Summary
Identification of high‐risk patients admitted to intensive care with COVID‐19 may inform management strategies. The objective of this meta‐analysis was to determine factors associated with mortality among adults with COVID‐19 admitted to intensive care by searching databases for studies published between 1 January 2020 and 6 December 2020. Observational studies of COVID‐19 adults admitted to critical care were included. Studies of mixed cohorts and intensive care cohorts restricted to a specific patient sub‐group were excluded. Dichotomous variables were reported with pooled OR and 95%CI, and continuous variables with pooled standardised mean difference (SMD) and 95%CI. Fifty‐eight studies (44,305 patients) were included in the review. Increasing age (SMD 0.65, 95%CI 0.53–0.77); smoking (OR 1.40, 95%CI 1.03–1.90); hypertension (OR 1.54, 95%CI 1.29–1.85); diabetes (OR 1.41, 95%CI 1.22–1.63); cardiovascular disease (OR 1.91, 95%CI 1.52–2.38); respiratory disease (OR 1.75, 95%CI 1.33–2.31); renal disease (OR 2.39, 95%CI 1.68–3.40); and malignancy (OR 1.81, 95%CI 1.30–2.52) were associated with mortality. A higher sequential organ failure assessment score (SMD 0.86, 95%CI 0.63–1.10) and acute physiology and chronic health evaluation‐2 score (SMD 0.89, 95%CI 0.65–1.13); a lower PaO2:FIO2 (SMD −0.44, 95%CI −0.62 to −0.26) and the need for mechanical ventilation at admission (OR 2.53, 95%CI 1.90–3.37) were associated with mortality. Higher white cell counts (SMD 0.37, 95%CI 0.22–0.51); neutrophils (SMD 0.42, 95%CI 0.19–0.64); D‐dimers (SMD 0.56, 95%CI 0.43–0.69); ferritin (SMD 0.32, 95%CI 0.19–0.45); lower platelet (SMD −0.22, 95%CI −0.35 to −0.10); and lymphocyte counts (SMD −0.37, 95%CI −0.54 to −0.19) were all associated with mortality. In conclusion, increasing age, pre‐existing comorbidities, severity of illness based on validated scoring systems, and the host response to the disease were associated with mortality; while male sex and increasing BMI were not. These factors have prognostic relevance for patients admitted to intensive care with COVID‐19.
Keywords: COVID‐19, critical care, meta‐analysis, mortality
Introduction
COVID‐19 requiring admission to ICU has been associated with a high mortality [1], with data reporting a mortality of 41.6% [1]. A more recent meta‐analysis which included the African COVID‐19 Critical Care Outcomes Study reported ICU mortality of 31.5% [2]. Despite the poor outcomes, the current clinical problem remains that the factors associated with ICU mortality are poorly described [3]. Understanding the factors associated with mortality may allow for appropriate risk stratification and management of these critically ill patients.
With the large volume of peer‐reviewed publications relating to COVID‐19 patient outcomes, it may be possible to describe the factors associated with mortality among patients admitted to ICU. Currently, we are only aware of systematic reviews which have described factors associated with mortality with unselected cohorts of COVID‐19 patients [4], and not critically ill patients with COVID‐19. The objective of this systematic review and meta‐analysis of observational studies was to determine which factors are associated with mortality in adult patients with COVID‐19 admitted to ICU.
Methods
This study is reported in accordance with the PRISMA statement [5]. We included all observational studies (prospective and retrospective) of adult patients with COVID‐19 admitted to ICU, reporting mortality or survival outcomes stratified by patient factors, risk scores and haematological results of interest. We excluded studies with mixed cohorts (i.e. not limited to patients admitted to ICU); ICU cohorts restricted to a specific patient sub‐group; studies investigating drug efficacy; and review articles.
Studies were identified through a comprehensive and systematic search of the following databases: MEDLINE, Embase, the Cochrane Library, Africa‐Wide Information via EBSCOhost and SciELO Citation Index via Web of Science. Databases were searched from 1 January 2020 to 10 November 2020, with an updated search on 6 December 2020. The search encompassed terms relating to COVID‐19 and intensive care. This search was supplemented by a manual search up to 21 February 2021. The full search strategy can be found in the online Supporting Information (Appendix S1).
Results from each database were imported to Mendeley reference management software (Elsevier, Amsterdam, Netherlands) and duplicates removed. Titles and abstracts were screened for eligibility by two authors independently based on predefined criteria. The full texts of articles possibly eligible for inclusion were reviewed independently by two authors. Discrepancies were resolved by a third reviewer (ET or BB). Two reviewers independently extracted data from eligible texts (and relevant supplementary material) using a standardised piloted form. Discrepancies were resolved by mutual agreement or by a third reviewer (ET or BB). The reference lists of other reviews were screened for further eligible texts.
We extracted the following information for each study: study design (prospective or retrospective); study location; and the length and location of follow up. For dichotomous variables we collected data on the number of patients who died and survived, stratified by: patient factors (sex; smoking; hypertension; cardiovascular disease; pre‐existing respiratory disease; renal disease; diabetes; malignancy; cerebrovascular disease; liver disease); and respiratory support (invasive mechanical ventilation on ICU admission). The original study definitions for the presence of comorbidities were adopted for this review. For continuous variables, we collected summary data (mean and SD or median and IQR) for the overall cohort, survivors and those who died for: patient factors (age; BMI); intensive care risk stratification scores (sequential organ failure assessment (SOFA) score; acute physiology and chronic health evaluation‐2 (APACHE‐2) score; respiratory support (PaO2:FIO2 ratio on admission to ICU); and haematological factors (D‐dimer; ferritin; platelets; haemoglobin; white blood cells; neutrophils; lymphocytes).
We used a modified Newcastle‐Ottawa Scale to assess the methodological quality of each included study [6], and this is shown in the online Supporting Information (Appendix S2). Studies scoring 7–9 points were considered high quality, with studies scoring ≤ 6 considered low quality. Modified Newcastle‐Ottawa Scale assessments were conducted independently by two reviewers.
We summarised cohort characteristics for dichotomous variables by calculating the proportion of those with each factor in the overall cohort, survived and died groups; for continuous variables, we calculated the pooled estimate mean and 95%CI for the overall cohort, survived and died groups. To assess the association of the factors of interest with mortality we calculated the pooled OR and 95%CI for dichotomous variables and the pooled standardised mean difference (SMD) and 95%CI for continuous variables. Data reported as median and IQR or range were converted to mean and SD using the formula described by Wan et al. [7]. We assessed the τ² and I² statistics as measures of statistical inconsistency and heterogeneity, respectively. A random‐effects model was adopted if there was moderate (25–50%) or high (> 50%) between‐study heterogeneity as assessed by the I² test. The random‐effects meta‐analysis was conducted using the Sidik–Jonkman method. The analysis was conducted using Stata version 16 (StataCorp. 2019, College Station, TX, USA). Funnel plots were generated to assess publication bias. A post‐hoc decision was taken to conduct a sensitivity analysis of haematological factors where we excluded studies when it was unclear if the haematological tests were conducted at ICU admission.
Results
Study screening and selection is shown in Fig. 1. In total, 6498 abstracts were screened, with 751 full‐text reviews. Fifty‐eight studies with 44,305 patients were included in the review [2, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65]. These included studies provided data on mortality or survival following ICU admission for 43,845 (99.0%) of the patients.
Figure 1.
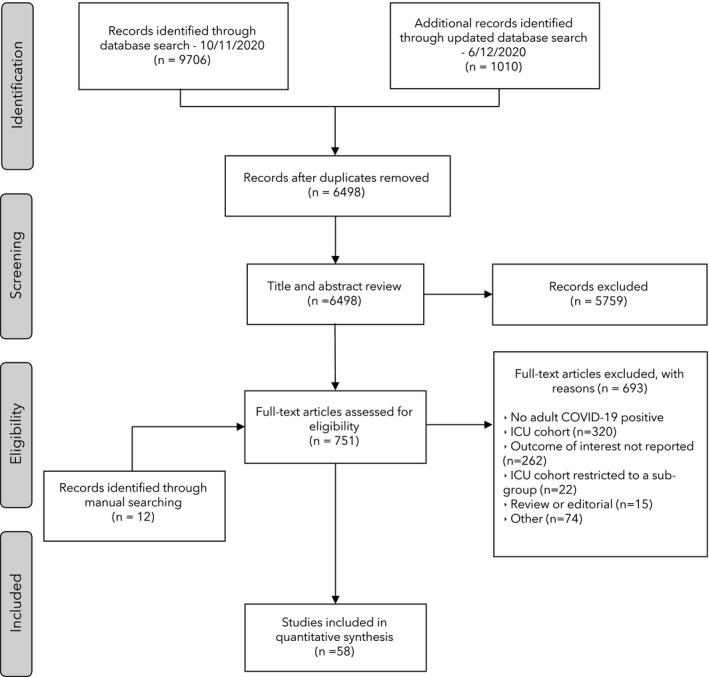
Flow diagram of study screening and inclusion.
The included study and patient cohort characteristics are shown in the online Supporting Information (Appendix S3) and the full reference list in the online Supporting Information (Appendix S4). Fifteen (25.9%) of the studies were prospective, and 12 (20.7%) were multicentre studies. The summarised cohort characteristics for each factor of interest are shown in Tables 1 and 2. There were predominantly male patients (68.9%) with a mean (95%CI) age of 61.8 (60.7–63.0) years. The two most common comorbidities were hypertension (47.7%) and diabetes (26.9%). The majority of patients required invasive mechanical ventilation on admission to ICU (54.0%) with a high mean (95%CI) SOFA score of 5.7 (5.1–6.3) and APACHE‐2 score of 15.7 (14.7–16.6). The Newcastle‐Ottawa Scale risk of bias assessment is shown in the online Supporting Information (Appendix S5). Overall, 45/58 (77.6%) studies were deemed to be high quality.
Table 1.
Characteristics of the dichotomous variable risk‐factors reported in patients with COVID‐19 admitted to ICU. Numbers are value, or value (proportion).
| Studies | Patients | Total with characteristic | With characteristic survived/total survived | With characteristic died/total died | |
|---|---|---|---|---|---|
| Sex; male | 55 | 43,355 | 29,889 (68.9%) | 17,587/26,144 (67.3%) | 12,302/17,211 (71.5%) |
| Renal disease | 28 | 13,926 | 952 (6.8%) | 420/7997 (5.3%) | 532/5929 (9.0%) |
| Cardiovascular disease | 41 | 16,205 | 2097 (12.9%) | 978/9378 (10.4%) | 1119/6827 (16.4%) |
| Hypertension | 45 | 20,496 | 9767 (47.7%) | 5374/12,240 (43.9%) | 4393/8256 (53.2%) |
| Respiratory disease | 41 | 16,353 | 1056 (6.5%) | 514/9406 (5.5%) | 542/6947 (7.8%) |
| Diabetes | 47 | 20,910 | 5627 (26.9%) | 3029/12,530 (24.2%) | 2598/8380 (31.0%) |
| Malignancy | 29 | 14,272 | 801 (5.6%) | 356/8102 (4.4%) | 445/6170 (7.2%) |
| Smoking | 21 | 12,627 | 1579 (12.5%) | 931/7801 (11.9%) | 648/4826 (13.4%) |
| Liver disease | 17 | 9674 | 223 (2.3%) | 109/5128 (2.1%) | 114/4546 (2.5%) |
| Cerebrovascular disease | 12 | 5013 | 266 (5.3%) | 124/2639 (4.7%) | 142/2374 (6.0%) |
| Mechanical ventilation on admission | 6 | 14,504 | 7826 (54.0%) | 4234/8546 (49.5%) | 3592/5958 (60.3%) |
Table 2.
Characteristics of the continuous variable risk‐factors reported in patients with COVID‐19 admitted to ICU. Numbers are value or pooled mean (95%CI).
| Studies | Patients | Overall pooled estimate | Survived pooled estimate | Died pooled estimate | |
|---|---|---|---|---|---|
| Age; y | 51 | 27,149 | 61.8 (60.7‐63.0) | 58.4 (57.1‐59.6) | 66.8 (65.4‐68.1) |
| BMI; kg.m‐2 | 21 | 21,243 | 28.9 (28.2‐29.7) | 28.7 (27.9‐29.5) | 29.0 (28.3‐29.7) |
| SOFA | 24 | 8650 | 5.7 (5.1‐6.3) | 4.7 (4.0‐5.4) | 7.0 (6.4‐7.7) |
| APACHE‐2 | 21 | 13,456 | 15.7 (14.7‐16.6) | 13.4 (12.3‐14.5) | 18.3 (17.2‐19.4) |
| PaO2:FIO2 ratio | 20 | 17,825 | 126.4 (117.2‐135.6) | 137.9 (126.6‐149.1) | 109.5 (98.1‐121.0) |
| D‐dimer | 32 | 9239 | * | * | * |
| Neutrophils | 14 | 2678 | * | * | * |
| White blood Cells | 31 | 8075 | * | * | * |
| Ferritin | 13 | 2345 | * | * | * |
| Haemoglobin | 17 | 4392 | * | * | * |
| Lymphocytes | 29 | 11,083 | * | * | * |
| Platelets | 27 | 9131 | * | * | * |
SOFA, sequential organ failure assessment; APACHE‐2, acute physiology and chronic health evaluation 2.
Pooled mean (95%CI) for haematological results were not estimated due to different units used to report these tests. Summary estimates of risk for mortality associated with haematological results (shown in Fig. 3) were calculated using standardised mean difference, which accounts for variation in reported units.
There was moderate or high heterogeneity across all analyses, and therefore all analyses were conducted with random‐effects models. Increasing age (SMD 0.65, 95%CI 0.53–0.77); smoking (OR 1.40, 95%CI 1.03–1.90); hypertension (OR 1.54, 95%CI 1.29–1.85); diabetes (OR 1.41, 95%CI 1.22–1.63); cardiovascular disease (OR 1.91, 95%CI 1.52–2.38); respiratory disease (OR 1.75, 95%CI 1.33–2.31); renal disease (OR 2.39, 95%CI 1.68–3.40); and malignancy (OR 1.81, 95%CI 1.30–2.52) were associated with mortality following ICU admission. A higher SOFA score (SMD 0.86, 95%CI 0.63–1.10) and APACHE‐2 score (SMD 0.89, 95%CI 0.65–1.13); a lower PaO2:FIO2 (SMD −0.44, 95%CI −0.62 to −0.26) and the need for mechanical ventilation at admission (OR 2.53, 95%CI 1.90–3.37) were all associated with mortality. Higher white cell counts (SMD 0.37, 95%CI 0.22–0.51); neutrophils (SMD 0.42, 95%CI 0.19–0.64); D‐dimers (SMD 0.56, 95%CI 0.43–0.69); ferritin (SMD 0.32, 95%CI 0.19–0.45); lower platelet counts (SMD −0.22, 95%CI −0.35 to −0.10); and lymphocyte counts (SMD −0.37, 95%CI −0.54 to −0.19), were all associated with mortality. Sex, BMI, cerebrovascular disease, liver disease and admission haemoglobin concentration were not associated with mortality following ICU admission (Figs. 2 and 3; see also online Supporting Information, Figures S1–S23).
Figure 2.

Summary estimates of risk for mortality following critical care admission associated with dichotomous variables (patient characteristics; comorbidities; and invasive mechanical ventilation (IMV) on admission).
Figure 3.
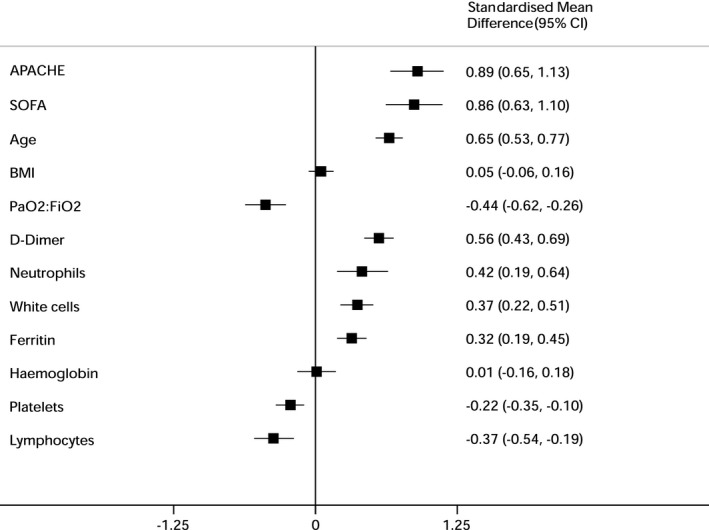
Summary estimates of risk for mortality following critical care admission associated with continuous variables (patient factors; risk scores; and haematological results at admission). SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation‐2.
Funnel plots are shown in the online Supporting Information (Figures S24–S46). These show asymmetry for BMI, cerebrovascular disease and serum ferritin. All other plots appear symmetrical. Sensitivity analyses are shown in the online Supporting Information (Figures S47–S53). Sensitivity analyses included only haematological tests where it was clear that these were taken at the time of admission to ICU. The findings of the sensitivity analysis did not differ from the main findings, except for the neutrophil count which crossed the line of no effect (SMD 0.29, 95%CI −0.05 to 0.62).
Discussion
The principal findings of this meta‐analysis are that increasing age; smoking; hypertension; diabetes; cardiovascular disease; respiratory disease; renal disease; and malignancy were associated with ICU mortality in patients with COVID‐19. At admission to ICU, higher SOFA and APACHE‐2 scores, a lower PaO2:FIO2 and the need for invasive mechanical ventilation were all associated with mortality. Higher white cell counts; neutrophils; D‐dimers; ferritin; lower platelet; and lymphocyte counts were also associated with mortality.
The findings confirm the association between diabetes, cardiovascular and respiratory comorbidities with mortality in COVID‐19 patients. However, the reported associations between male sex and increasing BMI are not supported by this meta‐analysis [66]. This meta‐analysis provides a large sample size with respect to these risk‐factors and is a robust estimate of risk associated with male sex and BMI. The previously described obesity paradox in which patients admitted to ICU with higher BMI have more favourable outcomes [67] is not supported by our findings. The previously described association between male sex and mortality [68, 69, 70] may need to be questioned further in light of these findings, particularly in the context of those admitted to ICU.
The associations with ICU mortality demonstrated in this meta‐analysis may provide direction for future COVID‐19‐specific prognostic research. Age may be a surrogate for frailty in patients with COVID‐19 [71]. The risk‐factors of hypertension, smoking and respiratory disease may all be partially related to increased risk associated with angiotensin‐converting enzyme (ACE) receptors, as seen by the increased expression of ACE‐2 receptors among smokers and patients with chronic obstructive pulmonary disease [72, 73]. The association between hypertension and cardiovascular disease, and increased mortality may potentially increase the risk of cardiac injury associated with the systemic inflammatory response to COVID‐19 infection [74, 75].
The inflammatory response associated with mortality appears to be dysregulated in response to COVID‐19, and it is likely to drive the high mortality in critically ill patients with COVID‐19 [76]. Previously, a smaller meta‐analysis has shown that a higher neutrophil:lymphocyte ratio is associated with mortality [77]. Our meta‐analysis supports this finding with a significantly higher neutrophil count and significantly lower lymphocyte count associated with mortality. Furthermore, the inflammatory effects of a high ferritin, high D‐dimers and low platelet counts could both precipitate or be the result of thrombotic and coagulopathic effects [78]. Our meta‐analysis suggests that there is little difference between the SOFA or APACHE‐2 risk stratification scores at critical care admission in patients with COVID‐19, although other studies have suggested that the APACHE‐2 score may be better at predicting mortality among severely ill patients with COVID‐19 than the SOFA score [64]. Simpler scores, such as the quick SOFA may not have equivalent prognostic performance to the SOFA or APACHE‐2 scores [79], but may have clinical utility in lower resource environments where access to a full blood profile is not universally available [2].
One limitation of this meta‐analysis is that it does not allow us to risk‐adjust between risk factors associated with ICU mortality. Risk adjustment is important in accurate prognostication for ICU admission. Some of the included studies have provided risk‐adjusted (multivariable adjusted) risk‐factors. Of the prospective observational studies, the largest studies which provide data are the Intensive Care National Audit and Research Centre (ICNARC) [25, 65], the COVID‐ICU Group study from Europe [18] and the African COVID‐19 Critical Care Outcomes Study (ACCCOS) [2]. These large prospective observational studies have confirmed an independent association with increasing age, immunosuppression, diabetes, cardiovascular and renal disease, ICU severity scores and a lower PaO2:FIO2 ratio with mortality [2, 18, 19]. While increasing respiratory and ventilatory support at admission were also associated with mortality in this meta‐analysis, these findings should be viewed with caution. They may reflect resource and management factors or deterioration before critical care admission, which may bias the estimates of risk. Without more nuanced data on the effect of resource availability and management strategies before admission, it is impossible to determine the association between the PaO2:FIO2 ratio and the need for invasive mechanical ventilation at admission on outcome. It is likely that the prognostic importance of these risk‐factors will be difficult to determine, especially as a recent meta‐analysis of early vs. late tracheal intubation in patients with COVID‐19 requiring mechanical ventilation did not show a difference in outcome between the two strategies [80].
This meta‐analysis did not assess some factors that may be prognostically important in critically ill patients with COVID‐19, such as a short duration of time between first symptoms and ICU admission [18] and HIV/AIDS [2]. Furthermore, the association between C‐reactive protein, interleukin‐6 or procalcitonin and mortality were also not evaluated [81]. The impact of therapies such as dexamethasone and tocilizumab were not examined in this meta‐analysis [82, 83].
Finally, this meta‐analysis is characterised by high heterogeneity despite using random‐effects models. This may be partly due to the different definitions used for the risk‐factors across the included studies.
In conclusion, increasing age, pre‐existing comorbidities and greater severity of illness are associated with mortality in patients admitted to ICU with COVID‐19, but male sex and increasing BMI were not. The host response to disease as manifested by various inflammatory and thrombotic markers and the severity of respiratory failure also predicts outcome. Requiring invasive mechanical ventilation on admission to ICU was a significant predictor of mortality although resources, management strategies and pre‐admission deterioration may all modify this risk‐factor.
Supporting information
Figure S1–S23. Forest plots demonstrating associations between characteristics and mortality in COVID‐19 patients admitted to critical care.
Figure S24–S46. Funnel plots of studies reporting the associations between characteristics and mortality in COVID‐19 patients admitted to critical care.
Figure S47–S53. Sensitivity analyses with forest plots demonstrating associations between characteristics and mortality in COVID‐19 patients admitted to critical care.
Appendix S1. Search strategy used for this systematic review.
Appendix S2. Modified Newcastle‐Ottawa Quality Assessment Scale.
Appendix S3. Study and cohort characteristics of studies included in the review.
Appendix S4. Reference list of studies included in the review.
Appendix S5. Methodological quality assessment of included studies.
Appendix S6. PRISMA checklist.
Acknowledgements
This study protocol was prospectively registered with PROSPERO (CRD42020212347). The authors acknowledge the assistance of specialist librarian D. Brey for the database search for this meta‐analysis. EHT acknowledges the South African Medical Research Council (Mid‐Career Scientist Grant). No other external funding or competing interests declared.
This article is accompanied by an editorial by Cook and Camporota, Anaesthesia 2021; 76: 1155–8.
Contributor Information
E. H. Taylor, @elliott_taylor1.
E. J. Marson, @ella_m31.
M. Elhadi, @ElhadiMuhammed.
K. D. M. Macleod, @Kieran_Macleod_.
R. Boden, @boden_regan.
B. M. Biccard, Email: bruce.biccard@uct.ac.za, @brucebiccard.
References
- 1.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. Anaesthesia 2020; 75: 1340–9. [DOI] [PubMed] [Google Scholar]
- 2.Biccard BM, Gopalan PD, Miller M, et al. Patient care and clinical outcomes for patients with COVID‐19 infection admitted to African high‐care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study. Lancet 2021; 397: 1885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor EH, Hofmeyr R, Torborg A, et al. Risk factors and interventions associated with mortality or survival in adult COVID‐19 patients admitted to critical care: a systematic review and meta‐analysis. Southern African Journal of Anaesthesia and Analgesia 2020; 26: 116–27. [Google Scholar]
- 4.Wu Y, Li H, Zhang Z, et al. Risk factors for mortality of coronavirus disease 2019 (COVID‐19) patients during the early outbreak of COVID‐19: a systematic review and meta‐analysis. Annals of Palliative Medicine 2021; 10: 5069–83. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. British Medical Journal 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 01/02/2021).
- 7.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aladağ N, Atabey RD. The role of concomitant cardiovascular diseases and cardiac biomarkers for predicting mortality in critical COVID‐19 patients. Acta Cardiologica 2021; 76: 132–9. [DOI] [PubMed] [Google Scholar]
- 9.Alharthy A, Aletreby W, Faqihi F, et al. Clinical characteristics and predictors of 28‐day mortality in 352 critically ill patients with covid‐19: a retrospective study. Journal of Epidemiology and Global Health 2020; 11: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amit M, Sorkin A, Chen J, et al. Clinical course and outcomes of severe covid‐19: a National Scale Study. Journal of Clinical Medicine 2020; 9: 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arvinte C, Singh M, Marik PE. Serum levels of vitamin c and vitamin d in a cohort of critically ill covid‐19 patients of a North American community hospital intensive care unit in May 2020: a pilot study. Medicine in Drug Discovery 2020; 8: 100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auld SC, Caridi‐Scheible M, Blum JM, et al. ICU and ventilator mortality among critically Ill adults with coronavirus disease 2019. Critical Care Medicine 2020; 48: e799–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayed M, Borahmah AA, Yazdani A, Sultan A, Mossad A, Rawdhan H. Assessment of clinical characteristics and mortality‐associated factors in COVID‐19 critical cases in Kuwait. Medical Principles and Practice 2020; 323: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezuidenhout MC, Wiese OJ, Moodley D, et al. Correlating arterial blood gas, acid‐base and blood pressure abnormalities with outcomes in COVID‐19 intensive care patients. Annals of Clinical Biochemistry 2021; 58: 95–101. [DOI] [PubMed] [Google Scholar]
- 15.Borobia A, Carcas A, Arnalich F, et al. A cohort of patients with covid‐19 in a major teaching hospital in Europe. Journal of Clinical Medicine 2020; 9: 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chand S, Kapoor S, Orsi D, et al. COVID‐19‐associated critical illness‐report of the first 300 patients admitted to intensive care units at a New York City medical center. Journal of Intensive Care Medicine 2020; 35: 963–70. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Wang J, Su N, Bao X, Li Y, Jin J. Simplified immune‐dysregulation index: a novel marker predicts 28‐day mortality of intensive care patients with COVID‐19. Intensive Care Medicine 2020; 46: 1645–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVID‐ICU Group on behalf of the REVA Network and the COVID‐ICU Investigators . Clinical characteristics and day‐90 outcomes of 4244 critically ill adults with COVID‐19: a prospective cohort study. Intensive Care Medicine 2021; 47: 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrando‐Vivas P, Doidge J, Thomas K, et al. Prognostic factors for 30‐day mortality in critically ill patients with coronavirus disease 2019: an observational cohort study. Critical Care Medicine 2021; 49: 102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrando C, Mellado‐Artigas R, Gea A, et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS‐CoV‐2 in Spain: a prospective, cohort, multicentre study. Revista Española de Anestesiología y Reanimación 2020; 67: 425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. Journal of the American Medical Association Internal Medicine 2020; 180: 1345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. Journal of the American Medical Association Internal Medicine 2020; 180: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haase N, Plovsing R, Christensen S, et al. Characteristics, interventions, and longer term outcomes of COVID‐19 ICU patients in Denmark‐A nationwide, observational study. Acta Anaesthesiologica Scandinavica 2021; 65: 68–75. [DOI] [PubMed] [Google Scholar]
- 24.Kayina CA, Haritha D, Soni L, et al. Epidemiological and clinical characteristics & early outcome of COVID‐19 patients in a tertiary care teaching hospital in India: a preliminary analysis. Indian Journal of Medical Research 2020; 152: 100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Intensive Care National Audit and Research Centre . ICNARC report on COVID‐19 in critical care‐19 February 2021. 2021. https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports (accessed 19/02/2021).
- 26.Klein SJ, Bellmann R, Dejaco H, et al. Structured ICU resource management in a pandemic is associated with favorable outcome in critically ill COVID‐19 patients. Wiener Klinische Wochenschrift 2020; 132: 653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.KocayİĞİt H, Özmen SÜner K, Tomak Y, et al. Characteristics and outcomes of critically ill patients with Covid‐19 in Sakarya, Turkey: a single center cohort study. Turkish Journal of Medical Sciences 2021; 51: 440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokoszka‐Bargieł I, Cyprys P, Rutkowska K, Madowicz J, Knapik P. Intensive care unit admissions during the first 3 months of the COVID‐19 Pandemic in Poland: a single‐center, Cross‐Sectional Study. Medical Science Monitor 2020; 26: e926974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson E, Brattström O, Agvald‐Öhman C, et al. Characteristics and outcomes of patients with COVID‐19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiologica Scandinavica 2021; 65: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Jiang J, Wang F, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID‐19 patients. Journal of Molecular and Cellular Cardiology 2020; 147: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long X, Zhang Z, Zou W, et al. Coagulopathy of patients with COVID‐19 is associated with infectious and inflammatory markers. Risk Management and Healthcare Policy 2020; 13: 1965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorente L, Martín MM, Argueso M, et al. Association between red blood cell distribution width and mortality of COVID‐19 patients. Anaesthesia, Critical Care and Pain Medicine 2021; 40: 100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Zhang Y, Cheng G, et al. Clinical characteristics and outcomes of adult critically ill patients with COVID‐19 in Honghu, Hubei Province. Nan Fang Yi Ke Da Xue Xue Bao 2020; 40: 778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittel AM, Panzer O, Wang DS, et al. Logistical considerations and clinical outcomes associated with converting operating rooms into an intensive care unit during the Covid‐19 pandemic in a New York City Hospital. Anesthesia and Analgesia 2021; 135: 1182–90. [DOI] [PubMed] [Google Scholar]
- 35.Nachtigall I, Lenga P, Jóźwiak K, et al. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID‐19 in Germany: an observational study. Clinical Microbiology and Infection 2020; 26: 1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadeem R, Thomas SJ, Fathima Z, et al. Pattern of anticoagulation prescription for patients with Covid‐19 acute respiratory distress syndrome admitted to ICU. Does it impact outcome? Heart and Lung 2021; 50: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ñamendys‐Silva SA, Alvarado‐Ávila PE, Domínguez‐Cherit G, et al. Outcomes of patients with COVID‐19 in the intensive care unit in Mexico: a multicenter observational study. Heart and Lung 2021; 50: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan P, Li Y, Xiao Y, et al. Prognostic assessment of COVID‐19 in the intensive care unit by machine learning methods: model development and validation. Journal of Medical Internet Research 2020; 22: e23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paranjpe I, Russak AJ, De Freitas JK, et al. Retrospective cohort study of clinical characteristics of 2199 hospitalised patients with COVID‐19 in New York City. British Medical Journal Open 2020; 10: e040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellaud C, Grandmaison G, Hoa Phong PHT, et al. Characteristics, comorbidities, 30‐day outcome and in‐hospital mortality of patients hospitalised with COVID‐19 in a Swiss area ‐ a retrospective cohort study. Swiss Medical Weekly 2020; 150: w20314. [DOI] [PubMed] [Google Scholar]
- 41.Peng X, Chen Y, Deng L, et al. Clinical features of critically ill patients infected with SARS‐CoV‐2 outside Wuhan with and without diabetes. International Journal of Diabetes in Developing Countries 2020; 40: 482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Primmaz S, Le Terrier C, Suh N, et al. Preparedness and reorganization of care for coronavirus disease 2019 patients in a swiss icu: characteristics and outcomes of 129 patients. Critical Care Explorations 2020; 2: e0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahim F, Amin S, Noor M, et al. Mortality of patients with severe covid‐19 in the intensive care unit: an observational study from a major COVID‐19 receiving hospital. Cureus 2020; 12: e10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rieg S, von Cube M, Kalbhenn J, et al. COVID‐19 in‐hospital mortality and mode of death in a dynamic and non‐restricted tertiary care model in Germany. PLoS One 2020; 15: e0242127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez A, Moreno G, Gómez J, et al. Severe infection due to the SARS‐CoV‐2 coronavirus: experience of a tertiary hospital with COVID‐19 patients during the 2020 pandemic. Medicina Intensiva 2020; 44: 525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Routsi C, Magira E, Kokkoris S, et al. Hospital resources may be an important aspect of mortality rate among critically Ill patients with COVID‐19: the Paradigm of Greece. Journal of Clinical Medicine 2020; 9: 3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayad B, Rahimi Z. Blood coagulation parameters in patients with severe COVID‐19 from Kermanshah Province, Islamic Republic of Iran. Eastern Mediterranean Health Journal 2020; 26: 999–1004. [DOI] [PubMed] [Google Scholar]
- 48.Serrano‐Martínez JL, Machado‐Casas JF, Redondo‐Orts M, Manzano‐Manzano F, Castaño‐Pérez J, Pérez‐Villares JM. Characteristics and results of a series of 59 patients with severe pneumonia due to COVID‐19 admitted in the ICU. Medicina Intensiva 2020; 44: 580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Socolovithc RL, Fumis RRL, Tomazini BM, et al. Epidemiology, outcomes, and the use of intensive care unit resources of critically ill patients diagnosed with COVID‐19 in Sao Paulo, Brazil: a cohort study. PLoS One 2020; 15: e0243269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sosa‐García JO, Gutiérrez‐Villaseñor AO, García‐Briones A, Romero‐González JP, Juárez‐Hernández E, González‐Chon O. Experience in the management of severe COVID‐19 patients in an intensive care unit. Cirugía y Cirujanos 2020; 88: 569–75. [DOI] [PubMed] [Google Scholar]
- 51.Taboada M, Rama P, Pita‐Romero R, et al. Critically ill COVID‐19 patients attended by anesthesiologists in northwestern Spain: a multicenter prospective observational study. Revista Española de Anestesiología y Reanimación 2021; 68: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka S, De Tymowski C, Assadi M, et al. Lipoprotein concentrations over time in the intensive care unit COVID‐19 patients: results from the ApoCOVID study. PLoS One 2020; 15: e0239573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Twigg HL, Khan SH, Perkins AJ, et al. Mortality rates in a diverse cohort of mechanically ventilated patients with novel coronavirus in the Urban Midwest. Critical Care Explorations 2020; 2: e0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vassiliou AG, Jahaj E, Ilias I, et al. Lactate kinetics reflect organ dysfunction and are associated with adverse outcomes in intensive care unit patients with COVID‐19 pneumonia: preliminary results from a Greek single‐centre study. Metabolites 2020; 10: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID‐19. American Journal of Respiratory and Critical Care Medicine 2020; 201: 1430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang ZH, Shu C, Ran X, Xie CH, Zhang L. Critically ill patients with coronavirus disease 2019 in a designated ICU: clinical features and predictors for mortality. Risk Management and Healthcare Policy 2020; 13: 833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wendel Garcia PD, Fumeaux T, Guerci P, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID‐19 in Europe: initial report of the international RISC‐19‐ICU prospective observational cohort. EClinicalMedicine 2020; 25: 100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie J, Wu W, Li S, et al. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID‐19) in China: a retrospective multicenter study. Intensive Care Medicine 2020; 46: 1863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, Yang X, Yang L, et al. Clinical course and predictors of 60‐day mortality in 239 critically ill patients with COVID‐19: a multicenter retrospective study from Wuhan, China. Critical Care 2020; 24: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respiratory Medicine 2020; 8: 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. Journal of Clinical Virology 2020; 127: 104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Liu P, Wang M, et al. The clinical data from 19 critically ill patients with coronavirus disease 2019: a single‐centered, retrospective, observational study. Journal of Public Health 2020. Epub 2 April. doi.org/10.1007/s10389-020-01291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, Huang B, Xia H, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiology and Infection 2020; 148: e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou X, Li S, Fang M, et al. Acute Physiology and Chronic Health Evaluation II Score as a predictor of hospital mortality in patients of coronavirus disease 2019. Critical Care Medicine 2020; 48: e657–e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Intensive Care National Audit and Research Centre . ICNARC report on COVID‐19 in critical care‐7 September 2020. 2020. https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports (accessed 19/02/2021).
- 66.Li Y, Ashcroft T, Chung A, et al. Risk factors for poor outcomes in hospitalised COVID‐19 patients: a systematic review and meta‐analysis. Journal of Global Health 2021; 11: 10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pepper DJ, Demirkale CY, Sun J, et al. Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Critical Care Medicine 2019; 47: 643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iaccarino G, Grassi G, Borghi C, et al. Gender differences in predictors of intensive care units admission among COVID‐19 patients: The results of the SARS‐RAS study of the Italian Society of Hypertension. PLoS One 2020; 15: e0237297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hollinger A, Gayat E, Féliot E, et al. Gender and survival of critically ill patients: results from the FROG‐ICU study. Annals of Intensive Care 2019; 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nature Communications 2020; 11: 6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hewitt J, Carter B, Vilches‐Moraga A, et al. The effect of frailty on survival in patients with COVID‐19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 2020; 5: e444–e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leung JM, Yang CX, Tam A, et al. ACE‐2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID‐19. European Respiratory Journal 2020; 55: 2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith JC, Sausville EL, Girish V, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS‐CoV‐2 receptor ACE2 in the respiratory tract. Developmental Cell 2020; 53: 514–29.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). Journal of the American Medical Association Cardiology 2020; 5: 811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID‐19) with myocardial injury and mortality. Journal of the American Medical Association Cardiology 2020; 5: 751–3. [DOI] [PubMed] [Google Scholar]
- 76.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). Journal of the American Medical Association 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Liu C, Mao Z, et al. Predictive values of neutrophil‐to‐lymphocyte ratio on disease severity and mortality in COVID‐19 patients: a systematic review and meta‐analysis. Critical Care 2020; 24: 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiang G, Hao S, Fu C, et al. The effect of coagulation factors in 2019 novel coronavirus patients: a systematic review and meta‐analysis. Medicine (Baltimore) 2021; 100: e24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu S, Yao N, Qiu Y, He C. Predictive performance of SOFA and qSOFA for in‐hospital mortality in severe novel coronavirus disease. American Journal of Emergency Medicine 2020; 38: 2074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID‐19: a systematic review and meta‐analysis of non‐randomized cohort studies. Critical Care 2021; 25: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji P, Zhu J, Zhong Z, et al. Association of elevated inflammatory markers and severe COVID‐19: a meta‐analysis. Medicine (Baltimore) 2020; 99: e23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid‐19. New England Journal of Medicine 2020; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abani O, Abbas A, Abbas F, et al. Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet 2021; 397: 1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1–S23. Forest plots demonstrating associations between characteristics and mortality in COVID‐19 patients admitted to critical care.
Figure S24–S46. Funnel plots of studies reporting the associations between characteristics and mortality in COVID‐19 patients admitted to critical care.
Figure S47–S53. Sensitivity analyses with forest plots demonstrating associations between characteristics and mortality in COVID‐19 patients admitted to critical care.
Appendix S1. Search strategy used for this systematic review.
Appendix S2. Modified Newcastle‐Ottawa Quality Assessment Scale.
Appendix S3. Study and cohort characteristics of studies included in the review.
Appendix S4. Reference list of studies included in the review.
Appendix S5. Methodological quality assessment of included studies.
Appendix S6. PRISMA checklist.


