Abstract
Several individuals have developed delayed localized cutaneous vaccine reactions to the two novel mRNA Covid‐19 vaccines. Clinical and histopathologic results of this case series study confirm that the localized injection‐site reactions to the mRNA COVID‐19 vaccines are delayed hypersensitivity reactions that, unlike immediate hypersensitivity reactions, are not a contraindication to vaccination.
Dear Editor,
Delayed local injection site reactions were reported in the clinical trials of the two novel mRNA COVID‐19 vaccines (Pfizer/BioNTech and Moderna). 1 , 2 With the increase in vaccination rates, awareness of associated adverse events (AEs) is needed. We report on a study of delayed localized hypersensitivity reactions to mRNA SARS‐CoV‐2 vaccines with histopathological confirmation.
The study was approved by the institutional ethics committee of Aristotle University (Thessaloniki, Greece). Informed consent for biopsy and for publication of case details and photographs was obtained from participants.
This was a retrospective study conducted at the First Dermatology Department of Aristotle University between 1 January and 20 June 2021. Patients’ demographics, vaccine information (manufacturer and first or second dose administration), medical history, allergies, COVID‐19 infection, prior history of vaccine reactions, time of onset and duration of injection‐site reaction (ISR) were recorded. Photographs were obtained for six patients during the study course and histopathological examination was performed for two patients. Patients who experienced a cutaneous reaction to the first vaccine dose were followed up until 1 month after the second dose.
Overall, 84 patients referred to the emergency department reporting ISRs after their first and/or second vaccine dose. All 84 patients were white with a mean age of 57 years (range 27–86 years), and the majority (n = 82; 97.6%) were women. Hypertension (n = 25; 29.8%), dyslipidaemia (n = 12; 14.3%) and diabetes mellitus (n = 8; 9.5%) were the most common comorbidities reported. Most participants (n = 80; 95.2%) did not have any history of cutaneous disease; the four who did had eczema (n = 3; 3.6%) and urticaria (n = 1; 1.2%).
All patients had received the Moderna vaccine and none had received the Pfizer/BioNTech vaccine. Most patients (n = 57; 68%) had not been previously infected with SARS‐CoV‐2 and the majority (n = 76; 90.5%) reported no relevant cutaneous reaction to any other vaccine type. Local ISRs preceded delayed large ISRs in 51 of the 84 patients (60.7%).
Delayed large ISRs occurred in 82 of the 84 patients after their first dose, occurring in 79 of the 84 patients (94%) approximately 9 days after the first dose (range 7–13 days). The plaques were mainly oedematous or indurated and homogeneous or annular, and subsided after a mean of 3 days (range 2–6 days) after starting treatment with topical corticosteroids and oral antihistaminic or anti‐inflammatory medication (Fig. 1a).
Figure 1.
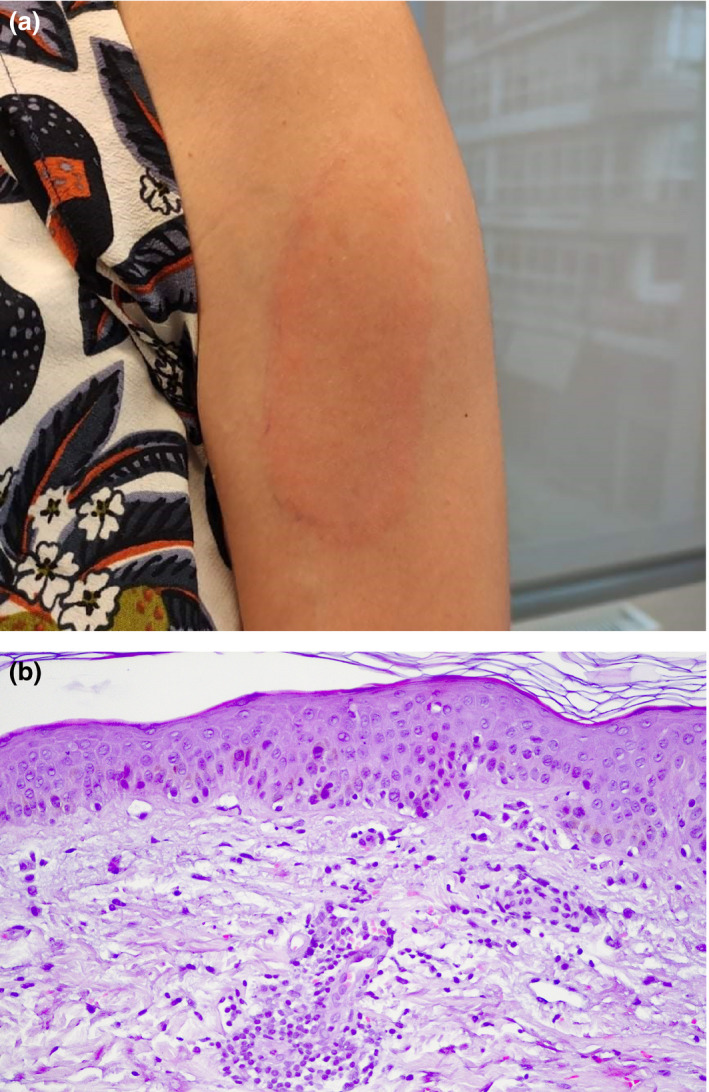
(a) Indurated erythematous patch at the injection site, 10 days after the first dose of the Moderna vaccine; (b) mild to moderate perivascular infiltrate predominantly of lymphocytes, mild dermal focal oedema with red cell extravasation and rare interstitial eosinophils (haematoxylin and eosin, original magnification × 100).
In addition, 37 of the 84 patients (44%) presented delayed large ISRs after their second vaccine dose; 2 of these reported having had no reaction to the first dose. ISRs after the second dose occurred sooner in the 35 patients (41.7%) who had experienced a relevant reaction to the first dose, with a mean onset of 2 days (range 1–6 days) after vaccine administration and with a similar clinical presentation. Of these, 7 (20%) experienced a more pronounced reaction with the second dose.
Histological findings were consistent with delayed localized hypersensitivity reaction, demonstrating mild to moderate perivascular lymphocytic infiltrate, mild dermal focal oedema with red cell extravasation and rare interstitial eosinophils (Fig. 1b).
Limited data on delayed hypersensitivity reactions have been published, primarily after administration of the Moderna vaccine. 3 , 4 , 5 In our study, although the number of Pfizer vaccines allocated to our region was seven times that of the number of Moderna vaccines, relevant reactions presented only in individuals who received the Moderna vaccination. Therefore, it is possible that delayed localized reactions may have been underestimated in the Moderna clinical trial, as they were actively monitored for only 7 days after vaccination.
In conclusion, ISRs can occur after administration of mRNA vaccines, and may be delayed. However, AEs to mRNA vaccines are minor and self‐limiting, and should not discourage vaccination.
Conflict of interest: the authors declare that they have no conflicts of interest.
IP and KB contributed equally to this manuscript and should be considered joint first authors.
References
- 1. Baden LR, El Sahly HM, Essink B et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Eng J Med 2021; 384: 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Eng J Med 2020; 383: 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID‐19 vaccine: a case series. JAMA Dermatol 2021; 157: 716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blumenthal KG, Freeman EE, Saff RR et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Eng J Med 2021; 384: 1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


