Thrombotic thrombocytopenic purpura (TTP) is caused by a severe deficiency of plasma protease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), which is responsible for cleavage of von Willebrand factor (vWF). Immune TTP (iTTP), which accounts for approximately two‐thirds of childhood TTP, occurs secondary to inhibitory autoantibodies against ADAMTS13. These autoantibodies prevent cleavage of vWF resulting in ultralarge multimers of vWF and, in turn, microangiopathic haemolytic anaemia, thrombocytopenia, and organ dysfunction. 1
A 14‐year‐old female presented to a community hospital with a two‐day history of fatigue, headache, confusion, and bruising. She had a long‐standing history of anxiety, iron deficiency, and postprandial abdominal pain. She received the first dose of the BNT162b2 vaccine two weeks prior to presentation. Her neurological examination and head computed tomography (CT) were both normal. Her laboratory investigations showed a haemolytic anaemia with a haemoglobin of 63 g/l, platelets <10 × 1012/l, bilirubin 68 µmol/l, lactate dehydrogenase (LDH) 626 µ/l, haptoglobin <0·10 g/l, and the occasional red cell fragment noted on blood film. She had a PLASMIC score of 6 and was transferred to our tertiary‐care paediatric centre with a suspicion of TTP.
The diagnosis of iTTP was confirmed with urgent ADAMTS13 activity testing showing a level of <1% and ADAMTS13 IgG of 72 µ/ml. She was started on oral prednisone 2 mg/kg and daily therapeutic plasma exchange (TPE) to replace 1·5× her plasma volume using cryosupernated plasma as the exchange solution. Her platelet count rapidly improved and after two consecutive days of a count greater than 150 × 1012/l, TPE was held (Day 5). Rituximab was administered to reduce the risk of long‐term relapse. Within 48 h she had an exacerbation as her platelet count dropped precipitously to 28 × 1012/l which resulted in restarting TPE. Twice daily TPE and a pulse of methylprednisolone was started on Day 9 as her platelet count was 15 × 1012/l. As a result of her early exacerbation, caplacizumab, a novel anti‐vWF nanobody was obtained via the Sanofi compassionate access programme. 2 Her platelet count increased to 177 × 1012/l within 48 h of starting caplacizumab and remained in the normal range (Fig 1). TPE was stopped on Day 12 at which point her ADAMTS13 activity was 19% and antibody level had fallen to 7 µ/ml. Steroids were weaned and caplacizumab continued for 30 days post TPE. She did not experience any bleeding during her therapy.
Fig 1.
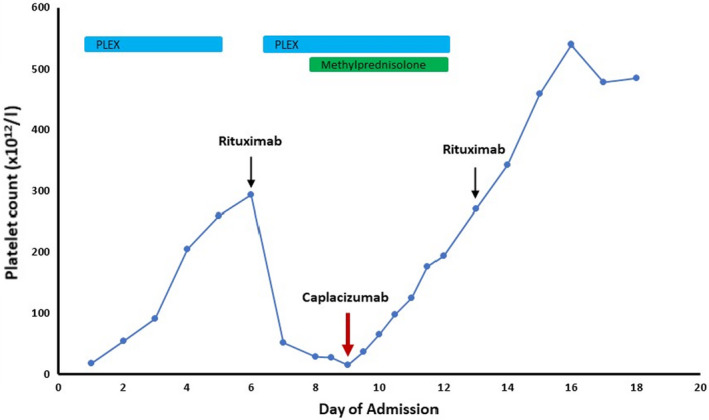
Platelet count over the course of therapy. Oral prednisone and daily therapeutic plasma exchange (TPE) were started upon admission. The patient had an exacerbation within 24 h of holding TPE to administer rituximab. She received a pulse of methylprednisolone and caplacizumab was administered on Day 9.
Throughout the course of her treatment, she appeared remarkably well, with no evidence of end‐organ damage. She had a normal blood pressure, serum creatinine, and no heamaturia nor albuminuria throughout her course. Ophthalmologic examination, head magnetic resonance imaging (MRI), and abdominal ultrasound were normal. There were no clinical features to support underlying autoimmune disease. Her anti‐nuclear and double‐stranded DNA antibodies were negative. Human immunodeficiency virus (HIV), Epstein–Barr virus (EBV), and cytomegalovirus (CMV) testing were negative. Her SARS‐CoV‐2 polymerase chain reaction (PCR) and anti‐SARS‐CoV‐2 total assay (nucleocapsid) pre‐TPE were both negative. IgA and IgG assays (Spike; S1) were tested following initiation of TPE and were borderline.
Immune thrombotic thrombocytopenic purpura is rare in childhood with an annual incidence of less than one in a million. 3 In the Oklahoma TTP registry, only two of 90 patients registered in 23 years were children under 18 years of age. 4 In adults, autoimmune disorders, pregnancy, drugs, HIV, malignancy, and organ transplantation have also been linked as possible triggers for TTP. 1 Childhood‐onset iTTP has a similarly increased association with autoimmune disorders, though most cases are idiopathic. 4 Vaccine‐associated iTTP has been previously noted with the pneumococcal, rabies, and influenza vaccines. 5 , 6 De Bruijn et al. first reported an adult patient who presented with a new diagnosis of iTTP following the first dose of the BNT162b2 vaccine. 7 Recently, an Israeli study reported a cluster of four adult cases of iTTP (two new and two relapses) within four weeks of the BNT162b2 vaccine, a rate higher than that usually seen. 8 Three of these cases were after the second dose and one after the first dose of the BNT162b2 vaccine.
Our patient’s presentation within two weeks of vaccination together with the lack of other possible causes are suspicious for the BNT162b2 vaccine playing a role, though we cannot assign causality. The presence of antibodies to the spike protein would have potentially supported this hypothesis. However, her presentation soon after the first vaccine dose and analysis being performed after initiation of TPE may have influenced the borderline assay in our patient. There is a family history of maternal immune thrombocytopenia (ITP) and thus it is possible the vaccine may have been the trigger to unmask an underlying vulnerability to autoimmunity.
There are no consensus guidelines for the management of paediatric TTP, with TPE, corticosteroids and rituximab serving as initial therapy options. This is in keeping with adult literature. The use of vincristine, cyclophosphamide, bortezomib, and splenectomy have been reported in refractory cases. 9
Caplacizumab is a nanobody which recognizes the vWF A1 domain and inhibits platelet binding to vWF. Two pivotal randomized controlled trials in adults demonstrated the use of caplacizumab was associated with a more rapid platelet response, and reduced risk of recurrence or a refractory course. 2 , 10 Consequently, the International Society on Thrombosis and Haemostasis (ISTH) guideline for the treatment of TTP suggests the use of caplacizumab in initially treating iTTP. 11 As a result of inhibiting vWF function, mucocutaneous bleeding is the most common side‐effect. The successful use of caplacizumab in paediatric iTTP has been reported in front‐line therapy or for refractory disease. 12 , 13 , 14 , 15 Our patient had an early exacerbation upon stopping TPE which responded very well to caplacizumab, with a rapid platelet response, discontinuation of TPE, and no bleeding.
We report the first paediatric case of de novo iTTP possibly associated with the BNT162b2 vaccine and highlight the need to consider TTP in thrombocytopenic children post vaccination. We strongly support the drive to vaccinate children and adults against COVID‐19 as the established benefits of the BNT162b2 vaccine in reducing COVID‐19 related morbidity and mortality far outweigh the risks of extremely rare side effects such as iTTP. To our knowledge, this is also the first paediatric patient treated with caplacizumab in Canada. Our results support the early consideration of the use of caplacizumab in children with iTTP.
Author contributions
AK and JG collected and analyzed the data and revised the manuscript. KA, SP, APS, SLG, MB, CC, SL, LB, S‐HSH and MJK analyzed the data and revised the manuscript. ST collected and analyzed the data, drafted the initial manuscript and revised the manuscript. All authors approve of the final manuscript as submitted.
Conflicts of interest statement
The authors have no conflicts of interest to disclose.
Patient consent statement
The patient provided written consent to publish this case report.
Acknowledgements
We would like to acknowledge the apheresis team and the haemostasis and thrombosis laboratory at London Health Sciences Centre for their support in caring for the patient.
References
- 1. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836–46. [DOI] [PubMed] [Google Scholar]
- 2. Scully M, Cataland SR, Peyvandi F, Coppo P, Knobl P, Kremer Hovinga JA, et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335–46. [DOI] [PubMed] [Google Scholar]
- 3. Joly BS, Coppo P, Veyradier A. Pediatric thrombotic thrombocytopenic purpura. Eur J Haematol. 2018;101(4):425–34. [DOI] [PubMed] [Google Scholar]
- 4. Siddiqui A, Journeycake JM, Borogovac A, George JN . Recognizing and managing hereditary and acquired thrombotic thrombocytopenic purpura in infants and children. Pediatr Blood Cancer. 2021;68(5):e28949. [DOI] [PubMed] [Google Scholar]
- 5. Dias PJ, Gopal S. Refractory thrombotic thrombocytopenic purpura following influenza vaccination. Anaesthesia. 2009;64(4):444–6. [DOI] [PubMed] [Google Scholar]
- 6. Kojima Y, Ohashi H, Nakamura T, Nakamura H, Yamamoto H, Miyata Y, et al. Acute thrombotic thrombocytopenic purpura after pneumococcal vaccination. Blood Coagul Fibrinolysis. 2014;25(5):512–4. [DOI] [PubMed] [Google Scholar]
- 7. de Bruijn S, Maes MB, De Waele L, Vanhoorelbeke K, Gadisseur A. First report of a de novo iTTP episode associated with an mRNA‐based anti‐COVID‐19 vaccination. J Thromb Haemost. 2021;19(8):2014–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maayan H, Kirgner I, Gutwein O, Herzog‐Tzarfati K, Rahimi‐Levene N, Koren‐Michowitz M, et al. Acquired thrombotic thrombocytopenic purpura: A rare disease associated with BNT162b2 vaccine. J Thromb Haemost. 2021.1–4. 10.1111/jth.15420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sayani FA, Abrams CS. How I treat refractory thrombotic thrombocytopenic purpura. Blood. 2015;125(25):3860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knobl P, Wu H, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511–22. [DOI] [PubMed] [Google Scholar]
- 11. Zheng XL, Vesely SK, Cataland SR, Coppo P, Geldziler B, Iorio A, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhoopalan SV, Hankins J, George J, Ryder A, Onder AM, Puri L. Use of caplacizumab in a child with refractory thrombotic thrombocytopenic purpura. Pediatr Blood Cancer. 2019;66(7):e27737. [DOI] [PubMed] [Google Scholar]
- 13. Boudali J, Hallak B, Haeck M, Sellier‐Leclerc AL, Ulrich M, Coppo P, et al. Immune‐mediated thrombotic thrombocytopenic purpura in childhood treated by caplacizumab, about 3 cases. J Nephrol. 2021. 10.1007/s40620-021-00992-5 [DOI] [PubMed] [Google Scholar]
- 14. Nagel MB, Ryder A, Lobbins M, Bhatt N. Refractory acquired thrombotic thrombocytopenic purpura treated with caplacizumab in a pediatric patient with systemic lupus erythematosus. Pediatr Blood Cancer. 2021;68(1):e28534. [DOI] [PubMed] [Google Scholar]
- 15. Dutt T, Shaw RJ, Stubbs M, Yong J, Bailiff B, Cranfield T, et al. Real‐world experience with caplacizumab in the management of acute TTP. Blood. 2021;137(13):1731–40. [DOI] [PubMed] [Google Scholar]


