed nonspecific features of mild superficial perivascular inflammation. The patient was commenced on potent topical steroids, and at follow‐up 7 days later the rash had almost completely resolved; prednisolone was then reduced to 40 mg, with the patient remaining on lansoprazole.
The patient received her second COVID Pfizer vaccination (BioNTech‐Pfizer COVID‐19 RNA vaccine) 2 weeks later, and within 2 days she had had a significant flare of her rash and presented with a further grade 3 eruption. There was no mucosal membrane involvement at that stage. Over the following 7 days, the rash worsened to grade 4 (Fig. 1), becoming erythrodermic with superficial blistering noted on the patient’s thigh and chest with associated mild mucosal and eye involvement, despite her being on prednisolone 30 mg. The findings of a further skin biopsy were consistent with a drug‐induced lichenoid dermatitis with scattered apoptotic bodies and lymphocytic infiltrate (Fig. 2). Direct immunofluorescence was negative. She was admitted to hospital and treated with two further doses of intravenous methylprednisolone 500 mg and her prednisolone dose was increased to 40 mg, while lansoprazole was switched to famotidine. An infective/septic screen and a viral reactivation screen, including Epstein–Barr virus, human herpesvirus (HHV)‐6, HHV‐7, hepatitis B and C viruses, and HIV were negative. She remained systemically well throughout her admission, with subsequent slow improvement of her rash over a 2‐week period.
Figure 1.
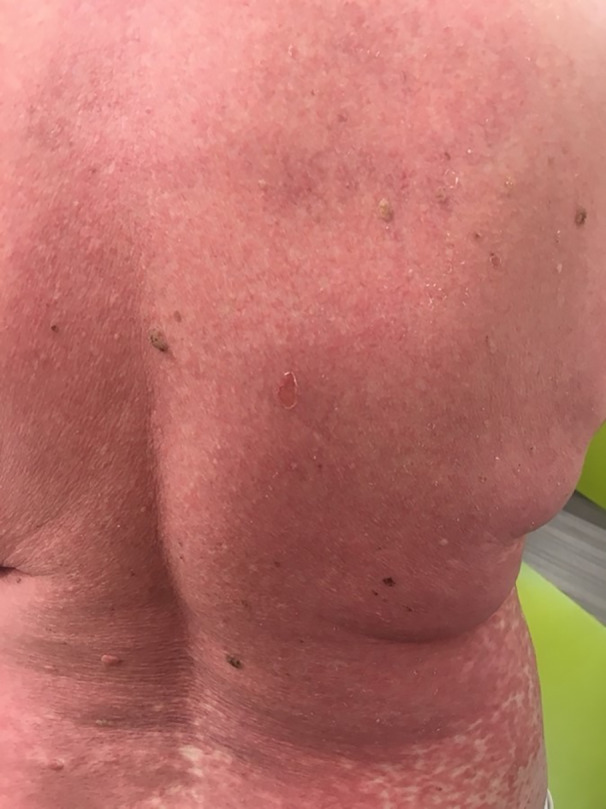
Image revealing grade 4 cutaneous skin toxicity (erythroderma and superficial blistering), with associated pruritis and skin tenderness.
Figure 2.
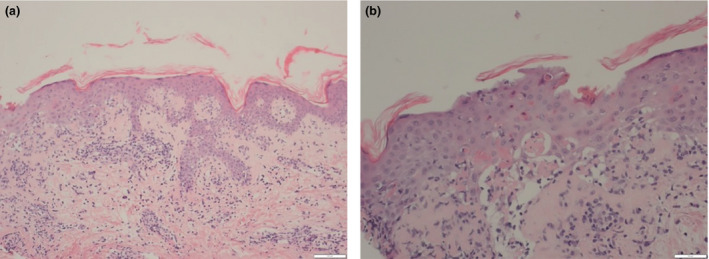
(a) A dense infiltrate at the dermoepidermal junction; and (b) evidence of a lichenoid infiltrate in the upper dermis with scattered apoptotic bodies. Haematoxylin and eosin, original magnification (a) × 100; (b) × 200.
Drug hypersensitivity reactions are the result of immune interactions with small molecular compounds or proteins used as drugs. 1 Delayed‐type hypersensitivity reactions (type IV hypersensitivity) are T‐cell‐mediated reactions that can be CD4+ and/or CD8+ dependent, with a target allergen presented via major histocompatibility molecules to T‐cell receptors. 2 Our patient had an initial drug rash that resolved on cessation of the co‐trimoxazole and she then developed a more severe form of the rash after her vaccine. We postulate that our patient’s original presentation was a reaction to the co‐trimoxazole, during which drug‐specific memory T cells were formed. The subsequent COVID‐19 vaccination then caused a surge in the T‐cell‐driven response from skin‐homing CD4+ T cells generated by the original delayed hypersensitivity reaction. This was on a background of recent CPI therapy, which in itself reduces the self‐tolerance response of T cells and boosts effector T‐cell responses.
Vaccinations have been reported to raise the potential of immune‐related adverse events (irAEs) in patients on CPIs. 3 Skin irAEs were the most commonly reported, followed by arthritis. 4 The Pfizer vaccine has been reported to cause mild skin reactions, which are generally self‐limiting. 5 An increased incidence of co‐trimoxazole‐induced rash in patients treated with CPIs has also been reported. 6 Current recommendations suggest that patients on CPIs can receive inactivated vaccines. 7
This case highlights the importance of possible exacerbation of irAEs in patients on CPIs, which can occur post‐vaccination, especially in the case of recent and active irAEs. This is likely to be an under‐reported phenomenon. Consideration by clinicians of timing of vaccinations should therefore be given in light of active or severe irAEs in patients taking CPIs. Further work is required to elucidate drug reactions and the effect of vaccinations in patients on CPIs.
Acknowledgement
We thank the patient for her informed consent for publication of her case details and images.
Conflict of interest: the authors declare that they have no conflicts of interest.
References
- 1. Demoly P, Adkinson NF, Brockow K et al. International consensus on drug allergy. Allergy 2014; 69: 420–37. [DOI] [PubMed] [Google Scholar]
- 2. Abbas AK. Hypersensitivity disorders. Chapter 19. In: Cellular and Molecular Immunology. 9th edn. (Lichtman AH, Pillai S, Abbas AK eds). Philadelphia: Elsevier, 2018. [Google Scholar]
- 3. Desage A‐L, Bouleftour W, Rivoirard R et al. Vaccination and immune checkpoint inhibitors. Am J Clin Oncol 2021; 44: 109–13. [DOI] [PubMed] [Google Scholar]
- 4. Läubli H, Balmelli C, Kaufmann L et al. Influenza vaccination of cancer patients during PD‐1 blockade induces serological protection but may raise the risk for immune‐related adverse events. J Immunother Cancer 2018; 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edwards C, Larkin J, Fearfield L et al. Increased incidence of co‐trimoxazole induced rash in patients treated with immune checkpoint inhibitors. Br J Dermatol 2020; 183(Suppl): P087. [DOI] [PubMed] [Google Scholar]
- 7. Gauci M‐L, Coutzac C, Houot R et al. SARS‐CoV‐2 vaccines for cancer patients treated with immunotherapies: recommendations from the French society for ImmunoTherapy of Cancer (FITC). Eur J Cancer 2021; 48: 121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


