Abstract
Background
Circulating testosterone levels have been found to be reduced in men with severe acute respiratory syndrome coronavirus 2 infection, COVID‐19, with lower levels being associated with more severe clinical outcomes.
Objectives
We aimed to assess total testosterone levels and the prevalence of total testosterone still suggesting for hypogonadism at 7‐month follow‐up in a cohort of 121 men who recovered from laboratory‐confirmed COVID‐19.
Materials and methods
Demographic, clinical, and hormonal values were collected for all patients. Hypogonadism was defined as total testosterone ≤9.2 nmol/L. The Charlson Comorbidity Index was used to score health‐significant comorbidities. Descriptive statistics and multivariable linear and logistic regression models tested the association between clinical and laboratory variables and total testosterone levels at follow‐up assessment.
Results
Circulating total testosterone levels increased at 7‐month follow‐up compared to hospital admittance (p < 0.0001), while luteinizing hormone and 17β‐estradiol levels significantly decreased (all p ≤ 0.02). Overall, total testosterone levels increased in 106 (87.6%) patients, but further decreased in 12 (9.9%) patients at follow‐up, where a total testosterone level suggestive for hypogonadism was still observed in 66 (55%) patients. Baseline Charlson Comorbidity Index score (OR 0.36; p = 0.03 [0.14, 0.89]) was independently associated with total testosterone levels at 7‐month follow‐up, after adjusting for age, BMI, and IL‐6 at hospital admittance.
Conclusions
Although total testosterone levels increased over time after COVID‐19, more than 50% of men who recovered from the disease still had circulating testosterone levels suggestive for a condition of hypogonadism at 7‐month follow‐up. In as many as 10% of cases, testosterone levels even further decreased. Of clinical relevance, the higher the burden of comorbid conditions at presentation, the lower the probability of testosterone levels recovery over time.
Keywords: comorbidities, COVID‐19, follow‐up, male, SARS‐CoV‐2, testosterone
1. INTRODUCTION
The new severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐induced disease COVID‐19 provokes a number of clinical sequelae associated with viral multiorgan tropism. 1 Host characteristics have been demonstrated to allow or even predispose to more or less unfavorable outcomes, thus including fatal events (e.g., age, sex‐related differences in terms of susceptibility to viral infection and adaptive immune responses, comorbidities, etc. 2 , 3 , 4 ).
Despite the testis is one of the very few organs with immune privilege, several observations reported that SARS‐CoV‐2 enters into the reproductive system through viral receptors highly expressed on testicular tissues. 5 , 6 As a consequence, SARS‐CoV‐2 was demonstrated to cause damage to both Sertoli and Leydig cells. 6 , 7 Accordingly, based on the hypothesis that also hormone‐related biological sex differences may have a relevant impact throughout COVID‐19 course, 8 a growing number of studies suggested that the viral infection per se may lower the production of testosterone (T), 9 , 10 , 11 with lower circulating T levels being associated with more severe clinical outcomes. 12 Moreover, the real‐life clinical evidence suggests that men with lower T levels at baseline could have worse outcomes because of COVID‐19, despite equal comorbidities at presentation, 13 thus confirming one of the most debated hypotheses during the initial speculation on this topic. 14 , 15 , 16 Conversely, given the reported quantitative and qualitative damage that SARS‐CoV‐2 infection seems to cause at the testicular level, 6 , 7 whether SARS‐CoV‐2 infection affects over time testis exocrine (i.e., spermatogenesis) and endocrine (i.e., steroidogenesis) function in men who have survived COVID‐19 17 , 18 , 19 is still to be clarified. Therefore, based on the preclinical and clinical observations that circulating total testosterone (tT) significantly decreases in men with COVID‐19, we aimed to investigate the levels of tT and the rate of patients with tT levels suggestive for hypogonadism 20 over the follow‐up in a cohort of male patients who eventually survived after COVID‐19.
2. METHODS
Data from a subcohort of 121 male patients belonging to the original cohort of men admitted to the emergency or clinical departments because of symptomatic SARS‐CoV‐2‐induced disease at a single academic hospital between February 29 and May 2, 2020 12 and who eventually recovered from COVID‐19 were analyzed.
Data were collected through patient interview or medical chart review, and followed the principles outlined in the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations. On obtaining written individual patient's consent, clinical data from all patients were retrieved using a dedicated case report form (CRF), according to an institutional protocol (Covid‐BioB, ClinicalTrials.gov NCT04318366; Ethical Committee approval number 34/int/2020). 21
As previously described, 12 the Charlson Comorbidity Index (CCI) was used to score health‐significant comorbidities, 22 and measured body mass index (BMI) was obtained for each patient. Likewise, at hospital admission, patients were subdivided into mild, moderate, and severe acute respiratory distress syndrome (ARDS), according to standard definitions. 23 Moreover, baseline chest radiography findings of SARS‐CoV‐2 pneumonia severity were scored in every patient with the Radiographic Assessment of Lung Edema (RALE) score to evaluate the extent and density of alveolar opacities on chest radiographs the same day of the admission. 24 Accordingly, 12 patients with COVID‐19 were divided into four groups according to the outcome after hospital admission: Group 1: patients in good clinical conditions and discharged home from the emergency department; Group 2: patients admitted in the internal medicine unit until possible discharge home; and Group 3: patients invasively ventilated in the intensive care unit (ICU), and subsequently successfully extubated and discharged either to the internal medicine unit or home. Furthermore, a validated composite risk score based on the characteristics at the time of first hospital admission was calculated for every patient (i.e., Critical‐ill COVID‐19 score). 25
2.1. Biochemical measurements
Baseline venous blood samples were drawn in all patients at hospital admission (between 7 am and 11 am, after an overnight fast), and kept at 4°C until serum or plasma were separated by centrifugation. Serum and plasma aliquots were then stored at −80°C until assay. For the specific purposes of this analysis, on each sample for every patient at baseline we measured follicle‐stimulating hormone (FSH; FSH: LIAISON FSH ‐ [REF] 312251), luteinizing hormone (LH; LH: LIAISON LH ‐ [REF] 312201), tT (testosterone: LIAISON Testosterone ‐ [REF] 310410), and 17β‐estradiol (E2; E2: LIAISON Estradiol II Gen ‐ [REF] 310680; DiaSorin SpA, Saluggia, Italy). Furthermore, interleukin‐6 (IL‐6) was measured by ECLIA (Elecsys IL‐6, COBAS ROCHE) in every patient. Moreover, LIAISON SARS‐CoV‐2 S1/S2 IgM and LIAISON SARS‐CoV‐2 S1/S2 IgG serological tests were used to assess SARS‐CoV‐2 IgM and IgG in all patients. Routine blood tests encompassed serum biochemistry (including complete blood count with differential and C‐reactive protein [CRP] as inflammation markers).
2.2. Post‐discharge follow‐up
As of February 1, 2021, a total of 121 patients had available follow‐up at 7 ± 2 months since hospital discharge after complete recovery from COVID‐19 (laboratory‐confirmed negativity for SARS‐CoV‐2). Updated clinical data, including specific therapeutic compounds (i.e., antiviral therapy, systemic corticosteroid treatment or some biological disease‐modifying anti‐rheumatic drugs [bDMARDs] 26 ) used throughout the hospitalization after first hospital admittance and till the final hospital discharge were collected through individual CRF for every patient. 21
Likewise, a further serum sample was stored in our institution biobank during the post‐discharge follow‐up visits for all patients. For every patient, we measured FSH, LH, tT, E2, IL‐6, and IgM/IgG against SARS‐CoV‐2 spike protein, as previously described. 12
2.3. Outcomes
Primary outcomes were tT levels at baseline and at 7‐month follow‐up assessment, along with the overall rate of patients with tT levels suggestive for hypogonadism (according to a tT threshold of 9.2 nmol/L) 20 at both dates. Secondary outcome was to detail the overall rate of patients with tT levels suggestive for hypogonadism (according to a tT threshold of 12 nmol/L). 27
2.4. Statistical methods
Distribution of data was tested with the Shapiro–Wilk test. Data are presented as medians (and IQR) or frequencies (proportions). We used Wilcoxon signed‐rank test or chi‐square test in order to compare hormonal levels and other demographics, clinical, and laboratory characteristics between COVID‐19 patients at both assessment dates.
To test the hypothesis that complete recovery from COVID‐19 could be associated with changes of tT levels over time, we performed a landmark analysis with logistic and linear models testing probability and predictors of increased tT levels and magnitude of tT increase up to 7‐month follow‐up; both models were adjusted for baseline clinical factors and markers of systemic inflammation as a measure of disease severity at first hospital admission (e.g., IL‐6). As follow‐up tT levels could also be influenced by a number of treatments received during hospitalization, we also performed a sensitivity analysis to test the association of treatments received and the probability of tT change at follow‐up. Statistical analyses were performed using Stata 14.0 (StataCorp, College Station, TX, USA). All tests were two‐sided, and statistical significance level was determined at p < 0.05.
3. RESULTS
Table 1 depicts patients characteristics at hospital admission and at 7‐month follow‐up. Analysis of clinical and laboratory parameters revealed significantly lower levels of LH and E2 (all p ≤ 0.02) at follow‐up, with higher circulating tT compared with baseline (p < 0.0001) (Figure 1; Table 1). Overall, tT levels increased in 106 (87.6%) patients, but remained almost stable in three (2.5%) and further decreased in 12 (9.9%) patients at follow‐up as compared with values at hospital admittance. Of all, a tT level <9.2 nmol/L was still observed in 66 (55%) patients at follow‐up compared with 115 (95%) at admittance (p < 0.0001) (Table 1). Median (IQR) tT level at follow‐up was slightly above the threshold for hypogonadism (9.99 [6.7–13.4] nmol/L) (Table 1). Likewise, according to a 12 nmol/L threshold, as many as 79 (66%) patients were suggestive for hypogonadism at follow‐up compared with 117 (97%) at admittance (p < 0.0001) (Table 1).
TABLE 1.
Demographic, clinical, and laboratory characteristics of patients at admission compared to 6‐month follow‐up (n = 121)
| Admission | Follow‐up | p‐Value a | |
|---|---|---|---|
| Age (years) | 57 (49–65) | 57 (49–65) | |
| Ethnicity | |||
| White European | 101 (83) | 101 (83) | |
| Latin American | 15 (12) | 15 (12) | |
| African | 4 (3.3) | 4 (3.3) | |
| Far‐East Asian | 1 (0.8) | 1 (0.8) | |
| BMI (kg/m2) | 0.001 | ||
| <25 | 20 (17) | 21 (18) | |
| 25–29.9 | 41 (33) | 43 (36) | |
| ≥30 | 60 (50) | 56 (46) | |
| Comorbidities | |||
| CCI | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | <0.001 |
| CCI (score) | |||
| 0 | 72 (60) | 90 (74) | <0.001 |
| 1 | 27 (22) | 19 (16) | |
| ≥2 | 22 (18) | 12 (10) | |
| Cardiovascular diseases | 16 (13.2) | ||
| Arterial hypertension | 45 (37) | ||
| Diabetes mellitus | 17 (14) | ||
| Chronic kidney disease | 4 (3.3) | ||
| COVID‐19 severity at admission | |||
| ARDS (PaO2:FiO2) | |||
| None | 40 (33) | ||
| Mild ARDS (300–200 mm Hg) | 51 (41) | ||
| Moderate ARDS (100–200 mm Hg) | 14 (12) | ||
| Severe ARDS (<100–200 mm Hg) | 16 (13) | ||
| RALE score at admission | 7.0 (4.0–14.0) | ||
| Critical‐Ill COVID‐19 at admission | 95.1 (73.1–113.1) | ||
| Groups | |||
| 1 | 4 (3.5) | ||
| 2 | 93 (76.3) | ||
| 3 | 24 (20.2) | ||
| Laboratory parameters | |||
| WBC, 109/L | 7.2 (5.2–9.1) | 6.4 (5.5–8.0) | 0.01 |
| Neutrophils, 109/L | 5.3 (3.5–7.0) | 3.4 (2.8–4.4) | <0.0001 |
| Lymphocytes, 109/L | 1.0 (0.7–1.5) | 2.2 (1.9–2.8) | <0.0001 |
| NLR | 5.2 (2.8–8.2) | 1.5 (1.1–2.2) | <0.0001 |
| Platelets, 109/L | 250 (190–335) | 240 (195–266) | 0.0003 |
| Creatinine, mg/dl | 1.0 (0.9–1.1) | 1.0 (0.7–1.6) | 0.5 |
| C‐reactive protein, mg/L | 83.4 (28.7–140.4) | 87.1 (22.3–135.9) | 0.7 |
| IL‐6, pg/ml | 32.3 (9.5–76.5) | 26.5 (5.2–74.3) | 0.3 |
| FSH, mU/ml | 5.7 (3.9–8.4) | 7.8 (5.8–11.0) | 0.08 |
| LH, mU/ml | 5.0 (3.6–6.5) | 4.1 (2.9–5.9) | 0.02 |
| tT, nmol/L | 2.6 (1.4–4.9) | 9.99 (6.7–13.4) | <0.0001 |
| Hypogonadism (tT < 9.2 nmol/L) | 115 (95) | 66 (55) | <0.0001 |
| Hypogonadism (tT < 12 nmol/L) | 117 (97) | 79 (66) | <0.0001 |
| E2, pg/ml | 34.5 (19.7–42.2) | 30.4 (26.5–35.1) | 0.001 |
Note: Data are n (%) or median (IQR). Groups were as follows: Group 1: patients in good clinical conditions and discharged home from the emergency department; Group 2: patients who have been admitted in the internal medicine unit until possible discharge at home; and Group 3: patients invasively ventilated in the intensive care unit, and subsequently successfully extubated and discharged either to the internal medicine unit or at home.
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; CCI, Charlson Comorbidity Index; E2, 17β‐estradiol; FiO2, fractional concentration of oxygen in inspired air; FSH, follicle‐stimulating hormone; IL‐6, interleukin‐6; LH, luteinizing hormone; NLR, neutrophil/lymphocytes ratio; PaO2, partial pressure of oxygen in arterial blood; RALE, radiographic assessment of lung edema; tT, total testosterone.
p‐Value according to the Wilcoxon signed‐rank test and chi‐square test, as indicated.
FIGURE 1.
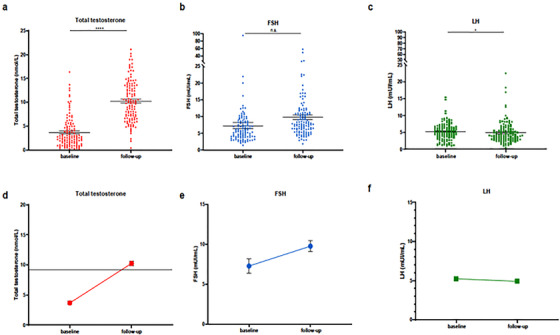
Scatter plot, vertical. Sex‐related hormonal analyses at baseline compared to 6‐month follow‐up in COVID‐19 patients. (A and D) Total testosterone. (B and E) Follicule‐stimulating hormone (FSH). (C and F) Luteinizing hormone (LH). *p = 0.01; ****p < 0.0001
Of 121, 103 (85%) patients received anti‐virals, 53 (44%) bDMARDs and 22 (18%) systemic corticosteroid treatment, respectively.
Multivariable logistic regression analyses revealed that CCI (OR 0.36; p = 0.03 [0.14, 0.89]) and IL‐6 levels (OR 1.43; p = 0.04 [1.00, 2.05]) at hospital admittance were independently associated with tT levels at 7‐month follow‐up, after adjusting for age and BMI (Table 2).
TABLE 2.
Logistic regression models predicting increase in total testosterone levels at 7‐month follow‐up
| Increased testosterone levelsMVAOR; p‐value [95% CI] | |
|---|---|
| Age | 1.00; 1 [0.89, 1.12] |
| BMI | 1.13; 0.4 [086, 1.48] |
| CCI | 0.36; 0.03 [0.14, 0.89] |
| IL‐6 | 1.43; 0.04 [1.00, 2.05] |
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; IL‐6, interleukin‐6; MVA, multivariable analyses; OR, odds ratio.
At multivariable linear regression analyses, the higher the CCI score at admittance, the lower the delta of increase of tT from baseline to 7‐month follow‐up, after adjusting for age, BMI, and IL‐6 levels (Table 3).
TABLE 3.
Linear regression models predicting the magnitude of total testosterone level increases in the whole cohort (n = 121)
| (A) | Multivariable model B; p‐value [95% CI] |
|---|---|
| Age | −0.01; 0.8 [−0.10, 0.08] |
| BMI | −0.20; 0.8 [−0.42, 0.03] |
| CCI | −1.05; 0.01 [−1.84, −0.25] |
| IL‐6 | 0.00; 0.4 [−0.00, 0.01] |
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; IL‐6, interleukin‐6.
Figure 2 graphically depicts the probability (%) of tT levels recovery at 7‐month follow‐up according to CCI at hospital admittance.
FIGURE 2.
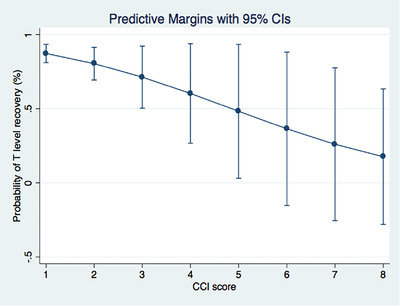
Interaction test assessing the probability (%) that circulating total testosterone levels could increase according to Charlson Comorbidity Index (CCI) score at hospital admittance
As a sensitivity analysis, we performed logistic and linear regression analyses testing the influence of treatment (any type) received during hospitalization and the probability of tT increase at follow‐up; therapy with either systemic corticosteroid, bDMARDs, antivirals or bDMARDs + antivirals was not associated with the probability of tT increase or the magnitude of tT change at follow‐up (Table 4).
TABLE 4.
(A) Logistic regression testing association between medical treatments and increased testosterone at follow‐up; and (B) linear regression testing association between medical treatments and magnitude of testosterone increase at follow‐up
| A) | OR | 95% CI | p‐Value |
|---|---|---|---|
| Corticosteroids | 0.30 | 0.01, 6.48 | 0.4 |
| Antivirals | 3.19 | 0.81, 12.53 | 0.10 |
| bDMARDs | 2.52 | 0.73, 8.73 | 0.15 |
| bDMARDs + corticosteroids | 3.34 | 0.10, 109.90 | 0.5 |
| B) | Coefficient | 95% CI | p‐Value |
|---|---|---|---|
| Corticosteroids | −3.19 | −9.93, 3.56 | 0.4 |
| Antivirals | 1.59 | −1.00, 4.17 | 0.2 |
| bDMARDs | 0.88 | −0.80, 2.56 | 0.3 |
| bDMARDs + corticosteroids | 3.42 | −3.70, 10.54 | 0.3 |
Abbreviations: bDMARDs, biological disease‐modifying anti‐rheumatic drugs; OR, odds ratio.
4. DISCUSSION
Current findings show that tT levels significantly increased at 7‐month follow‐up in men who recovered from COVID‐19. Overall, tT increased in almost 90% of patients, but further decreased in roughly 10% of the entire cohort as compared with values at hospital admittance. Still, 55% of men depicted tT concentrations suggestive for hypogonadism, 20 despite patients having recovered from COVID‐19 and the time elapsed. Of clinical relevance, the higher the burden of comorbid conditions at presentation, the lower the probability of tT levels recovery throughout the follow‐up. Interestingly enough, treatments (any, including antiviral therapy, systemic corticosteroids or bDMARDs) used for curing SARS‐CoV‐2 infection did not impact tT levels.
Our observations further stressed the clinical importance of recent findings showing that SARS‐CoV‐2 infection status was independently associated with tT levels suggestive for hypogonadism already at hospital admittance, 9 , 10 , 11 , 12 and that lower tT levels were associated with poorer clinical outcomes (i.e., greatest need of ICU and highest risk of death) in men with COVID‐19. 9 , 10 , 11 , 12 , 13
Here, in a relatively large group of patients from the original cohort of men who had been admitted at a single academic hospital because of symptomatic SARS‐CoV‐2 infection between February 29 and May 2, 2020, 12 we had the option to continue and describe the terrible impact of COVID‐19 in terms of overall health of most of infected males, and in particular on the testicular endocrine function, which seemed to be particularly affected over time. Indeed, according to our original results, we had postulated five hypotheses in order to attempt and explain the potential importance of low T levels in terms of sex differences in COVID‐19 severity. 12
First, we had considered that low tT levels may simply be a marker of illness severity, thus recapitulating what has already been reported for viral infections. 2 , 28 , 29 Current findings may partially support this hypothesis, as the progressive—although slow and only partial—recovery of circulating T levels would seem to go in the direction of a tT also acting as a marker of severity of an overall severely compromised health condition. Second hypothesis was that androgens per se are poorly protective over the immune response in males as compared with the actual ability of estrogens (along with progesterone) to provide adequate protection to females, even stimulating the humoral response to viral infections 30 , 31 , 32 , 33 ; as a consequence, male T production undergoes a dramatic impact because of SARS‐CoV‐2 infection, but T levels per se could not elicit an effective counteracting response to the inflammatory and immunological outcome resulting from viral infection. 34 , 35 The third hypothesis we postulated had considered a background condition of chronic low T levels that could facilitate overall greater incidence, higher severity, and greater probability of fatal events in men compared to women. 34 Up to now, no data could either corroborate or rebut the idea that baseline preinfection tT levels were responsible for worse clinical outcomes in men with COVID‐19. Indeed, even recently, Dhindsa et al. 13 confirmed previous findings that in their cohort of male patients with severe COVID‐19 (i.e., ICU admission [OR, 0.15; p = 0.007] and ventilator use [OR, 0.29; p = 0.01]), baseline tT concentrations were lower compared to those of men with milder disease, where T levels were anyhow still lower than the reference range. Likewise, the greater the inflammatory state, the lower the circulating T levels. Overall, the authors had to admit that their study could not determine whether T was a marker or a mediator associated with COVID‐19 severity, as they did not know the pre‐illness serum T concentrations in their studied patients. 13 Conversely, while they supported the concept that T levels had already declined dramatically compared with their baseline concentrations, alternatively Dhindsa and colleagues could not exclude that those men who had developed severe COVID‐19 had T concentrations that were already chronically less than the reference range, even prior to their illness. Indeed, they discussed that men with chronically low T have a number of physical characteristics (e.g., decreased muscle mass and strength), which could have a role in contributing to the clearly observed decreased lung capacity and ventilator dependence. 13 , 36 Of paramount clinical importance, these authors have postulated the concept that long‐term T treatment has even potential to prevent respiratory compromise in illnesses and acute infections that target the respiratory tract. 13 , 37 Further studies should be addressed to answer this question.
As a fourth hypothesis, the interaction between SARS‐CoV‐2 and androgen‐regulated proteins to invade host cells has been discussed, including viral receptor angiotensin‐converting enzyme 2 (ACE2) for viral entry and cell surface protease transmembrane serine protease 2 (TMPRSS2) for viral S protein priming. 6 Here, we further confirmed this interaction, and the clinical importance that the impact of SARS‐CoV‐2 infection in the testes eventually promotes. Indeed, data demonstrated that SARS‐CoV‐2 infection is associated with sparse intratubular cells with swollen and vacuolated Sertoli cells along with a decreased number of Leydig cells in the testicular interstitium. 6 , 7 Thus, focusing on the possible harm to testicular steroidogenesis, SARS‐CoV‐2 per se was demonstrated to reduce Leydig cell population (as also indirectly depicted because of the dramatic decrease in terms of insulin‐like factor 3, the most abundantly expressed proteins in Leydig cells) and Leydig cell functions, eventually lowering T production. 5 , 6 , 7 Our findings further corroborate this pathophysiology observations.
Lastly, we had hypothesized that the virus–host interaction mechanism in males is different, and more acutely linked to SARS‐CoV‐2 infection per se, with tT levels suggestive for hypogonadism observed in almost 90% of patients with COVID‐19 already at hospital admission. 12 According to our speculation, circulating tT levels could have been negatively affected also by acute illness, 29 including acute viral infections. We found that SARS‐CoV‐2 infection status per se emerged to be independently associated with both lower tT levels and hypogonadism, further strengthening the speculation on the causal role of the infection in the androgenic collapse.
First strength of current observations is that this study is the first to comprehensively follow a group of men who had been considered over time after being included in the original case–control study 12 ; here, we report novel finding that tT slowly increases throughout the follow‐up timeframe in men who recovered from COVID‐19, but still median concentrations are only slightly above the threshold suggestive for hypogonadism (i.e., tT threshold suggested by the Endocrine Society is <9.2 nmol/L 20 ) even at 7‐month follow‐up. Second, we observed that almost 10% of the cohort of men who recovered from the disease suffered a further decrease in tT values. Along with the persistence of circulating levels suggestive for hypogonadism in 55% of patients, this observation could further support the concept that tT is clinically relevant in patients with COVID‐19, and future prospective studies should probably also consider to apply T therapy in those cases that present with baseline parameters suggestive of worse outcomes and more difficult tT recovery (i.e., greater CCI scores) already at hospital admittance. 38
Therefore, once again our findings outline the importance of assessing serum hormones in men presenting for COVID‐19; on the one hand, tT levels emerged as a potential early sentinel marker for subsequent worse outcomes during hospitalization, and on the other, as they deserve to be continuously assessed over time, being this true especially in men with greater burden of comorbid conditions at presentation that may suffer from slower and more difficult recovery of overall health.
Our study is not devoid of limitations. First, this was a single‐center‐based observational study that evaluated the recovery of tT concentrations in men after COVID‐19, thus raising the possibility of selection biases and limiting the generalizability of the findings. Therefore, although we could not make final interpretations of causality, such a specific tT levels behavior may support the hypothesis of a direct impact of SARS‐CoV‐2 infection in terms of testicular steroidogenesis. Second, as previously detailed, 12 this study is part of an institutional protocol, which lacks a pre‐infection hormonal milieu assessment in men with COVID‐19. Third, we did not assess free or bioavailable T concentrations. Finally, we are aware that longer follow‐up assessments of sex steroids in men who recovered from COVID‐19 are needed to even better investigate causality correlations.
5. CONCLUSIONS
Although total testosterone levels increase over time after COVID‐19, our findings indicate that more than 50% of men who recovered from the disease still showed low circulating testosterone levels suggestive for a condition of hypogonadism at 7‐month follow‐up. In as many as 10% of cases, testosterone levels even decreased throughout the follow‐up timeframe. Of clinical relevance, the higher the burden of comorbid conditions at presentation, the lower the probability of testosterone levels recovery over time.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
AS designed and led the study and wrote the report. MP, LB, CC, AL, AMF, GAR, CT, ML, LS, GC, AC, AZ, MT, PR‐Q, and FC took care of patients and acquired data. AS, PC, SG, IR, LD, FC, and FM analyzed the data and drafted the report.
ACKNOWLEDGMENTS
Unrestricted, liberal fund for research was received for this study from Gruppo Prada, Milan, Italy. All commercial LIAISON kits for the assessment of the entire hormonal milieu were provided free of charge by DiaSorin SpA, Saluggia, Italy.
Salonia A, Pontillo M, Capogrosso P, et al. Testosterone in males with COVID‐19: A 7‐month cohort study. Andrology. 2022;10:34–41. 10.1111/andr.13097
REFERENCES
- 1. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26:1017‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singhal S, Kumar P, Singh S, Saha S, Dey AB. Clinical features and outcomes of COVID‐19 in older adults: a systematic review and meta‐analysis. BMC Geriatr. 2021;21:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng WH, Tipih T, Makoah NA, et al. Comorbidities in SARS‐CoV‐2 patients: a systematic review and meta‐analysis. mBio. 2021;12:e03647‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corona G, Pizzocaro A, Vena W, et al. Diabetes is most important cause for mortality in COVID‐19 hospitalized patients: systematic review and meta‐analysis. Rev Endocr Metab Disord. 2021;22:275‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roychoudhury S, Das A, Jha NK, et al. Viral pathogenesis of SARS‐CoV‐2 infection and male reproductive health. Open Biol. 2021;11:200347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nie X, Qian L, Sun R, et al. Multi‐organ proteomic landscape of COVID‐19 autopsies. Cell. 2021;184:775‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang M, Chen S, Huang Bo, et al. Pathological findings in the testes of COVID‐19 patients: clinical implications. Eur Urol Focus. 2020;6:1124‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salonia A, Corona G, Giwercman A, et al. SARS‐CoV‐2, testosterone and frailty in males (PROTEGGIMI): a multidimensional research project. Andrology. 2021;9:19‐22. [DOI] [PubMed] [Google Scholar]
- 9. Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients. Andrology. 2021;9:88‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Çayan S, Uğuz M, Saylam B, Akbay E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID‐19) in SARS‐CoV‐2 infected male patients: a cohort study. Aging Male. 2020;23:1493‐1503. [DOI] [PubMed] [Google Scholar]
- 11. Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A. SARS‐CoV‐2 pneumonia affects male reproductive hormone levels: a prospective, cohort study. J Sex Med. 2021;18:256‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salonia A, Pontillo M, Capogrosso P, et al. Severely low testosterone in males with COVID‐19: a case‐control study. Andrology. 2021;9(4):1043‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhindsa S, Zhang N, Mcphaul MJ, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID‐19. JAMA Netw Open. 2021;4:e2111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rowland SP, O'brien Bergin E. Screening for low testosterone is needed for early identification and treatment of men at high risk of mortality from COVID‐19. Crit Care. 2020;24:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giagulli VA, Guastamacchia E, Magrone T, et al. Worse progression of COVID‐19 in men: is testosterone a key factor? Andrology. 2021;9:53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Auerbach JM, Khera M. Testosterone's role in COVID‐19. J Sex Med. 2021;18:843‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tur‐Kaspa I, Tur‐Kaspa T, Hildebrand G, Cohen D. COVID‐19 may affect male fertility but is not sexually transmitted: a systematic review. F S Rev. 2021;2:140‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruan Y, Hu B, Liu Z, et al. No detection of SARS‐CoV‐2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID‐19 male patients: a perspective and urogenital evaluation. Andrology. 2021;9:99‐106. [DOI] [PubMed] [Google Scholar]
- 19. Xu H, Wang Z, Feng C, et al. Effects of SARS‐CoV‐2 infection on male sex‐related hormones in recovering patients. Andrology. 2021;9:107‐114. [DOI] [PubMed] [Google Scholar]
- 20. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536‐2559. [DOI] [PubMed] [Google Scholar]
- 21. Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID‐19 pandemic emergency. Crit Care Resusc. 2020;22:91‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 23. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562‐572. [DOI] [PubMed] [Google Scholar]
- 24. Warren MA, Zhao Z, Koyama T, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73:840‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180:1081‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cavalli G, Larcher A, Tomelleri A, et al. Interleukin‐1 and interleukin‐6 inhibition compared with standard management in patients with COVID‐19 and hyperinflammation: a cohort study. Lancet Rheumatol. 2021;3:e253‐e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salonia A, Bettocchi C, Boeri L, et al, European Association of Urology guidelines on sexual and reproductive health‐2021 update: male sexual dysfunction. Eur Urol. 2021;80(3):333‐357. 10.1016/j.eururo.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 28. Pradhan A, Olsson P‐E. Sex differences in severity and mortality from COVID‐19: are males more vulnerable? Biol Sex Differ. 2020;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salonia A, Rastrelli G, Hackett G, et al. Paediatric and adult‐onset male hypogonadism. Nat Rev Dis Primers. 2019;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scully EP. Hidden in plain sight: sex and gender in global pandemics. Curr Opin HIV AIDS. 2021;16:48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ciarambino T, Para O, Giordano M. Immune system and COVID‐19 by sex differences and age. Womens Health (Lond). 2021;17:17455065211022262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vadakedath S, Kandi V, Mohapatra RK, et al. Immunological aspects and gender bias during respiratory viral infections including novel coronavirus disease‐19 (COVID‐19): a scoping review. J Med Virol. 2021;93:5295‐5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reyes‐García J, Montaño LM, Carbajal‐García A, Wang YX. Sex hormones and lung inflammation. Adv Exp Med Biol. 2021;1304:259‐321. [DOI] [PubMed] [Google Scholar]
- 34. Di Florio DN, Sin J, Coronado MJ, Atwal PS, Fairweather D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020;31:101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cutolo M, Smith V, Paolino S. Understanding immune effects of oestrogens to explain the reduced morbidity and mortality in female versus male COVID‐19 patients. Comparisons with autoimmunity and vaccination. Clin Exp Rheumatol. 2020;38:383‐386. [PubMed] [Google Scholar]
- 36. Mohan SS, Knuiman MW, Divitini ML, et al. Higher serum testosterone and dihydrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community‐dwelling men. Clin Endocrinol (Oxf). 2015;83:268‐276. [DOI] [PubMed] [Google Scholar]
- 37. Salciccia S, Del Giudice F, Eisenberg ML, et al. Testosterone target therapy: focus on immune response, controversies and clinical implications in patients with COVID‐19 infection. Ther Adv Endocrinol Metab. 2021;12:20420188211010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coelingh Bennink HJT, Foidart J‐M, Debruyne FMJ. Treatment of serious COVID‐19 with testosterone suppression and high‐dose estrogen therapy. Eur Urol. 2021. 10.1016/j.eururo.2021.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]


