Abstract
A highly transmissible severe acute respiratory coronavirus 2 (SARS‐CoV‐2) caused the coronavirus diseases 2019 (COVID‐19) pandemic, which resulted the highest morbidity and mortality rates among SARS‐CoV and MERS‐CoV. SARS‐CoV‐2 B.1.1.7 variant indicated the higher transmission among human‐to‐human and increasing hospitalisation. SARS‐CoV‐2 infection was observed in domestic animals showing human‐to‐pet transmission. In the current study, we report the first direct known human‐to‐cat transmission of the SARS‐CoV‐2 B.1.1.7 variant within the same family. Previous findings showed that companion animals can get infected by COVID‐19 patients after 3–6 weeks; however, according to our molecular findings, the cat was infected by the viral variant at the same period. Moreover, B.1.1.7 infection caused and developed several clinical symptoms including cardiac and ocular abnormalities. Overall, our findings determined the first direct and high transmission ability of the B.1.1.7 variant from COVID‐19 affected family members to cat. This result showed that the SARS‐CoV‐2 B.1.1.7 variant could have the highest transition capacity from human to domestic cat as shown for human‐to‐human. The governmental or worldwide policies should consider more detailed against the war with COVID‐19 pandemic.
Keywords: B.1.1.7, COVID‐19, domestic pet, SARS‐CoV‐2, Sphynx
Abbreviations
- CBC
complete blood count
- COVID‐19
coronavirus diseases 2019
- RT‐PCR
real‐time polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SST
serum separator tube
- STT
Schirmer tear test
Sarbecoviruses gave rise to two outbreaks during the 21st century resulting epidemics and pandemics, globally. 1 The ongoing pandemic coronavirus diseases 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which was first reported in Wuhan, China in December 2019. Subsequently, 147 million confirmed cases with more than 3.11 million deaths worldwide by 26 April 2021. The first SARS‐CoV‐2 infection was observed on 11 March 2020 in Northern Cyprus. 2 Since then, the government has allocated quarantine site for 14 days for both SARS‐CoV‐2 real‐time polymerase chain reaction (RT‐PCR) positive patients and their direct contacts. It is well known that genetic changes within the viral genome alter RNA virus virulence, transmissibility and pathogenesis. 3 Coronaviruses may undergo frequent recombination generating structural genetic variety due to the heir natural process. On the other hand, there is no known evidence about the recombinant sarbecovirus SARS‐CoV‐2. Despite the lack of molecular evidence, its particular binding capacity to host human angiotensin‐converting enzyme 2 (ACE2) receptor indicates that it originated from common ancestral with severe SARS‐like bat coronaviruses. 4 , 5
SARS‐CoV‐2 RNA polymerase proof‐reading shows higher replication activity, which results in genomic errors, increasing genetic diversity. Some variants such as D614G mutation within the spike (S) protein, which is referred as B.1 variant, became favoured and rapidly spread worldwide, consequently substituted the wild‐type SARS‐CoV‐2. 6 More remarkable variants have made appeared in several countries at the end of 2020. The first determined variant B.1.1.7 (20I/N501Y.V1) was identified in Kent, England, 7 the second important variant with N501Y mutation was first detected in South Africa named as B.1.351 (20J/N501Y.V2), and lately P.1 variant (20I/N501Y.V3) was detected in Brazil. It is worth to mention that these three variants have N501Y mutation in the S protein receptor‐binding domain that is crucial for binding to the human ACE2 receptor to process endocytosis. 7 , 8
The B.1.1.7 variant has increased transmissibility and infectivity ability and has now been identified in more than 85 countries including Northern Cyprus, 9 and caused national re‐lockdowns globally. The B.1.1.7 variant was first detected in January 2021 in Northern Cyprus, and 70% of SARS‐CoV‐2 positive cases were determined by April 2021 (unpublished data by Tuncel et al., 2021).
The SARS‐CoV‐2 ‘Cluster 5’ variant was detected in farmed minks associated with human to animal transmission and vice versa by Danish authorities. Likely, the variant was detected only in 12 patients and did not spread globally. 10 Since the COVID‐19 pandemic, SARS‐CoV‐2 infection has been reported in domestic pets, and it has been shown about human to animal transmission of the virus. Despite SARS‐CoV‐2 being highly susceptible in ferrets and cats, dogs have poor susceptibility and chicken, pigs and ducks are not susceptible to the virus. 11 The SARS‐CoV‐2 infection causes unapparent to mild digestive, respiratory problems, for instance, runny nose, cough, sneezing and conductivities in domestic pets. 12 , 13 , 14 Since the first human SARS‐CoV‐2 case detected in Northern Cyprus by March 2020, no domestic animal infection has been reported. To the best of our knowledge, we are reporting the first human to domestic cat transmission of SARS‐CoV‐2 with N501Y mutation in Northern Cyprus.
Materials and methods
Subject and sample collection
Two members of a family of four with 8‐year‐old male castrated Sphynx cat owners were tested SARS‐CoV‐2 RT‐PCR positive by 13 April 2021 and allocated quarantine site by the government. However, the other two family members were tested SARS‐CoV‐2 RT‐PCR negative but according to the government COVID‐19 pandemic regulation they were classified as contacted family members and allocated different quarantine site. The elder family member who has co‐morbidity (obesity and diabetes) with respiratory problems was taken to intensive care unit at the government state pandemic hospital. The youngest family member had back pain, cough and fewer as COVID‐19 symptoms. The cat stayed together in the same room with one of them. The SARS‐CoV‐2 RT‐PCR results were positive to previously negative detected family members by 17 April 2021 with back pain, cough and loss of smell. Since then, the cat was separated from the owner and relocated to a home, which was cared by a family friend for 3 days. The owners contacted Near East University Animal Hospital to report the situation. This study was approved by the Near East University Animal Experiments Local Ethics Committee (Approval number: 2020/120) and written informed consent was obtained from the owners for clinical and molecular analysis.
For this purpose, oropharyngeal, third eyelid inner side conjunctival and rectal swabs were taken from the cat at certain intervals, and that treated into viral transfer medium was collected from the cat every 3 days for 12 days for SARS‐CoV‐2 RT‐PCR test for traditional microbiological tests.
Blood samples were collected through the cephalic vein into vacuum tubes with a K3EDTA (0.5 mL, Ref. 450530, MiniCollect Tube, Greiner bio‐one, Kremsmünster, Austria) as an anticoagulant for complete blood count (CBC), a serum separator tube (5 mL, Ref 10175, BD Vacutainer, Plymouth, UK) for serum biochemistry and serology, and a lithium heparin tube (4 mL, Ref 368884, BD Vacutainer) for blood gas analysis. Serum separator tube blood sample was then centrifuged at 1500 g for 10 min after complete blood clot formation, and serum was separated.
Molecular microbiological and biochemical analyses
Nucleic acid extraction kit for viral DNA and RNA extraction and its automatic system (Ascend Biotechnology, Henan, China) was used to isolate nucleic acid from swab samples. Diagnovital® HS SARS‐CoV‐2 real‐time PCR kit (RTA Laboratories Inc., Gebze, Kocaeli, Turkey) was used to detect the SARS‐CoV‐2 directly from swab samples and extracted nucleic acids. The kit contains primer and probe set mixes particularly designed for SARS‐Cov‐N1 and N2 genes (FAM). Twenty microlitres of RT‐PCR reaction mix was prepared according to manufacturer's guidelines. Hibrigen® SARS‐CoV‐2 and N501Y mutation detection kit (Hibrigen Biotechnology AR‐GE San Tic Ltd Sti., Gebze, Kocaeli, Turkey) was used for the detection of the presence of N501Y mutation. The kit contains primer and probe sets specifically designed for SARS‐CoV‐2 RdRp gene (FAM) and nucleotide sequence harbouring the N501Y mutation (HEX). Twenty microlitre reaction mix was prepared with 10 μL of 2× One Step RT‐PCR Mix, 4 μL of Primer Probe Mix and 6 μL RNA sample. A positive control and a negative control, which were provided by the kit, were included for each run. RT‐qPCR was carried out using Insta Q96™ Plus Real‐time PCR Detection System (HiMedia Laboratories Pvt. Ltd., Mumbai, India). Analyses were carried out according to the manufacturer's instructions, and samples that have Ct (cycle threshold) values in both FAM and HEX channels were considered positive for the SARS‐CoV‐2 and the SARS‐CoV‐2 N501Y mutation, respectively. The confirmation of the B.1.1.7 variant was conducted by the detection of spike protein 69/70 deletion and N501Y variants by SNP SARS‐CoV‐2 real‐time PCR kit (SNP Biotechnology, Ankara, Turkey) according to manufacturer instructions.
CBC, serum biochemistry and serology were done at Diagnostic Laboratory, Animal Hospital, Near East University, using fresh blood samples. CBC was performed using an automatic analyser calibrated for veterinary use (BC‐2800Vet, Mindray, Shenzen, China). The assessed parameters were white blood cells (in ×109/L), including lymphocytes (in ×109/L), monocytes (in ×109/L) and granulocytes (Gran, in ×109/L); erythrocytes (red blood cells, in ×1012/μL), haemoglobin (in g/dL), haematocrit (in %), mean corpuscular volume (in fL); mean corpuscular haemoglobin (in pg); mean corpuscular haemoglobin concentration (in g/dL), platelets (in ×109/μL) and mean platelet volume (in fL). Serum biochemistry was performed using commercially available assay kits (Mindray Chemistry Reagents, Shenzen, China) and an automated clinical chemistry analyser (BS120, Mindray, Shenzen, China). Total protein (biuret method, in g/dL), albumin (bromocresol green method, in g/dL), total bilirubin (VOX method, in mg/dL), blood urea nitrogen (Urea/2.14; urease method, in mg/dL), total cholesterol (CHOD‐POD method, in mg/dL), creatinine (Jaffe method, in mg/dL) and triglycerides (GPO‐POD method, in mg/dL) were measured as serum metabolites, and also serum phosphorous concentration was measured (phosphomolybdate method, in mg/dL). Serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, creatine kinase, γ‐glutamyl transferase, lactate dehydrogenase, α‐amylase and lipase activities were quantified using the International Federation of Clinical Chemistry methods in U/L. Besides, pH, pCO2 (in mmHg), bicarbonate (HCO3 −, in mmol/L), base excess (in mmol/L), sodium (Na+, in mmol/L), potassium (K+, in mmol/L), ionized calcium (Ca++, in mmol/L), chloride (Cl−, in mmol/L), anion gap (in mmol/L), glucose (in mg/dL) and lactate (in mmol/L) levels were measured using veterinary calibrated Epoc blood gas analyses system (Ref 10736516, Epocal Inc., Ottawa, ON, Canada).
Indirect immunofluorescence antibody test (IFAT) was performed to detect feline coronavirus specific IgG antibodies (FCoV IgG). Standardised assay kits were used for this purpose, supplied by MEGACOR Diagnostik GmbH, Austria (Ref 821K10FK1). Indirect immunofluorescence antibody test titres greater than or equal to 1:100 were considered seropositive according to assay instructions. A rapid immunoassay (SNAP Combo FeLV Ag/FIV ab, Ref 99‐06033, IDEXX, Westbrook, ME, USA) was performed to detect the presence of feline leukaemia virus p27 antigen (FeLV Ag) and antibodies specific to feline immunodeficiency virus (FIV Ab). The tests were assayed following manufacturers' instructions.
Clinical examination
After the necessary precautions were taken, the cat was brought to the Near East Animal Hospital for a detailed clinical examination. In the general examination, routine clinical examination methods, inspection, palpation and auscultation examination methods were applied. Blood pressure, pulse, heart rhythm measurements were made, and then routine eye examination of the bulbus oculi and eye attachment organs was started. For this purpose, penlight was used to evaluate direct and indirect pupillary light reflexes; direct ophthalmoscope (Riester Otoscope/Ophtalmoscope, Germany) was used for cornea, anterior chamber, lens and fundus examination. Schirmer tear test (STT) for tear function evaluation, fluorescent staining test for corneal erosions and rebound tonometry (Icare Tonovet, Finland) for intraocular pressure measurement were conducted. Ocular reflex examinations were performed, including the manage response.
Subsequently, three‐sided direct radiographs of the patient, thoracic and abdominal, were taken (ECORAY HF‐525 PLUS VET, South Korea). Standard left parasternal apical imaging, right parasternal apical long axis and short axis imaging, colour Doppler, tissue Doppler imaging and modified Simpson methods were used for echocardiographic evaluation. Echocardiographic examination was performed with a GE LOGIQ e R7 CONSOLE ultrasound machine with built‐in DTI capacity equipped with a 4–7 MHz flat phased array probe.
Results
Molecular findings
The SARS‐CoV‐2 RT‐PCR results from oropharyngeal, eye and rectum swab samples are shown in Table 1. During first and second sampling both oropharyngeal swabs and extracted nucleic acid from oropharyngeal swabs were SARS‐CoV‐2 RT‐PCR positive (Ct: 33.90 and Ct: 31.21; Ct: 38.72 and Ct: 36.06, respectively). However, eye and rectum samples were negative. Figure 1 displays the RNA sample RT‐PCR result and its Ct curve. Third and fourth sampling showed SARS‐CoV‐2 RT‐PCR negative in all samples including nucleic acid isolations as well.
Table 1.
SARS‐CoV‐2 RT‐PCR results
| Sample type | SARS‐CoV‐2 RT‐PCR results | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 6 | Day 9 | Day 12 | |||||
| Swab Ct | RNA Ct | Swab Ct | RNA Ct | Swab Ct | RNA Ct | Swab Ct | RNA Ct | |
| Oropharyngeal | 33.90 | 31.21 | 38.72 | 36.06 | – | – | – | – |
| Eye | – | – | – | – | – | – | – | – |
| Rectum | – | – | – | – | – | – | – | – |
Figure 1.
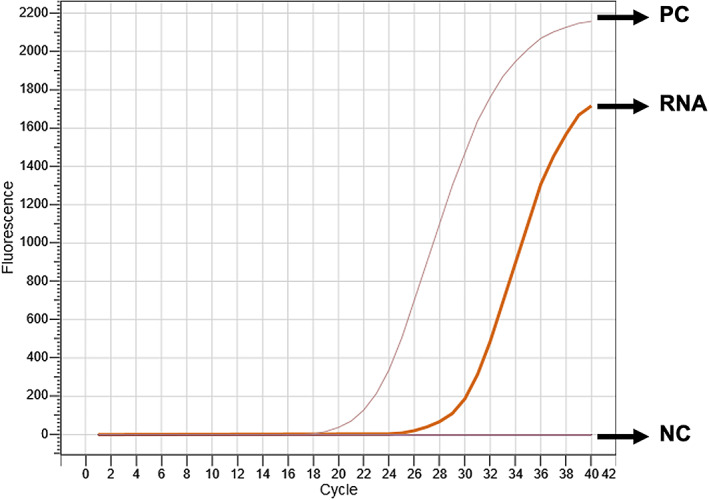
Oropharyngeal RNA showed SARS‐CoV‐2 positive result with Ct: 31.21. NC, negative control; PC, positive control; RNA, cat's RNA sample.
Oropharyngeal RNA was further analysed for detection of the B.1.1.7 variant, and the presence of the N501Y mutation (Ct: 32.24) and the absence of the 69/70del indicated the B.1.1.7 variant (Figure 2).
Figure 2.
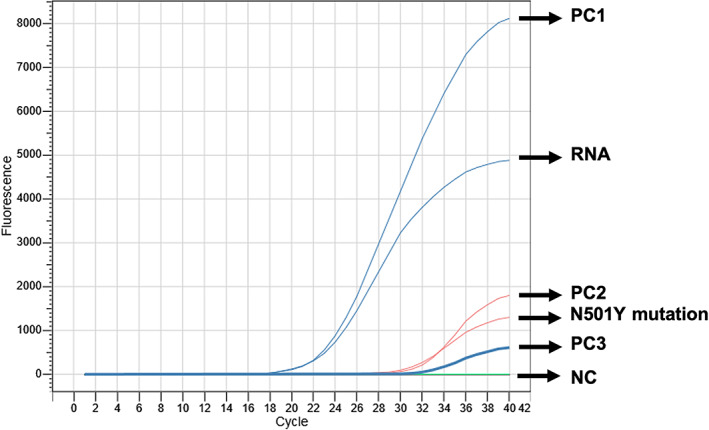
Further molecular genotyping analysis detected B.1.1.7 variant resulting the presence of the N501Y mutation and the absence of the 69/70del mutation. N501Y, existence of the N501Y mutation at Cat's RNA; PC1, positive control for SARS‐CoV‐2; PC2, positive control for N5017 mutation; PC3, positive control for 69/70del mutation; NC, negative controls; RNA, Cat's RNA sample.
CBC, serum biochemistry and serology findings
The result of CBC is presented in Table 2. A slight increase in the mean corpuscular volume, which indicates the average red blood cell volume, was detected.
Table 2.
Complete blood count results of the case
| Test | Result | Reference interval |
|---|---|---|
| WBC (×109/L) | 14.5 | 6.0–20.0 |
| Lym (×109/L) | 3.3 | 1.0–7.0 |
| Mon (×109/L) | 0.9 | <2.0 |
| Gran (×109/L) | 10.3 | 2.0–15.0 |
| RBC (×1012/μL) | 7.8 | 5.0–10.0 |
| HGB (g/dL) | 13.3 | 9.0–15.0 |
| HCT (%) | 43.4 | 28.0–19.0 |
| MCV (fL) | 55.9 | 39.0–52.0 |
| MCH (pg) | 17.1 | 13.0–21.0 |
| MCHC (g/dL) | 30.6 | 30.0–38.0 |
| PLT (×109/μL) | 292 | 100–514 |
| MPV (fL) | 11.7 | 5.0–12.0 |
Gran, granulocytes; HCT, haematocrit; HGB, haemoglobin; Lym, lymphocytes; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; Mon, monocytes; MPV, mean platelet volume; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
Note: The volume percentage of red blood cells in blood (HCT) and mean corpuscular volume was slightly higher.
High HCO3 −, total protein, glucose, lactate concentration, decreased total cholesterol concentration, increased lactate dehydrogenase enzyme activity, hypocalcaemia and hypophosphatemia were present in serum biochemistry (Table 3).
Table 3.
Laboratory results of serum biochemistry
| Test | Result | Reference interval |
|---|---|---|
| pH | 7.33 | 7.25–7.40 |
| pCO2 (mmHg) | 49.1 | 33.0–51.0 |
| HCO3 − (mmol/L) | 26.3 | 13.0–25.0 |
| BE (mmol/L) | 0.50 | −5.0–2.0 |
| Na+ (mmol/L) | 156 | 147–162 |
| K+ (mmol/L) | 3.1 | 2.9–4.2 |
| Ca++ (mmol/L) | 0.72 | 1.20–1.32 |
| Cl− (mmol/L) | 120 | 112–129 |
| Anion gap (mmol/L) | 14 | 10–27 |
| Glucose (mg/dL) | 136 | 60–131 |
| Lactate (mmol/L) | 2.78 | 0.50–2.70 |
| Albumin (g/dL) | 3.0 | 2.1–3.3 |
| Albumin:Globulin ratio (g/dL) | 0.56 | 0.45–1.19 |
| ALP (U/L) | 32 | 25–93 |
| ALT (U/L) | 46 | 6–83 |
| α‐Amylase (U/L) | 1275 | 532–2008 |
| Total bilirubin (mg/dL) | 0.10 | <0.20 |
| BUN (mg/dL) | 27 | 20–30 |
| CK (U/L) | 226 | 73–388 |
| Phosphorus (P) | 3.8 | 4.5–8.1 |
| Gamma‐GT | <4.0 | 1.3–5.1 |
| Cholesterol (mg/dL) | 92 | 95–130 |
| Creatinine (mg/dL) | 1.37 | 0.80–1.80 |
| LDH (U/L) | 331 | 63–273 |
| Lipase (U/L) | 51 | 0–83 |
| Total protein (g/dL) | 8.30 | 5.4–7.8 |
| Triglycerides (mg/dL) | 42 | 25–133 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; BE, base excess; BUN, blood urea nitrogen; CK, creatine kinase; GT, glutamyl transferase; LDH, lactate dehydrogenase.
Note: Serum biochemistry results indicated that higher HCO3−, total protein, glucose, lactate concentration, increased lactate dehydrogenase enzyme activity, hypocalcaemia and hypophosphatemia and decreased total cholesterol concentration.
Feline coronavirus specific IgG antibodies, feline leukaemia virus p27 antigen and feline immunodeficiency virus tests performed using serology methods were negative.
Microbiological findings
In order to determine whether the purulent discharge in the left eye had a relationship with SARS‐CoV‐2, microbiological swab samples were taken from here, and bacteria isolation and identification were performed. As a result of these tests, Staphylococcus xylous and Corynebacterium spp. were isolated from the sample.
Clinical findings
On the third day of separation from the COVID‐19 owner, detailed clinical examination was performed to 8‐year‐old male patient. The patient had previously diagnosed skin lesions on his head and his right leg. The general condition was good with a 5.5 kg body weight; however, his breathing was slightly faster compared with previous visits, including abnormal palpation and auscultation problems were observed. The body temperature was 36.3°C (37.1°C–39.1°C), which was lower than the expected range. His food appetite has decreased compared to few days before.
During the electrocardiogram (ECG) and colour Doppler USG examination, heart rate was taken as 187/min. Hypertension findings were detected by blood pressure measurements. Standard left parasternal apical imaging, right parasternal apical long axis and short axis imaging, colour Doppler and modified Simpson methods were used for echocardiographic evaluation. Aortic root width was normal, mitral and tricuspid valves were functional. Myocardial echogenicity was found to be variable. There was no systolic dysfunction. The enlargement of the right atrium and ventricle, high peak E velocity and E velocity indicate the shortening of the deceleration time, the presence of restrictive physiology and diastolic dysfunction.
During ocular examination, purulent discharge and conjunctivitis on the left eye were detected. The STT was conducted for both eyes. The left eye scores 18.5 mm/min and the right eye scores 16.3 mm/min for the STT (10–20 mm/min). 15 Direct and indirect pupillary light reflex, eye blink reflexes and manage response were evaluated as positive.
No abnormal findings were found in direct ophthalmoscopy results used in cornea, anterior chamber, lens and fundus examination. There were no pathology findings at the retinal vessels on fundus examination. Intra ocular pressure findings were within normal values for both right and left eyes, 25 mm/hg and 24 mm/hg, respectively (15–30 mm/hg). 15 On ocular examination, the only pathology was determined as left‐sided muco‐purulent tear discharge due to conjunctivitis.
The 3D thorax radiographs were taken to check any putative pathological finding caused by SARS‐CoV‐2 infection. On ventral‐dorsal radiograph, broncho interstitial patterns in the cranial lobes were observed, which might be compatible with a very mild amount of peri bronchial infiltrate. These findings were not diffuse. Thorax images were generally good looking considering the age of the patient. The dimensions of the heart were within the normal limits of three intercostal spaces. The radiological imaging findings of the heart, both atriums were minimally cambered and curved to lateral side (Figure 3).
Figure 3.
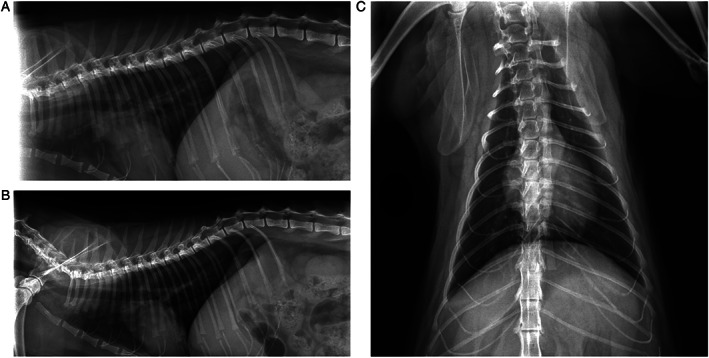
2D thorax radiograph result of the patient to examine the potential pathological results of the SARS‐CoV‐2 infections. (A) Right lateral thorax graphy, (B) left lateral thorax graphy, (C) ventral‐dorsal thorax graphy.
Discussion
The SARS‐CoV‐2 B.1.1.7 variant showed the highest transmission and infection activity so far among other identified SARS‐CoV‐2 variants since first discovered in December 2020. 16 The potential infection of the B.1.1.7 variant in domestic animals by their pet parents ought to urge COVID‐19 pandemic dynamics. 11 , 12 , 13 , 14 Previous studies indicated that SARS‐CoV‐2 binds ACE2 that is generally expressed in epithelial cells of tracheobronchial submucosal glands in cats as humans. 17 , 18 , 19 Previously, SARS‐CoV‐2 infection in cats was determined by serological 20 and molecular PCR analysis, 21 especially in outbred and naturally infected animals. To the authors' knowledge, this is the first study showing direct humans‐to‐cat transmission of the SARS‐CoV‐2 B.1.1.7 variant within the same household. There is no evidence of zoonotic or pet‐to‐pet transmissions of SARS‐CoV‐2 and its variants yet.
The oropharyngeal RNA of the cat displayed SARS‐CoV‐2 RT‐PCR positive result with B.1.1.7 variant on third and 6 days of separation from the pet parents, and the cat became SARS‐CoV‐2 RT‐PCR negative on the ninth day and 12 days later. As its companions became SARS‐CoV‐2 RT‐PCR negative after 10 days of the infection. Probably, the cat might have transmitted the virus directly from them as given higher Ct values.
The other notable findings of this investigation shed light to putative clinical outcomes of SARS‐CoV‐2 B.1.1.7 variant infected cats including cardiac abnormalities and secondary bacterial infections causing conjunctivitis. Despite a SARS‐CoV‐2 infected cat from Spain, 22 other domestic animals showed mild upper‐respiratory‐disease. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 21 Despite its clinical variety, the B.1.1.7 variant increased the COVID‐19 disease worldwide, resulting in growing hospitalisation and mortality. 23 However, the clinical effect of the SARS‐CoV‐2 B.1.1.7 variant infection in animals was not yet identified. In the current study, the clinical general examination and biochemical analysis of the B.1.1.7 infected Sphynx cat did not show serious symptoms. On the other hand, during radiological screening, small amounts of peribranchial infiltrative compatible lesions in the cranial lobes of the lungs were observed. Previously, Musso et al showed SARS‐CoV‐2 infected cat in Italy displayed pneumonia, however, there is no evidence for B.1.17. variant. 24
In the detailed eye examination, eye measurements were found in normal range for cat. Conjunctivitis and mucopurulent discharge were observed in the left eye. This pathogenicity has increased the bacterium activation of S. xylous and Corynebacterium spp., which might be the secondary infections agents for SARS‐CoV‐2. Recent studies showed that conjunctivitis was observed in domestic animal with SARS‐CoV‐2 infection. 12 , 13 , 14 During the ECG and colour Doppler USG examination, heart rate was taken as 187/min. Cardiac examination revealed the right atrium and ventricle enlargement and restrictive physiology and diastolic dysfunction. Since the cat did not have a cardiac history before, the virus infection might encourage multisystem inflammatory failure, which is also observed in patients with COVID‐19. 25 , 26 This finding has been supported by Ferasin et al, in their study, naturally SARS‐CoV‐2 infected domestic animals (cats and dogs) showed severe myocarditis in the United Kingdom. 21
These findings need to support with other domestic animals that are infected by SARS‐CoV‐2 B.1.1.7 variant. However, SARS‐CoV‐2 RT‐PCR positive patients and their contacts have been allocated governmental sites immediately. Thus, pet parents could not by followed‐up with their companion animals before. Our findings will light up the government and public awareness and contribute to the war against SARS‐CoV‐2 infection.
Conclusion
Overall, the direct human‐to‐cat transmission of the B.1.1.7 variant was demonstrated in this study. Moreover, the high transmission ability of the viral variant infection and its pathogenetic consequences in humans as well as animals should be more discussed and investigated.
Conflicts of interest and sources of funding
The authors declare no conflicts of interest or sources of funding for the work presented here.
Acknowledgments
Dedicated to the memory of our friend and colleague Prof. Kursad Turgut, who always believed in the success of this study. You are gone but your belief in us has made this journey possible.
Curukoglu, A. , Ergoren, MC. , Ozgencil, FE. , Sayiner, S. , Ince, ME. and Sanlidag, T. , First direct human‐to‐cat transmission of the SARS‐CoV‐2 B.1.1.7 variant. Aust Vet J. 2021;99:482–488. 10.1111/avj.13109
References
- 1. Baric RS. Emergence of a highly fit SARS‐CoV‐2 variant. N Engl J Med 2020;383:2684–2686. [DOI] [PubMed] [Google Scholar]
- 2. Baddal B, Bostanci A, Suer K et al. SARS‐CoV‐2 was already in circulation in Northern Cyprus in the prepandemic period. J Infect 2021;s0163‐4453(00097‐09), 21–22. 10.1016/j.jinf.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam TT‐Y, Zhu H, Guan Y et al. Genomic analysis of the emergence, evolution, and spread of human respiratory RNA viruses. Annu Rev Genomics Hum Genet 2016;17:193–218. [DOI] [PubMed] [Google Scholar]
- 4. Boni MF, Lemey P, Jiang X et al. Evolutionary origins of the SARS‐CoV‐2 sarbecovirus lineage responsible for the COVID‐19 pandemic. Nat Microbiol 2020;5:1408–1417. [DOI] [PubMed] [Google Scholar]
- 5. Liu K, Tan S, Niu S et al. Cross‐species recognition of SARS‐CoV‐2 to bat ACE2. Proc Natl Acad Sci 2021;118:e2020216118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korber B, Fischer WM, Gnanakaran S et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 virus. Cell 2020;182:812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. PHE . P.H.E. Investigation of novel SARS‐CoV‐2 variant: variant of concern 202012/01: technical briefing 6. London, PHE, 2021. Available at:. www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-of-concern-20201201. Accessed 13 February 2021. [Google Scholar]
- 8. Cao Y, Li L, Feng Z et al. Comparative genetic analysis of the novel coronavirus (2019‐nCoV/SARS‐CoV‐2) receptor ACE2 in different populations. Cell Discov 2020;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PANGO lineages. Available at: https://cov-lineages.org/global_report_B.1.1.7.html. Accessed 10 May 2021.
- 10. European Centre for Disease Prevention and Control . Detection of new SARS‐CoV‐2 variants related to mink – 12 November 2020. Stockholm, ECDC, 2020. [Google Scholar]
- 11. Shi J, Wen Z, Zhong G et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS‐coronavirus 2. Science 2020;368:1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barrs VR, Peiris M, Tam KWS et al. SARS‐CoV‐2 in quarantined domestic cats from COVID‐19 households or close contacts, Hong Kong, China. Emerg Infect Dis 2020;26:3071–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hosie MJ, Epifano I, Herder V et al. Respiratory disease in cats associated with human‐to‐cat transmission of SARS‐CoV‐2 in the UK. bioRxiv, 2020.2009.2023.309948 2020. [Google Scholar]
- 14. Sit THC, Brackman CJ, Ip SM et al. Infection of dogs with SARS‐CoV‐2. Nature 2020;586:776–778. 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Şaroğlu M. Kedi ve Köpeklerde Göz Hastalıkları. İstanbul, Nobel kitapevi, 2013;85–117. [Google Scholar]
- 16. Public Health England . Investigation of novel SARS‐CoV‐2 variant, Variant of Concern 202012/01 Technical briefing 2–28 December 2020. London, PHE, 2020. [Google Scholar]
- 17. Yan R, Zhang Y, Li Y et al. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science 2020;367:1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nat Microbiol 2020;5:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Cell Editorial Team . COVID‐19: navigating uncertainties together. Cell 2020;181:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Q, Zhang H, Huang K et al. SARS‐CoV‐2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv 2020. 10.1101/2020.04.01.021196. [DOI] [Google Scholar]
- 21. Ferasin L, Fritz M, Ferasin H et al. Myocarditis in naturally infected pets with the British variant of COVID‐19. bioRxiv, 2021.03.18.435945 2021. 10.1101/2021.03.18.435945. [DOI] [Google Scholar]
- 22. Segalés J, Puig M, Rodon J et al. Detection of SARS‐CoV‐2 in a cat owned by a COVID‐19−affected patient in Spain. Proc Natl Acad Sci 2020;117:24790–24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Challen R, Brooks‐Pollock E, Read IM et al. Risk of mortality in patients infected with SARS‐CoV‐2 variant of concern 202012/1: matched cohort study. BMJ 2021;372:n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Musso N, Costantino A, La Spina S et al. New SARS‐CoV‐2 infection in a pet cat with severe lung disease in Italy. Preprints 2020, 2020070398. 10.20944/preprints202007.0398.v1 [DOI]
- 25. Morris SB, Schwartz NG, Patel P et al. Case series of multisystem inflammatory syndrome in adults associated with SARS‐CoV‐2 infection ‐ United Kingdom and United States, march‐august 2020. MMWR Morb Mortal Wkly Rep 2020;69:1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valverde I, Singh Y, Sanchez‐de‐Toledo J et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID‐19 infection in Europe. Circulation 2021;143:21–32. [DOI] [PubMed] [Google Scholar]


