Abstract
Background
We sought to evaluate the association between vitamin D deficiency and the severity of coronavirus disease 2019 (COVID‐19) infection.
Methods
Multiple databases from 1 January 2019 to 3 December 2020 were searched for observational studies evaluating the association between vitamin D deficiency and severity of COVID‐19 infection. Independent reviewers selected studies and extracted data for the review. The main outcomes of interest were mortality, hospital admission, length of hospital stay and intensive care unit admission.
Results
Seventeen observational studies with 2756 patients were included in the analyses. Vitamin D deficiency was associated with significantly higher mortality (odds ratio [OR]: 2.47, 95% confidence interval [CI]: 1.50–4.05; 12 studies; hazard ratio [HR]: 4.11, 95% CI: 2.40–7.04; 3 studies), higher rates of hospital admissions (OR: 2.18, 95% CI: 1.48–3.21; 3 studies) and longer hospital stays (0.52 days; 95% CI: 0.25–0.80; 2 studies) as compared to nonvitamin D deficient status. Subgroup analyses based on different cut‐offs for defining vitamin D deficiency, study geographic locations and latitude also showed similar trends.
Conclusions
Vitamin D deficiency is associated with greater severity of COVID‐19 infection. Further studies are warranted to determine if vitamin D supplementation can decrease the severity of COVID‐19.
Keywords: COVID‐19, hospital admission, mortality, vitamin D
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) was initially identified in Wuhan, China in late 2019 and subsequently became a global pandemic. 1 The illness varies widely in severity with affected individuals being asymptomatic or developing mild, moderate or severe illness, which can be fatal. The case fatality rates have been different between geographic regions and countries. 2 The specific risk factors for a severe illness identified so far include older age, male sex, obesity, cardiovascular disease, chronic lung disease, diabetes mellitus and cancer. 1 , 3 Additionally, race is an important factor as several studies have shown that Black, Hispanic and Asian individuals accounted for a disproportionately higher number of hospitalisations and deaths due to COVID‐19 in the United Kingdom and the United States. 4 Many of the abovementioned risk factors are not modifiable, hence it is important to identify modifiable factors that might contribute to COVID‐19 infection severity. Diet and nutrition have important implications in immune functioning and infection risk, especially vitamin D level. 5 Vitamin D may be one potentially modifiable risk factor postulated to modulate COVID‐19 infection severity. 6
Vitamin D, in addition to its role in skeletal health, may modulate immune regulation. 7 The vitamin D receptor is present in a variety of cells involved in immune regulation, including monocytes, activated T and B lymphocytes and dendritic cells. Vitamin D has been shown to impact cytokine synthesis, lymphocyte proliferation, antibody production, monocyte activation and cell‐mediated immunity. 7 A systematic review and meta‐analysis of 25 randomised, double‐blind placebo‐controlled trials of supplementation with vitamin D3 or vitamin D2 of any duration found that vitamin D supplementation was beneficial in reducing the risk of acute respiratory tract infection. 8 There is a conflicting opinion on the role of vitamin D in impacting the risk of COVID‐19, with some studies suggesting that vitamin D deficiency increases the risk of COVID‐19 infection 9 , 10 while others did not find a significant association. 11 Similarly, there is conflicting evidence on whether vitamin D deficiency is associated with greater severity of COVID‐19 infection. 12 , 13 , 14 , 15 , 16 , 17
To further investigate this relationship and obtain greater clarity on this issue, we conducted this systematic review and meta‐analysis to evaluate the association between vitamin D deficiency and the severity of COVID‐19 infection.
2. METHODS
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines were followed to report this systematic review and meta‐analysis. 18
2.1. Data sources and searches
We conducted a comprehensive database search, including MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews and Scopus, from 1 January 2019 to 3 December 2020. Reference mining of existing systematic reviews/meta‐analyses, preprint medical literature from medRxiv. org, and relevant primary studies were conducted to identify additional studies. An experienced medical librarian, with input from the study investigators, developed the search strategy (Figure 1) and conducted the literature search.
Figure 1.
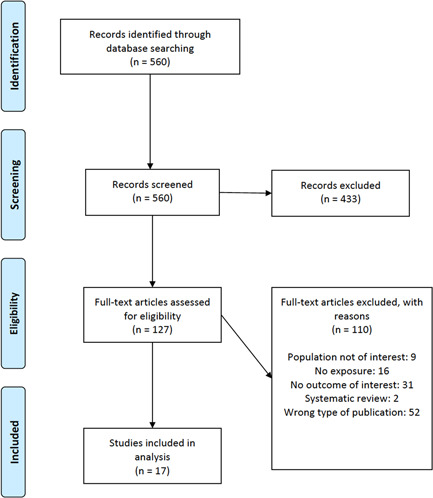
Flowchart outlining the protocol adopted in this systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines [Color figure can be viewed at wileyonlinelibrary.com]
2.2. Study selection
Eligible studies (1) included patients with laboratory‐confirmed COVID‐19 diagnosis (polymerase chain reaction [PCR]); (2) documented evaluation of total serum 25‐hydroxyvitamin D (25(OH)D) level within 3 months of COVID‐19 diagnosis; (3) availability of a comparison group: comparing vitamin D deficiency to nondeficiency (includes normal vitamin D status and insufficiency); and (4) reported outcomes of interest (mortality, hospital admission, length of hospital stay and intensive care unit [ICU] admission). As the 25(OH)D cut‐offs for defining vitamin D deficiency are controversial, 19 we used the 25(OH)D cut‐offs defined by each study. We included observational studies without restrictions on publication language, study location, or patient population. Studies were excluded if they evaluated vitamin D supplements, vitamin D insufficiency only, or suspected COVID‐19 cases (including those evaluated by radiology only, without confirmatory PCR or antigen‐based testing). We also excluded in vitro studies, and studies without original data (e.g., opinion, editorial and narrative review).
Pairs of reviewers, working independently, screened titles and abstracts of all references. Studies included by either reviewer were included for full‐text screening. Pairs of independent reviewers screened the full text of the eligible studies. Conflicts between the reviewers were resolved by a third investigator.
2.3. Data extraction and quality assessment
A pilot‐tested standardised data extraction form was developed to extract study characteristics and outcomes of interest. Reviewers worked independently to extract study details. A second reviewer reviewed data and resolved inconsistencies.
We evaluated the risk of bias of the included studies using the modified Newcastle–Ottawa Scale, in terms of representativeness of study cohort, ascertainment of exposure, comparability between groups, outcome data source and assessment of outcome (Appendix Table S1). 20
2.4. Data synthesis and analysis
Odds ratio (OR) or hazard ratio (HR) for binary outcomes (mortality, hospital admission, ICU admission) and mean difference for the continuous outcome (length of hospital stay) were extracted or calculated. The DerSimonian–Laird random‐effects model with Hartung–Knapp–Sidik–Jonkman variance correction was used to combine studies if the number of studies included in the analysis was larger than 3. 21 The fixed‐effect model based on the Mantel and Haenszel method was adopted when the number of studies was 3 or less. Treatment arm continuity corrections were used to adjust double‐zero‐event studies (i.e., 0 event in both groups). 22 Heterogeneity across studies was measured using the I 2 indicator, in which I 2 > 50% suggests substantial heterogeneity. To further explore heterogeneity, we conducted prespecified subgroup analyses based on serum 25(OH)D cut‐off levels used to define vitamin D deficiency (12, 20 and 25 ng/ml) and geographic regions (Europe, Asia, Middle East and North America). The latitudes of the study areas were evaluated, which were identified as the latitudinal coordinates of the geographic centroid of the study areas extracted from Google Map and categorised based on 5° increments from the equator. Publication bias was evaluated quantitatively using the asymmetry test of funnel plots and Egger's regression test when the number of studies included in a meta‐analysis was larger than 10. Two‐sided p‐value less than .05 was deemed to be statistically significant. All statistical analyses were conducted using Stata version 16.1 (Stata LLP Corp).
3. RESULTS
Our literature search identified 560 citations. Seventeen observational studies with 2756 eligible patients who met our inclusion criteria were included in the analyses (Figure 1 and Table 1). 12 , 13 , 14 , 15 , 16 , 17 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 The studies included only adult patients; and the majority (70.6%) evaluated only hospitalised COVID‐19 cases. Vitamin D deficiency was defined as total 25(OH)D) level less than 12 ng/ml (seven studies), less than 20 ng/ml (eight studies) and less than 25 ng/ml (one study). Mendy et al. 16 did not specify the 25(OH)D levels used to classify vitamin D deficiency and instead based the diagnosis on the International Classifications of Diseases 10 code for deficiency. Nine studies were conducted in Europe, one in the United States, five in Asia and two in the Middle East. Details of study characteristics can be found in Table 1.
Table 1.
Study characteristics
| Author, year (country) | Study period | Methods used for confirmation of COVID‐19 | Comparisons | Patients characteristics |
|---|---|---|---|---|
| De Smet, 2020 1 (Belgium) | 1 March 2020 to 7 April 2020 | RT‐PCR | ≥20 ng/ml | 109 Patients, aged 73 years (IQR: 53–81), 53.2% female, 100% Caucasian, 100% inpatients |
| <20 ng/mL | 109 Patients, aged 67 years (IQR: 52–79), 33.0% female, 100% inpatients | |||
| Carpagnano, 2020 2 (Italy) | 11 March 2020 to 30 April 2020 | RT‐PCR | All groups | 42 Patients, aged 65 ± 13 years, 28.6% female, BMI: 28.5 ± 5, 26.2% DM, 100% inpatients |
| Jain, 2020 3 (India) | 5 June 2020 to 17 July 2020 | RT‐PCR | All groups | 154 Patients, aged 46.1 ± 7.6 years, 44.8% female, BMI: 27.1 ± 4.6, 4.56% obese, 100% inpatients |
| Hars, 2020 4 (Switzerland) | 13 March 2020 to 14 April 2020 | RT‐PCR | All groups | 160 Patients, aged 85.9 ± 6.6 years, 59.4% female, 100% Caucasian, 100% inpatients |
| Cereda, 2020 5 (Italy) | March 2020 to April 2020 | RT‐PCR | ≥20 ng/ml | 30 Patients, aged 77.5 years (IQR: 65–86), 56.7% female, 100% Caucasian, BMI: 24.4 (IQR: 20.8–26.2), 36.7% DM, 100% inpatients |
| <20 ng/ml | 99 Patients, aged 77 years (IQR: 64–85), 42.4% female, 100% Caucasian, BMI: 24.7 (IQR: 22.9–27.9), 28.9% type 2 DM, 100% inpatients | |||
| Luo, 2020 6 (China) | Technology, 7 February 2020 to 21 March 2020 | RT‐PCR | All groups | 335 Patients, aged 56 years (IQR: 43.0–64.0), 55.8% female, 100% Chinese, BMI: 23.5 ± 3.1, 100% inpatients |
| Hernandez, 2020 7 (Spain) | 10 March 2020 to 31 March 2020 | RT‐PCR | ≥20 ng/ml | 35 Patients, aged 58 years (IQR: 45–69), 51.4% female, BMI: 29.8 ± 4.1, 17.1% DM, 100% inpatients |
| <20 ng/ml | 162 Patients, aged 62 years (IQR: 48–70.3), 34.6% female, BMI 29 ± 4.9, 17.3% DM, 100% inpatients | |||
| Abrishami, 2020 8 (Iran) | 28 February 2020 to 19 April 2020 | RT‐PCR | All groups | 73 Patients, aged 55.2 ± 15.0 years, 36.0% female, 15.1% DM, 100% inpatients |
| Ye, 2020 9 (China) | 16 February 2020 to 16 March 2020 | PCR | All groups | 62 Patients, aged 43 years (IQR: 32–59), 63.0% female, 100% Chinese, 8.3% DM, 100% inpatients |
| Baktash, 2020 10 (United Kingdom) | 1 March 2020 to 30 April 2020 | RT‐PCR | ≤12 ng/ml | 39 Patients, aged 79.4 ± 9.5 years, 38.5% female, 74.4% Caucasian, BMI: 25 (IQR: 23–32), 43.6% DM, 100% inpatients |
| >12 ng/ml | 31 Patients, aged 81.2 ± 7.2 years, 41.9% female, 67.7% Caucasian, BMI: 24 (IQR: 20–27), 29.0% DM, 100% inpatients | |||
| Mendy, 2020 11 (United States) | 13 March 2020 to 31 May 2020 | PCR | All groups | 689 Patients, aged 49.5 ± 34.1 years, 47.0% female, 29.2% Caucasian, 25.5% Black, 32.5% Hispanic, 18.6% obese, 24.7% DM |
| Im, 2020 12 (Korea) | February 2020 to June 2020 | RT‐PCR | ≤20 ng/ml | 38 Patients, 57.9% female, 100% inpatients |
| >20 ng/dl | 12 Patients, 58.3% female, 100% inpatients | |||
| Mardani, 2020 13 (Iran) | March 2020 | RT‐PCR | All groups | 63 Patients, aged 43.3 ± 14.5 years, 44.4% female |
| Macaya, 2020 14 (Spain) | 5 March 2020 to 31 March 2020 | RT‐PCR | All groups | 80 Patients, aged 67.7 years, 56.3% female, BMI: 27, 28.8% obese, 40.0% DM |
| Pizzini, 2020 15 (Austria) | From 29 April 2020 | RT‐PCR | All groups | 109 Patients, aged 58 ± 14 years, 40.0% female, BMI: 27 ± 14, 18.0% DM, 80% inpatients |
| Radujkovic, 2020 16 (Germany) | 18 March 2020 to 18 June 2020 | RT‐PCR | <12 ng/ml | 41 Patients, aged 66 years (IQR: 53–78), 43.9% female, 19.5% DM, 100% inpatients |
| ≥12 ng/ml | 144 Patients, aged 58 years (IQR: 47–67), 50% female, 19.4% DM, 100% inpatients | |||
| Anjum, 2020 17 (Pakistan) | March 2020 to July 2020 | RT‐PCR | All groups | 140 Patients, aged 42.5 ± 14.7 years, 41.4% female, BMI: 23.5 ± 3.6 |
Abbrevations: COVID‐19, coronavirus disease 2019; DM, diabetes mellitus; IQR, interquartile range; RT‐PCR, real‐time reverse transcription‐polymerase chain reaction.
The overall risk of bias of the included studies was high due to a lack of control of confounding variables (Appendix Table S1). We did not find potential publication bias for mortality, though we were unable to statistically evaluate publication bias for the other outcomes.
Of the 17 included studies, 14 compared vitamin D deficiency with normal vitamin D status, while the remaining 3 studies quantified vitamin D status qualitatively, which was categorised as deficiency, insufficiency and normal. For these three studies, outcomes in those with vitamin D deficiency were compared with the normal and insufficiency group combined. Vitamin D deficiency was associated with significantly higher mortality (OR: 2.47, 95% CI: 1.50–4.05; I 2 = 30.5%; 12 studies; HR: 4.11, 95% CI: 2.40–7.04; I 2 = 11.6%; 3 studies), higher rates of hospital admission (OR: 2.18, 95% CI: 1.48–3.21; I 2 = 0%, 3 studies) and longer hospital stays (+0.52 days; 95% CI: 0.25–0.80; I 2 = 89.6%; 2 studies) (Table 1 and Figure 2). We found no significant difference in ICU admissions (Table 2).
Figure 2.
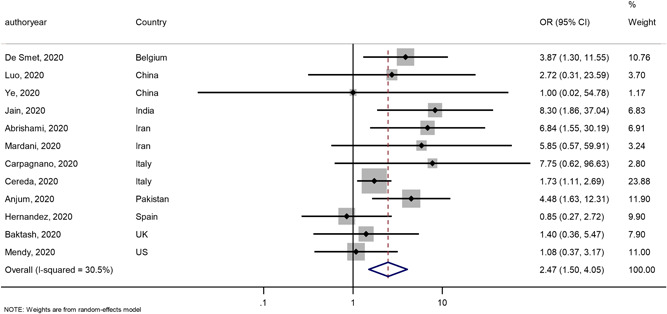
Forest plot showing odds ratio (OR) and 95% confidence interval (CI) for mortality for each of the 12 studies that reported this outcome [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Comparison of vitamin D deficiency versus nondeficiency
| Outcome | Number of studies | Findings |
|---|---|---|
| Mortality | 12 | OR: 2.47, 95% CI: 1.50–4.05; I 2 = 30.5% |
| 3 | HR: 4.11, 95% CI: 2.40–7.04; I 2 = 11.6% | |
| Hospital admission | 3 | OR: 2.18, 95% CI: 1.48–3.21; I 2 = 0% |
| Length of hospital stay | 2 | 0.52 days; 95% CI: 0.25–0.80; I 2 = 89.6% |
| ICU admission | 4 | OR: 5.44, 95% CI: 0.38–78.42; I 2 = 83.1% |
Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
We examined the difference in the severity of COVID‐19 infection based on different 25(OH)D cut‐offs used for defining vitamin D deficiency, geographical location and latitude (Appendix Tables S2–S4). Findings on subgroup analyses are listed in Appendix Tables S2–S4. The significant difference between patients with vitamin D deficiency and nondeficiency levels remained in most of the subgroup analyses (25(OH)D cut‐offs for the definition of vitamin D deficiency, geographic location and latitude).
4. DISCUSSION
In this systematic review and meta‐analysis of 17 studies, we found that vitamin D deficiency was associated with higher mortality and higher rates of hospital admissions with longer hospital stays in adult patients with COVID‐19. These findings need further validation as our findings represent an association, not causation, which may have far‐reaching implications for public health initiatives.
The association between vitamin D deficiency and COVID‐19 infection severity as reflected by greater mortality, higher rate of hospital admissions and longer hospital stay are hypothesised to be due to the immunomodulatory effect of vitamin D. 7 , 34 These effects may be mediated in part by vitamin D reducing the ‘cytokine storm,’ which has been implicated in severe COVID‐19 infection. Vitamin D decreases the production of Th1 cells 35 and suppresses the progress of inflammatory cascade by altering the proinflammatory cytokine signatures. Calcitriol (1,25(OH)2D3), the active metabolite of vitamin D, has been shown to inhibit the production of proinflammatory cytokines, such as interferon gamma, tumour necrosis factor alpha, interleuki‐2 (IL‐2), IL‐17, nuclear factor‐κB and IL‐21 and toll‐like receptors on monocytes and lead to upregulation of IL‐4, IL‐5 and IL‐10. 36 In human umbilical vein cord cells, calcitriol also decreased the expression of adhesion molecules, as well as lipopolysaccharide‐induced expression of receptor of the advanced glycation end product and IL‐6. 36 1,25(OH)D has been shown to result in upregulation of IkBalpha (an NF‐κN inhibitor) in alveolar A549 cells infected with respiratory syncytial virus and in primary human tracheobronchial epithelial cells. 37 Additionally, vitamin D upregulates the expression of angiotensin‐converting enzyme 2 (ACE2), the main host cell receptor of COVID‐19 and also downregulates renin at the transcriptional level. 38 , 39 , 40 The combination of vitamin D's effects on the inflammatory pathway and ACE2 expression may be uniquely applicable to the disease pathogenesis and severity of COVID‐19. 39
Our findings are largely in agreement with two recent systematic reviews on this topic. 41 , 42 but differ from findings from a recent review by Bassatne et al. 43 who reported a statistically nonsignificant trend between serum 25(OH)D level less than 20 ng/ml and an increased risk of mortality and ICU admission. These differences are related to very different criteria used for the selection of studies in the review by Bassatne et al. 43 We included studies with PCR‐confirmed positive COVID‐19 with 25(OH)D levels drawn within 3 months of COVID‐19 diagnosis and a comparison group (vitamin D deficiency vs. nondeficiency). As a result, more studies were included in the meta‐analyses reported in the current study.
Our study has several strengths. We included only studies with laboratory confirmation of COVID‐19 infection. We were able to examine the associations between vitamin D deficiency and severity in COVID‐19 using different cut‐offs for defining vitamin D deficiency. We found that these associations were noted at both 25(OH)D cut‐offs (<12 and <20 ng/ml). We also conducted additional analysis on any variation in the association between vitamin D deficiency and COVID‐19 infection severity among different latitudes, as the mortality from COVID‐19 has been noted to be lower in countries south of latitude 35° North. 6 We also examined the relationship between vitamin D deficiency and COVID‐19 severity in different geographic and cultural regions as ethnic and cultural factors likely play a role independent of latitude.
A limitation of our study is the inability to independently assess the impact of associated confounding variables, such as obesity, dark skin colour, non‐White race, diabetes and advancing age, all of which are risk factors for both greater severity of COVID‐19 infection and vitamin D deficiency. Most of the included studies did not adjust for any confounding variables, including weight status, race and age and therefore these results mainly represent an association and may not predict causality. Another limitation is that the age range in our study was limited as most studies included middle age and elderly patients. Children were not included in our study, as there were not enough studies assessing COVID‐19 infection severity and vitamin D levels. Hence, our results may not be generalisable to the pediatric population. The cut‐offs used to define vitamin‐D deficiency varied among the studies due to a lack of consensus on optimal levels of 25(OH)D. 19 However, subgroup analyses suggested these associations remain regardless of the 25(OH)D concentration used for the definition for deficiency.
Therapies currently aimed at decreasing COVID‐19 severity, such as monoclonal antibodies and remdesivir, may not be accessible in low‐income regions and therefore further studies are warranted to determine if a low‐cost intervention, such as vitamin D supplementation, can decrease COVID‐19 infection severity. Multiple ongoing clinical trials are evaluating the efficacy of vitamin D supplementation early in the course of COVID‐19 infection (https://clinicaltrials.gov/ct2/show/NCT04536298, https://clinicaltrials.gov/ct2/show/NCT04449718). Vitamin D deficiency may also have implications for Post‐Acute Sequelae of SARS‐CoV‐2 infection and this would be an area of future research.
5. CONCLUSION
Vitamin D deficiency is associated with greater COVID‐19 infection severity as measured by rates of mortality, hospital admission and duration of hospital stay. Longitudinal interventional studies are warranted to determine if vitamin D supplementation can decrease COVID‐19 infection severity.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. All authors have given final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. ZW, SK and AJ participated in conception and design of the study; ZW, AJ, KL, SJ, SC, AC, PT and SK were involved in data acquisition; ZW, TN and KM performed data analysis; ZW, AJ, KL, SJ. SC, TN, KM, AC, PT and SK were involved in drafting the manuscript or revising it critically for important intellectual content.
Supporting information
Supporting information.
Wang Z, Joshi A, Leopold K, et al. Association of vitamin D deficiency with COVID‐19 infection severity: Systematic review and meta‐analysis. Clin Endocrinol (Oxf). 2022;96:281‐287. 10.1111/cen.14540
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sorci G, Faivre B, Morand S. Explaining among‐country variation in COVID‐19 case fatality rate. Sci Rep. 2020;10(1):18909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Escobar GJ, Adams AS, Liu VX, et al. Racial disparities in COVID‐19 testing and outcomes: retrospective cohort study in an integrated health system. Ann Intern Med. 2021:M20‐6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients. 2019;11(8):1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: vitamin D deficiency and COVID‐19 severity—plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2021;289(1):97‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15(14):2579‐2585. [DOI] [PubMed] [Google Scholar]
- 8. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID‐19 test results. JAMA Netw Open. 2020;3(9):e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katz J, Yue S, Xue W. Increased risk for COVID‐19 in patients with vitamin D deficiency. Nutrition. 2020;84:111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hastie CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID‐19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14(4):561‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D level on hospital admission associated with COVID‐19 stage and mortality. Am J Clin Path. 2020;25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cereda E, Bogliolo L, Klersy C, et al. Vitamin D 25OH deficiency in COVID‐19 patients admitted to a tertiary referral hospital. Clin Nutr. 2020;02:02‐2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopez A, Cruz ML, Chompre G, et al. Vitamin D status in hospitalized patients with SARS‐CoV‐2 infection. J Clin Endocrinol Metab. 2020;27:27‐2186. [Google Scholar]
- 15. Baktash V, Hosack T, Patel N, et al. Vitamin D status and outcomes for hospitalised older patients with COVID‐19. Postgrad Med J. 2020;27:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mendy A, Apewokin S, Wells AA, Morrow AL. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID‐19 patients. MedRxiv. 2020;27:27. [Google Scholar]
- 17. Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID‐19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giustina A, Adler RA, Binkley N, et al. Controversies in vitamin D: summary statement From an International Conference. J Clin Endocrinol Metab. 2019;104(2):234‐240. [DOI] [PubMed] [Google Scholar]
- 20. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐analyses. Oxford; 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed June 1, 2021. [Google Scholar]
- 21. Rover C, Knapp G, Friede T. Hartung‐Knapp‐Sidik‐Jonkman approach and its modification for random‐effects meta‐analysis with few studies. BMC Med Res Methodol. 2015;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med. 2004;23(9):1351‐1375. [DOI] [PubMed] [Google Scholar]
- 23. Luo X, Liao Q, Shen Y, Li H, Cheng L. Vitamin D deficiency is inversely associated with COVID‐19 incidence and disease severity in Chinese people. J Nutr. 2020;13:13‐2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abrishami A, Dalili N, Mohammadi Torbati P, et al. Possible association of vitamin D status with lung involvement and outcome in patients with COVID‐19: a retrospective study. Eur J Nutr. 2020;30:30‐2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye K, Tang F, Liao X, et al. Does serum vitamin D level affect COVID‐19 infection and its severity? A case‐control study. [published online ahead of print October 13, 2020]. J Am Coll Nutr. 2020:1‐8. 10.1080/07315724.2020.1826005 [DOI] [PubMed] [Google Scholar]
- 26. Carpagnano GE, Di Lecce V, Quaranta VN, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID‐19. J Endocrinol Invest. 2020;09:765‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hars M, Mendes A, Serratrice C, et al. Sex‐specific association between vitamin D deficiency and COVID‐19 mortality in older patients. Osteoporos Int. 2020;31(12):2495‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Im JH, Je YS, Baek J, Chung M‐H, Kwon HY, Lee J‐S. Nutritional status of patients with COVID‐19. Int J Infect Dis. 2020;100:390‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mardani R, Alamdary A, Mousavi Nasab SD, Gholami R, Ahmadi N, Gholami A. Association of vitamin D with the modulation of the disease severity in COVID‐19. Virus Res. 2020;289:198148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Macaya F, Espejo Paeres C, Valls A, et al. Interaction between age and vitamin D deficiency in severe COVID‐19 infection. Nutr Hosp. 2020;37(5):1039‐1042. [DOI] [PubMed] [Google Scholar]
- 31. Pizzini A, Aichner M, Sahanic S, et al. Impact of vitamin D deficiency on COVID‐19‐A prospective analysis from the CovILD registry. Nutrients. 2020;12(9):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Radujkovic A, Hippchen T, Tiwari‐Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D deficiency and outcome of COVID‐19 patients. Nutrients. 2020;12(9):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anjum S, Suleman S, Yasmeen G, Ikram Shah M, Afridi S. Examine the association between severe Vitamin D deficiency and mortality in patients with Covid‐19. Pak J Med Health Sci. 2020;14(3):1184‐1186. [Google Scholar]
- 34. Bilezikian JP, Bikle D, Hewison M, et al. Mechanisms in endocrinology: vitamin D and COVID‐19. Eur J Endocrinol. 2020;183(5):R133‐R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ardizzone S, Cassinotti A, Trabattoni D, et al. Immunomodulatory effects of 1,25‐dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: An in vitro study. Int J Immunopathol Pharmacol. 2009;22(1):63‐71. [DOI] [PubMed] [Google Scholar]
- 36. Talmor Y, Bernheim J, Klein O, Green J, Rashid G. Calcitriol blunts pro‐atherosclerotic parameters through NFkappaB and p38 in vitro. Eur J Clin Invest. 2008;38(8):548‐554. [DOI] [PubMed] [Google Scholar]
- 37. Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF‐kappaB‐linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184(2):965‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin‐angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89‐90(1‐5):387‐392. [DOI] [PubMed] [Google Scholar]
- 39. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharideinduced acute lung injury via regulation of the reninangiotensin system. Mol Med Rep. 2017;16(5):7432‐7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pereira M, Dantas Damascena A, Galvao Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID‐19: systematic review and meta‐analysis. Crit Rev Food Sci Nutr. 2020:1‐9. [DOI] [PubMed] [Google Scholar]
- 42. Yisak H, Ewunetei A, Kefale B, et al. Effects of vitamin D on COVID‐19 infection and prognosis: a systematic review. Risk Manag Healthc Policy. 2021;14:31‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El‐Hajj Fuleihan G. The link between COVID‐19 and vItamin D (VIVID): a systematic review and meta‐analysis. Metabolism. 2021;119:154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


