Abstract
Introduction
We aimed to identify the associations between the lymphocytes (LYM) absolute count on admission and clinical outcomes in COVID‐19 patients.
Methods
In this retrospective study, 224 COVID‐19 patients who were admitted to General Hospital of Central Theater Command of the PLA from January 22 to April 4, 2020, were consecutively included. These patients were divided into the lymphopenia group and the nonlymphopenia group according to whether the LYM count on admission was below the normal range.
Results
During hospitalization, patients in the lymphopenia group have a much higher all‐cause mortality (14.5% vs 0.0%; P < .001) and an evidently longer length of hospital stay (24.0 vs 17.5 days; P < .001) than patients in the nonlymphopenia group. The correlation analysis results indicated that the LYM count was negatively correlated with the values of NEU (R = −.2886, P < .001), PT (R = −.2312, P < .001), FIB (R = −.2954, P < .001), D‐D (R = −.3554, P < .001), CRP (R = −.4899, P < .001), IL‐6 (R = −.5459, P < .001), AST (R = −.2044, P < .01), Cr (R = −.1350, P < .05), CPK (R = −.2119, P < .01), CK‐Mb (R = −.1760, P < .01), and LDH (R = −.4330, P < .001), and was positively correlated with the count of PLT (R = .2679, P < .001). In addition, LYM as a continuous variable was associated with 97% decreased risk of in‐hospital mortality in the fully adjusted models (OR = 0.03, 95%CI, 0.00‐0.37, P < .001).
Discussion
LYM screening on admission is a critical predictor for assessment of disease severity and clinical outcomes in patients with COVID‐19, and lymphopenia substantially correlates with poor clinical outcomes.
Keywords: clinical outcomes, COVID‐19, lymphocytes
1. INTRODUCTION
As of February 3, 2021, Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has led to more than 100 000 000 confirmed infections and 2 200 000 deaths all over the world. On the basis of the clinical symptoms severity, COVID‐19 patients are usually classified as mild, severe, and critical types. The total mortality rate of COVID‐19 patients is about 4% according to the statistical information from World Health Organization (WHO). However, the risk of adverse clinical outcomes appears a dramatic rise in COVID‐19 patients with severe/critical illness. Therefore, it is quite important to find the clinical indicators that can reasonably evaluate disease progression and effectively predict clinical outcomes in COVID‐19 patients.
Many previous studies 1 , 2 , 3 , 4 , 5 , 6 have showed that lymphopenia often appears in COVID‐19 patients especially in severe/critical types and that a potential correlation exists between lymphocytes (LYM) count and disease progression in SARS‐CoV‐2 infections. Nevertheless, whether LYM screening could be an independent predictor for clinical outcomes (including mortality and length of hospital stay) in hospitalized COVID‐19 patients needs to be further elucidated. In the present study, we aimed to clarify the clinical significance of LYM screening and its association with clinical outcomes in COVID‐19 patients, which might be helpful for determining the important biomarkers for COVID‐19.
2. METHODS
2.1. Study design and participants
For this retrospective cohort study, COVID‐19 patients who were admitted to General Hospital of Central Theater Command of the PLA from January 22 to April 4, 2020, were consecutively included. The clinical outcomes (discharge from hospital or death) were recorded up to April 4, 2020. All the patients were diagnosed according to WHO interim guidance. As the flow chart (Figure 1) shown, the general information and laboratory data of 356 patients were obtained. Patients who were tested negative for SARS‐CoV‐2 nucleic acid on admission, being transferred from/to other hospitals during hospitalization, having missing baseline data, or under 18 years old were excluded, and 224 patients were finally included in this research. A patient was categorized as severe case if any of the below clinical scenes additionally appeared: (1) respiratory rate ≥30/min; (2) oxygen saturation ≤93%; and (3) arterial partial pressure of oxygen (PaO2) / fraction of inspired oxygen (FiO2) ≤ 300 mm Hg (1 mm Hg = 0.133 kPa). The enrolled patients were divided into two groups (the lymphopenia group and the nonlymphopenia group) according to whether the LYM absolute count on admission was below the normal range. This study was approved by the Hospital Ethics Committee of General Hospital of Central Theater Command of the PLA (No. [2020]003‐1), and oral informed consent was obtained from each patient.
FIGURE 1.
Study population
2.2. Data collection
The general information (age, gender, clinical types, signs and symptoms, coexisting disorders, and clinical outcomes) and laboratory findings (lymphocytes [LYM, normal range 1.1‐3.2 × 109/L], neutrophils [NEU, normal range 1.8‐6.3 × 109/L], hemoglobin [Hb, normal range 115‐160 g/L], platelet [PLT, normal range 125‐350 × 109/L], prothrombin time [PT, normal range 10‐14 s], activated partial thromboplastin time [APTT, normal range 23.5‐39.1 s], fibrinogen [FIB, normal range 2‐4.98 g/L], D‐dimers [D‐D, normal range 0‐243 ng/mL], C‐reactive protein [CRP, normal range 0‐10 mg/L], interleukin‐6 [IL‐6, normal range 0‐7 pg/mL], alanine aminotransferase [ALT, normal range 7‐40 U/L], aspartate aminotransferase [AST, normal range 13‐35 U/L], creatinine [Cr, normal range 45‐110 μmol/L], creatine phosphokinase [CPK, normal range 0‐195 U/L], creatine kinase‐MB [CK‐Mb, normal range 0‐24 U/L], and lactate dehydrogenase [LDH, normal range 109‐225 U/L]) of the patients on admission were extracted from electronic medical records as of April 4, 2020. For LYM classification, the relevant results (absolute count of total T cells [normal range 955‐2860 /μL], CD8+ T cells [normal range 320‐1250 /μL], CD4+ T cells [normal range 550‐1440 /μL], natural killer [NK] cells [normal range 150‐1100 /μL], total B cells [normal range 90‐560 /μL], and ratio of CD4+/CD8+ [normal range 0.71‐2.78]) of patients on admission were recorded and analyzed.
Pharyngeal swab specimens were collected from patients for SARS‐CoV‐2 RNA test, and real‐time RT‐PCR was performed using the nucleic acid testing kit (Daan, Guangzhou, China) as previously described. 7
2.3. Statistical analysis
Continuous variables were described as the means and standard deviations or medians and interquartile ranges (IQR) values. Categorical variables were expressed as the counts and percentages. Independent group t tests were applied to continuous variables that were normally distributed; otherwise, the Mann‐Whitney test was used. Categorical variables were compared using the chi‐square tests, while the Fisher exact test was used when the data were limited. Linear fitting curve was drawn for analyzing the association between the LYM count and length of hospital stay, while logistic fitting curve was drawn for analyzing the association between the LYM count and all‐cause death. Univariate and multivariate logistic regression models were used to evaluate the relationships between baseline variables and all‐cause death of patients during hospitalization, then unadjusted and adjusted odds ratio (ORs) and 95% confidence intervals (CIs) were calculated. In the multivariate adjusted models, age, gender, hypertension, cardiovascular and/or cerebrovascular disease (CCD), NEU, D‐D, CRP, Cr, and LDH were included. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 22.0 software. A two‐sided α of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Clinical characteristics and laboratory findings
The clinical characteristics and laboratory data of 224 patients were shown in Table 1. Of these cases, the median age was 56.0 years (IQR, 40.8‐67.2), and 134 (59.8%) patients were men. Nearly 30% of the patients were severe cases on admission. The most common symptoms at illness onset were fever (187 [83.5%]), cough (141 [62.9%]), and fatigue (84 [37.5%]) among these patients. Of these patients, hypertension (63 [28.1%]), CCD (37 [16.5%]), and diabetes (28 [12.5%]) were the most common coexisting disorders. The median length of hospital stay of these patients was 21.0 days (IQR, 14.8‐29.0), and the all‐cause mortality in hospital was 7.1% (16/224).
TABLE 1.
Clinical characteristics and laboratory findings of 224 COVID‐19 patients
| Characteristics | All patients (N = 224) | Lymphopenia (N = 110) | Nonlymphopenia (N = 114) | P value |
|---|---|---|---|---|
| Age, years | 56.0 (40.8‐67.2) | 60.0 (49.0‐71.0) | 54.0 (37.2‐66.0) | .005 |
| Age groups | ||||
| 0‐59 y | 126 (56.2) | 54 (49.1) | 72 (63.2) | .047 |
| ≥60 y | 98 (43.8) | 56 (50.9) | 42 (36.8) | |
| Gender | ||||
| Male | 134 (59.8) | 75 (68.2) | 59 (51.8) | .018 |
| Female | 90 (40.2) | 35 (31.8) | 55 (48.2) | |
| Clinical types | ||||
| Severe | 61 (27.2) | 51 (46.4) | 10 (8.8) | <.001 |
| Nonsevere | 163 (72.8) | 59 (53.6) | 104 (91.2) | |
| Signs and symptoms | ||||
| Fever | 187 (83.5) | 98 (89.1) | 89 (78.1) | .026 |
| Cough | 141 (62.9) | 70 (63.6) | 71 (62.3) | .834 |
| Fatigue | 84 (37.5) | 47 (42.7) | 37 (32.5) | .112 |
| Coexisting disorders | ||||
| Hypertension | 63 (28.1) | 35 (31.8) | 28 (24.6) | .290 |
| CCD | 37 (16.5) | 22 (20.0) | 15 (13.2) | .231 |
| Diabetes | 28 (12.5) | 13 (11.8) | 15 (13.2) | .920 |
| Respiratory disease | 19 (8.5) | 11 (10.0) | 8 (7.0) | .575 |
| Malignancy | 16 (7.1) | 11 (10.0) | 5 (4.4) | .170 |
| Laboratory findings | ||||
| LYM (×109/L; 1.1‐3.2) | 1.1 (0.8‐1.5) | 0.8 (0.5‐1.0) | 1.5 (1.3‐2.0) | <.001 |
| NEU (×109/L; 1.8‐6.3) | 3.0 (2.2‐4.6) | 3.4 (2.4‐5.7) | 2.7 (2.2‐3.6) | <.001 |
| Hb (g/L; 115‐160) | 127.0 (118.0‐140.0) | 129.0 (117.0‐139.0) | 127.0 (118.0‐141.0) | .707 |
| PLT (×109/L; 125‐350) | 188.5 (149.8‐236.2) | 164.5 (138.0‐224.2) | 203.5 (173.2‐246.2) | <.001 |
| PT (s; 10‐14) | 12.0 (11.4‐12.8) | 12.3 (11.7‐13.0) | 11.8 (11.2‐12.4) | <.001 |
| APTT (s; 23.5‐39.1) | 32.3 (30.2‐34.7) | 32.5 (30.1‐34.7) | 32.2 (30.3‐34.6) | .703 |
| FIB (g/L; 2‐4.98) | 4.1 (3.6‐4.7) | 4.3 (3.8‐4.8) | 4.0 (3.5‐4.5) | .002 |
| D‐D (ng/mL; 0‐243) | 154.5 (86.0‐336.2) | 215.0 (114.0‐524.5) | 116.0 (67.0‐227.5) | <.001 |
| CRP (mg/L; 0‐10) | 11.0 (4.6‐44.1) | 29.2 (8.8‐69.4) | 9.5 (3.0‐15.2) | <.001 |
| IL‐6 (pg/mL; 0‐7) | 13.1 (4.4‐32.6) | 27.1 (9.9‐49.0) | 5.8 (2.6‐18.1) | <.001 |
| ALT (U/L; 7‐40) | 23.0 (16.0‐34.0) | 25.0 (18.0‐35.0) | 21.0 (14.0‐29.2) | .010 |
| AST (U/L; 13‐35) | 29.0 (23.0‐41.0) | 31.0 (25.0‐46.8) | 26.0 (21.0‐35.0) | <.001 |
| Cr (μmol/L; 45‐110) | 65.0 (54.2‐79.0) | 67.5 (55.2‐80.0) | 63.0 (53.0‐77.0) | .067 |
| CPK (U/L; 0‐195) | 113.0 (79.5‐177.0) | 122.5 (84.5‐236.8) | 105.0 (72.0‐147.0) | .006 |
| CK‐Mb (U/L; 0‐24) | 17.0 (15.0‐20.0) | 18.0 (15.0‐21.0) | 16.0 (14.0‐19.0) | .014 |
| LDH (U/L; 109‐225) | 220.0 (179.8‐276.0) | 249.5 (196.8‐351.8) | 197.0 (162.5‐231.8) | <.001 |
| Clinical outcome | ||||
| Length of stay, days | 21.0 (14.8‐29.0) | 24.0 (18.2‐32.8) | 17.5 (13.0‐23.0) | <.001 |
| All‐cause death | 16 ( 7.1) | 16 (14.5) | 0 (0.0) | <.001 |
Data are median (IQR), n (%), where N is the total number of patients with available data. P values comparing the two groups’ patients are from χ² test, Fisher's exact test, or Mann‐Whitney U test. Lymphopenia, Patients with lymphopenia on admission. Nonlymphopenia, Patients with nonlymphopenia on admission.
ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CCD, cardiovascular and/or disease; CK‐Mb, creatine kinase‐MB; CPK, creatine phosphokinase; Cr, creatinine; CRP, C‐reactive protein; D‐D, D‐dimers; FIB, fibrinogen; Hb, hemoglobin; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; LYM, lymphocytes; NEU, neutrophils; PLT, platelet; PT, prothrombin time.
The median age of patients in the lymphopenia group was much older than that of patients in the nonlymphopenia group (60.0 vs 54.0 years; P < .01), and the proportion of patients aged over 60 in the lymphopenia group were significantly higher than that of patients in the nonlymphopenia group (50.9% vs 36.8%; P < .05). In addition, the male cases possessed a higher proportion in the lymphopenia group than that in the nonlymphopenia group (68.2% vs 51.8%; P < .05). The severe cases owned a markedly higher proportion in the lymphopenia group than that in the nonlymphopenia group (46.4% vs 8.8%; P < .001). The proportion of patients with fever at illness onset in the lymphopenia group was slightly higher than that of patients with fever in the nonlymphopenia group (89.1% vs 78.1%; P < .05). During hospitalization, patients in the lymphopenia group have a much higher all‐cause mortality (14.5% vs 0.0%; P < .001) and an evidently longer length of hospital stay (24.0 vs 17.5 days; P < .001) than patients in the nonlymphopenia group. Moreover, most of the laboratory indicators shown in Table 1 were significantly different between the two groups’ patients. The correlation analysis results indicated that the LYM count was negatively correlated with the values of NEU (R=−0.2886, P < .001), PT (R = −0.2312, P < .001), FIB (R = −0.2954, P < .001), D‐D (R = −0.3554, P < .001), CRP (R = −0.4899, P < .001), IL‐6 (R = −0.5459, P < .001), AST (R = −0.2044, P < .01), Cr (R = −0.1350, P < .05), CPK (R = −0.2119, P < .01), CK‐Mb (R = −0.1760, P < .01), and LDH (R = −0.4330, P < .001), and was positively correlated with the count of PLT (R = 0.2679, P < .001). These results demonstrate that LYM has close associations with various clinical indicators related to coagulation, inflammatory reaction, and important organ (heart, liver, and kidney) function.
3.2. Lymphocyte classification
The LYM classification results of 224 patients on admission were shown in Table 2. Compared with patients in the nonlymphopenia group, patients in the lymphopenia group possessed much lower median values of total T cells (505.0 vs 1140.0; P < .001), CD8+ T cells (162.0 vs 335.5; P < .001), CD4+ T cells (291.0 vs 680.0; P < .001), natural killer [NK] cells (106.0 vs 199.0; P < .001), and total B cells (113.0 vs 228.0; P < .001). Furthermore, patients in the lymphopenia group have a higher percentage value of each of these indicators below the normal range compared with patients in the nonlymphopenia group (P < .001). These results point out that COVID‐19 patients with lymphopenia usually show a decrease in all types of LYM and that COVID‐19 patients with normal LYM also show a decrease in one or more types of LYM. Simultaneously, the median ratio of CD4+/CD8+ of patients in the lymphopenia group was also lower than that of patients in the nonlymphopenia group (1.5 vs 1.9; P < .05).
TABLE 2.
Lymphocyte classification results of 224 COVID‐19 patients on admission
| All patients (N = 224) | Lymphopenia (N = 110) | Nonlymphopenia (N = 114) | P value | |
|---|---|---|---|---|
| Total T cells (/μL; 955‐2860) | 746.5 (481.0‐1148.0) | 505.0 (319.3‐684.3) | 1140.0 (895.5‐1481.3) | <.001 |
| Decreased | 140 (62.5) | 99 (90.0) | 41 (36.0) | <.001 |
| CD8+ T cells (/μL; 320‐1250) | 247.0 (152.5‐367.0) | 162.0 (85.0‐244.0) | 335.5 (247.0‐458.0) | <.001 |
| Decreased | 147 (65.6) | 96 (87.3) | 51 (44.7) | <.001 |
| CD4+ T cells (/μL; 550‐1440) | 420.5 (270.0‐711.0) | 291.0 (165.8‐385.8) | 680.0 (451.3‐930.5) | <.001 |
| Decreased | 136 (60.7) | 98 (89.1) | 38 (33.3) | <.001 |
| Natural killer [NK] cells (/μL; 150‐1100) | 155.0 (94.0‐244.0) | 106.0 (65.0‐199.3) | 199.0 (129.0‐277.5) | <0.001 |
| Decreased | 108 (48.2) | 70 (63.6) | 38 (33.3) | <0.001 |
| Total B cells (/μL; 90‐560) | 150.5 (97.8‐264.0) | 113.0 (75.0‐152.3) | 228.0 (148.3‐341.0) | <.001 |
| Decreased | 46 (20.5) | 37 (33.6) | 9 (7.9) | <.001 |
| CD4+/CD8+ (0.71‐2.78) | 1.7 (1.2‐2.4) | 1.5 (1.1‐2.3) | 1.9 (1.3‐2.6) | .044 |
| Decreased | 9 (4.0) | 8 (7.3) | 1 (0.9) | .036 |
| Increased | 39 (17.4) | 16 (14.5) | 23 (20.2) | .267 |
Data are median (IQR), n (%), where N is the total number of patients with available data. P values comparing the confirmed patients and the suspected patients are from χ² test, Fisher's exact test, or Mann‐Whitney U test. Lymphopenia, Patients with lymphopenia on admission. Nonlymphopenia, Patients with nonlymphopenia on admission.
3.3. Associations of baseline variables with clinical outcomes
The results of univariate logistic regression models (Table 3) manifested that age (OR = 1.10, 95%CI, 1.05‐1.15, P < .001), hypertension (OR = 3.67, 95%CI, 1.30‐10.32, P < .05), CCD (OR = 3.43, 95%CI, 1.16‐10.10, P < .05), NEU (OR = 1.34, 95%CI, 1.17‐1.54, P < .001), D‐D (OR = 1.00 95%CI, 1.00‐1.00, P < .05), CRP (OR = 1.01, 95%CI, 1.01‐1.02, P < .001), Cr (OR = 1.04, 95%CI, 1.02‐1.06, P < .001), and LDH (OR = 1.01, 95%CI, 1.00‐1.01, P < .001) were positively correlated with the risk of in‐hospital death. However, LYM (OR = 0.02, 95%CI, 0.00‐0.11, P < .001) was negatively correlated with the risk of in‐hospital death. The logistic fitting curve (Figure 2A) also indicated that the death risk in hospital of COVID‐19 patients was significantly reduced with the increase in LYM count on admission. The linear fitting curve (Figure 2B) also showed that the length of hospital stay of surviving COVID‐19 patients was gradually decreased with the increase in LYM count on admission. Therefore, these results give an idea that lymphopenia on admission is closely related to adverse clinical outcomes.
TABLE 3.
The unadjusted association between baseline variables and all‐cause death of 224 COVID‐19 patients during hospitalization
| Variable | OR (95% CIs) | P value |
|---|---|---|
| Age, years | 1.10 (1.05, 1.15) | <.001 |
| Gender | ||
| Female | 1.0 | .452 |
| Male | 1.52 (0.51, 4.53) | |
| Hypertension | ||
| No | 1.0 | .014 |
| Yes | 3.67 (1.30, 10.32) | |
| CCD | ||
| No | 1.0 | .026 |
| Yes | 3.43 (1.16, 10.10) | |
| Diabetes | ||
| No | 1.0 | >.99 |
| Yes | 1.0 (0.21, 4.65) | |
| Malignancy | ||
| No | 1.0 | .886 |
| Yes | 0.86 (0.11, 6.94) | |
| Respiratory disease | ||
| No | 1.0 | |
| Yes | 2.77 (0.71, 10.74) | .141 |
| LYM, 109/L | 0.02 (0.00, 0.11) | <.001 |
| NEU, 109/L | 1.34 (1.17, 1.54) | <.001 |
| Hb, g/L | 1.01 (0.98, 1.04) | .67 |
| PLT, 109/L | 1.00 (0.99, 1.00) | .305 |
| PT, s | 1.24 (0.91, 1.69) | .174 |
| APTT, s | 1.04 (0.95, 1.14) | .426 |
| FIB, g/L | 1.47 (0.83, 2.61) | .184 |
| D‐D, ng/mL | 1.00 (1.00, 1.00) | .027 |
| CRP, mg/L | 1.01 (1.01, 1.02) | <.001 |
| IL‐6, pg/mL | 1.01 (1.00, 1.02) | .265 |
| ALT, U/L | 0.98 (0.94, 1.02) | .274 |
| AST, U/L | 1.01 (0.98, 1.03) | .656 |
| Cr, μmol/L | 1.04 (1.02, 1.06) | <.001 |
| CPK, U/L | 1.00 (1.00, 1.00) | .465 |
| CK‐Mb, U/L | 1.02 (0.98, 1.06) | .307 |
| LDH (U/L; 109‐225) | 1.01 (1.00, 1.01) | <.001 |
Abbreviations: ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CCD, cardiovascular and/or cerebrovascular disease; CK‐Mb, creatine kinase‐Mb; CPK, creatine phosphokinase; Cr, creatinine; CRP, C‐reactive protein; D‐D, D‐dimers; FIB, fibrinogen; Hb, hemoglobin; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; LYM, lymphocytes; NEU, neutrophils; OR, odds ratio; PLT, platelet; PT, prothrombin time.
FIGURE 2.
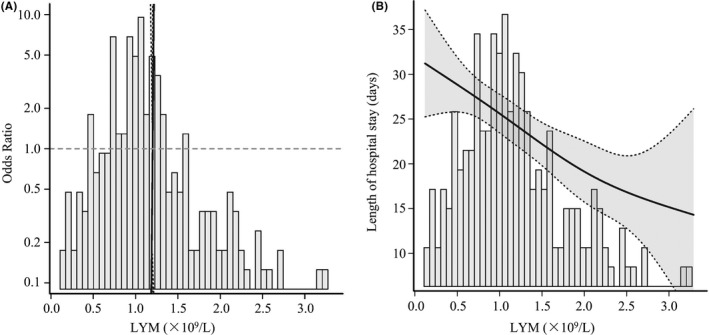
Fitting curve between the LYM count and clinical outcomes in COVID‐19 patients. A, The logistic fitting curve was drawn to assess the relation between the LYM count and in‐hospital death risk. B, The linear fitting curve was drawn to assess the relation between the LYM count and length of hospital stay. LYM, lymphocytes
The results of multivariate logistic regression models assessing the relations of LYM on admission and in‐hospital death were shown in Table S1. In the unadjusted model, LYM was significantly and negatively correlated with the risk of in‐hospital death. Adjustment for demographic variables and coexisting disorders (history of hypertension and history of CCD) did not weaken the associations between LYM and in‐hospital death (OR = 0.01, 95%CI, 0.00‐0.14, P < .001). Further adjusting for the baseline levels of NEU, D‐D, CRP, Cr, and LDH also did not affect the relationships in the fully adjusted models. LYM as a continuous variable was associated with 97% decreased risk of in‐hospital mortality in the fully adjusted models (OR = 0.03, 95%CI, 0.00‐0.37, P < .001). Taken above all, the normal LYM count of COVID‐19 patients on admission seems to be a protective factor that can effectively reduce in‐hospital mortality.
4. DISCUSSION
This retrospective cohort study included 224 COVID‐19 patients, and the associations between the LYM count on admission and clinical outcomes were displayed. The clinical characteristics, laboratory data, and clinical outcomes of these patients were shown according to whether the LYM count on admission was below the normal range. The results indicated that the patients with lymphopenia possessed much higher proportions of older, male, and severe cases than the patients without lymphopenia, which implies that the older and male COVID‐19 patients are more likely to have lymphopenia and that LYM may be a reliable baseline variable assessing COVID‐19 disease condition and progression. The LYM count was closely correlated with various laboratory indicators related to important physiological functions such as coagulation, inflammatory response, and functions of vital organs, which prompts that LYM may play a major role in multiple pathophysiological development pathways of COVID‐19. In addition, the COVID‐19 patients with lymphopenia were always associated with a higher death risk or a longer hospital stay. The results of univariate and multivariate logistic regression models further demonstrated that LYM was associated with 97% decreased risk of in‐hospital mortality. A meta‐analysis reported by Huang et al also indicates that lymphopenia correlates with several poor clinical outcomes including death, acute respiratory distress syndrome (ARDS), ICU care, and severe illness. 8 Thus, LYM seems to be an independent predictor for illness severity, treatment efficacy, and clinical outcomes in COVID‐19 patients.
LYM and their subsets act as a critical role in the maintenance of immune system function. In this study, lymphopenia occurred in nearly 50% (110/224) of the COVID‐19 patients, suggesting an impairment of the immune system during the course of SARS‐CoV‐2 infection. Moreover, decreases in CD8+ T cells, CD4+ T cells, NK cells, and B cells were also observed in these patients, indicating SARS‐CoV‐2 infection can also lead to dysregulation in the levels of all LYM subsets. 9 , 10 , 11 These similar alterations were also found in patients infected by MERS‐CoV and SARS‐CoV. 12 , 13 , 14 It has been suggested that lymphopenia might be caused by the exudation of circulating LYM into inflammatory lung tissues, and directly by virus attachment or indirectly by immune injuries from inflammatory mediators. 15 This study illustrates that the LYM count on admission has close associations with disease severity and clinical outcomes in COVID‐19 patients and may be a critical biomarker for COVID‐19 surveillance. However, multicenter and larger sample size studies are needed to further explore the relationships between the LYM count and clinical outcomes in COVID‐19 patients.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
LL and LN conceived the study and designed experimental procedures; YL, YW, YZ, LC, GK, and ZX collected patients’ samples. LL and LN wrote the article. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
Supporting information
Table S1
Nie L, Liu Y, Weng Y, et al. Lymphocytes screening on admission is essential for predicting in‐hospital clinical outcome in COVID‐19 patients: A retrospective cohort study. Int J Lab Hematol. 2021;43:1302–1308. 10.1111/ijlh.13640
Funding information
This work was supported by the Wuhan Young and Middle‐aged Medical Backbone Talents Training Project (Wuweitong [2019] 87th), and the Military Medical Science and Technology Youth Cultivation Project (20QNPY092).
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin C, Zhou L, Hu Z, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID‐19) in Wuhan. China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henry BM, de Oliveira MHS , Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 6. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID‐19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu L, Liu W, Zheng Y, et al. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in 238 admitted hospital patients. Microbes Infect. 2020;22(4–5):206‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang I, Pranata R. Lymphopenia in severe coronavirus disease‐2019 (COVID‐19): systematic review and meta‐analysis. J Intensive Care. 2020;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221(11):1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang W, Berube J, McNamara M, et al. Lymphocyte subset counts in COVID‐19 patients: a meta‐analysis. Cytometry A. 2020;97(8):772‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui W, Fan Y, Wu W, Zhang F, Wang JY, Ni AP. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin Infect Dis. 2003;37(6):857‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He Z, Zhao C, Dong Q, et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9(6):323‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


