Dear Editor,
The COVID‐19 pandemic has profoundly impacted people's lives across the globe. Vaccines are perhaps the only silver lining in this dark cloud as they mitigate the severity of the disease. However, as more people are vaccinated, various adverse events (AEs) including cutaneous AEs have been reported. Cutaneous vasculitis is one such rare AE with a few cases reported. We report a case of cutaneous small vessel vasculitis (cSVV) with a strikingly asymmetrical distribution, following COVID‐19 vaccination.
A 31‐year‐old woman presented with a 3‐day history of painful purpuric lesions on her legs. She reported no comorbidities, systemic problems or prior medications. Her medical history was unremarkable apart from receipt of her second dose of inactivated viral vaccine (COVAXIN®; Bharat Biotech, Hyderabad, India) 4 days prior to development of the lesion; she had not developed any similar lesion after the first dose, which had been given 4 weeks before the second dose.
On examination, tender palpable purpura was noted predominantly on the left leg, with pitting oedema (Fig. 1a,b).
Figure 1.
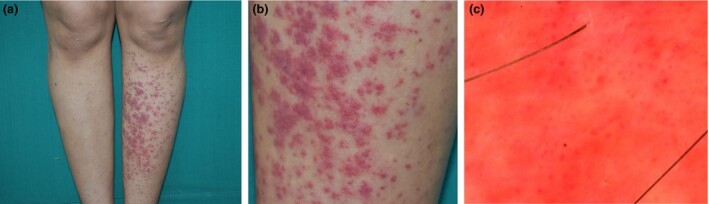
(a) Palpable purpura predominantly involving the left leg; (b) closer view. (c) Dermoscopy (polarized mode) revealing red dots and blotches on a red–orange background.
Dermoscopy revealed irregularly arranged red blotches with an orange–red background (Fig. 1c).
Histology showed an abundant upper and mid‐dermal perivascular infiltrate comprised predominantly of eosinophils and lymphocytes with a few neutrophils, along with erythrocyte extravasation, perivascular fibrin and perivascular oedema (Fig. 2a,b). The patient's financial constraints precluded direct immunofluorescence.
Figure 2.
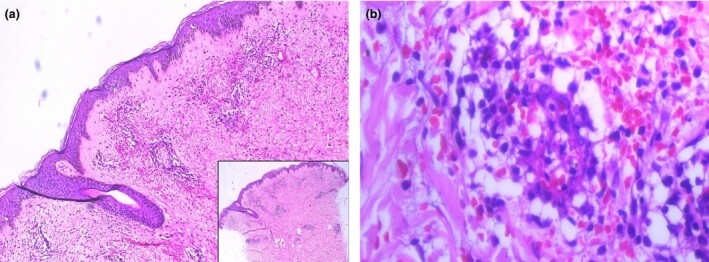
(a) Superficial and mid‐dermal perivascular infiltrate with erythrocyte extravasation; (b) fibrin cuffing around dermal blood vessels with eosinophils, lymphocytes and, to a lesser extent, neutrophils. Haematoxylin and eosin, original magnification (a) × 100; inset × 25; (b) × 400.
Routine biochemical and immunological investigations were within normal limits. Reverse transcription (RT)‐PCR for COVID‐19 and throat swab were negative.
The final diagnosis was cSVV secondary to COVAXIN®. The patient was advised rest and leg elevation, and prescribed antihistaminics for a week, after which the lesions resolved with hyperpigmentation. She is being followed up monthly and remains asymptomatic.
Immunological phenomena with vaccines are not unknown, having been noted with hepatitis B, human papillomavirus and influenza vaccines.1 This could be due to molecular mimicry and immunological crossreactivity due to vaccine antigens.2
With the increase in the number of people receiving COVID‐19 vaccines, multiple reports of cutaneous AEs to COVID‐19 vaccines have emerged. The largest series of 414 patients described local reactions as the commonest AEs.3 Only three cases of cutaneous vasculitis following COVID‐19 vaccination have been reported.3 Another report described a single patient with aggravation of pre‐existing vasculitis after mRNA vaccine.2 There are multiple reports of COVID‐19 manifesting with vasculitis, but the negative COVID‐19 PCR test excluded this possibility in our patient.
The obvious asymmetry of the purpura in this case was intriguing. While working, our patient tended to sit with her right leg habitually crossed over the left, thus due to the resultant reduction of gravity and hydrostatic pressure, the purpura were less severe on the right leg. The few previous reports of similar cases also considered the cause to be the dependent position of the affected limb, while the limb which is kept horizontally tends to be spared.4, 5
Eosinophil predominance, an unusual histopathological aspect of the present case, has been noted in only a few groups of diseases,6 and has not been described previously in vaccine‐induced vasculitis.
Awareness and reporting of systemic and cutaneous AEs of vaccines by physicians is important. Further studies are needed to determine the association of vasculitis with vaccine constituents. A wait‐and‐watch policy in such cases could be adopted before further investigations, as in our patient resolution was noted in 10 days without recurrence.
Acknowledgement
We thank the patient for their written informed consent to publication of the case details.
Contributor Information
V. Kharkar, Department of Dermatology Seth G. S. Medical College and K. E. M. Hospital Mumbai India
T. Vishwanath, Department of Dermatology Seth G. S. Medical College and K. E. M. Hospital Mumbai India
S. Mahajan, Department of Dermatology Seth G. S. Medical College and K. E. M. Hospital Mumbai India
R. Joshi, Department of Dermatology P. D. Hinduja National Hospital and Medical Research Centre Mumbai India
P. Gole, Department of Dermatology Seth G. S. Medical College and K. E. M. Hospital Mumbai India
References
- Orbach H, Agmon‐Levin N, Zandman‐Goddard G. Vaccines and autoimmune diseases of the adult. Discov Med 2010; 9: 90–7. [PubMed] [Google Scholar]
- Cohen SR, Prussick L, Kahn JS et al. Leukocytoclastic vasculitis flare following the COVID‐19 vaccine. Int J Dermatol 2021. 10.1111/ijd.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin B, Salman A, Tuglular S et al. Unilateral cutaneous vasculitis: an uncommon presentation and a possible explanation. Indian J Dermatol Venereol Leprol 2015; 81: 518–19. [DOI] [PubMed] [Google Scholar]
- Gershinsky Y, Levi R, Heyman SN. Unilateral purpuric rash in a patient with acute renal failure. Isr Med Assoc J 2011; 13: 515. [PubMed] [Google Scholar]
- Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol 2014; 10: 474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


