Adults and children with acute leukaemia are at high risk of serious illness and death from COVID‐19, compared with the general population. 1 , 2 Furthermore, delayed or interrupted leukaemia treatment due to SARS‐CoV‐2 infection may also result in poor prognosis, especially in aggressive forms of leukaemia. Initial studies of the general population have suggested that anti‐SARS‐CoV‐2 monoclonal antibodies (mAbs) may play a promising role in COVID‐19 treatment. 3 However, drug safety and efficacy data in patients with leukaemia are lacking. In this report, we describe the favourable evolution of an eight‐year‐old female patient with ambiguous lineage acute leukaemia, who presented at diagnosis with an active SARS‐CoV‐2 infection and was treated with combined mAbs.
The patient presented with fever, bone pain and bleeding symptoms (petechiae and spontaneous ecchymosis). Blood counts showed marked hyperleukocytosis (107 × 109/l with 85% blasts), mild non‐regenerative normocytic anaemia (105 g/l), moderate thrombocytopenia (86 × 109/l) and normal neutrophil counts. The patient was subsequently referred to a tertiary centre. Bone marrow aspirate analysis revealed massive infiltration by medium‐sized blast cells with a high nucleocytoplasmic ratio and non‐granular cytoplasm. Immunophenotype analysis revealed ambiguous lineage leukaemia with CD19+, cCD79a+, CD22+ and CD10− cells, of which 10% co‐expressed monocytic markers (MPO, CD14, CD64, CD33). Cytogenetic analysis revealed the complex karyotype 46,XX,‐10,‐12,‐17,‐21,+4mar[23]/46,XX[2]. Fluorescence in‐situ hybridization analysis was negative for the BCR‐ABL1, ETV6‐RUNX1, TCF3‐PBX1 and TCF3‐HLF fusions, KMT2A rearrangements and intra‐chromosomal amplification of chromosome 21. Multiplex ligation‐dependent probe amplification revealed an AF15–ZNF384 fusion.
Upon admission, the patient also tested positive for SARS‐CoV‐2 B.1.1.7, via real‐time polymerase chain reaction (RT‐PCR; multiplex TaqPath COVID‐19; ThermoFisher Scientific, Waltham, MA, USA). Although the patient had a fever, she did not present with COVID‐19 respiratory symptoms. The computed tomography (CT) scan showed mild ground‐glass opacity. The patient probably contracted COVID‐19 via household transmission, as her sister and both parents also tested positive for SARS‐CoV‐2. The patient’s mother was pregnant and was the first member of the family to develop COVID‐19 symptoms.
To mitigate the risk of a severe form of COVID‐19, the patient was treated with a combination of the anti‐SARS‐CoV‐2 mAbs bamlanivimab and etesevimab (off‐label). This therapeutic decision was made by a board of experts that included representatives of the French drug agency (ANSM) and the manufacturer (Eli Lilly). The day after admission, the patient received 700 mg (i.e., approx. 25 mg/kg) of bamlanivimab and 1,400 mg (i.e., approx. 50 mg/kg) of etesevimab (adult doses). The COVID‐19 treatment was well tolerated, and anti‐leukaemic corticosteroids were then administered starting three days after the mAbs infusion. The evolution of the clinical, haematological, immunological and viral parameters is shown in Fig 1. Nucleocapsid antibodies were not detected, thus indicating that the patient did not develop a natural immune response to SARS‐CoV‐2 (data not shown). After the infusion, we detected very high serum levels of neutralizing anti‐spike antibodies (i.e., infused mAbs). Although mAbs levels gradually decreased over time, the antibody titers remained high and subsequent mAbs administration was not needed. Viral clearance was observed 10 days after mAbs administration, with no recurrence after a >10‐week follow‐up.
Fig 1.
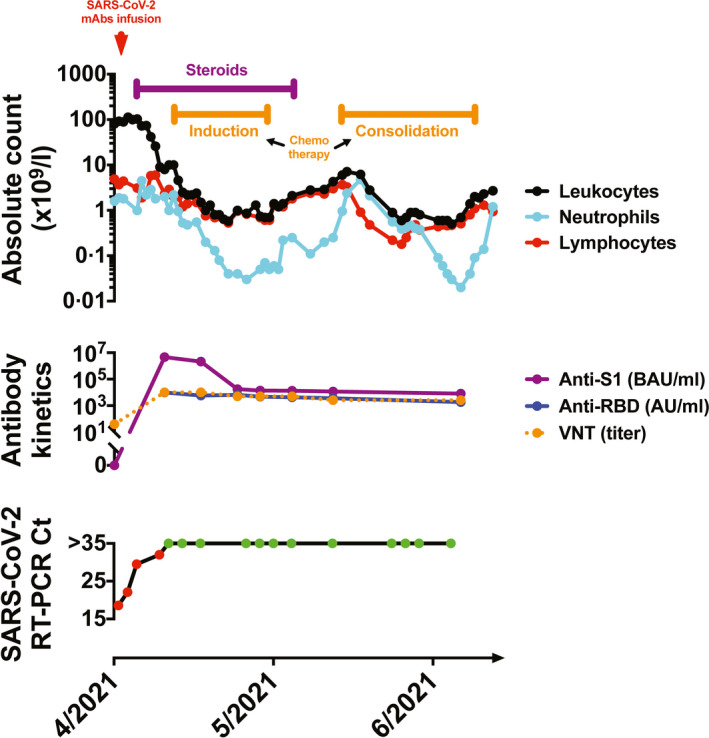
Evolution of the clinical, haematological, immunological and viral parameters over the course of the treatment. Top panel: Evolution of the leukocyte, neutrophil and lymphocyte levels over the course of the treatment. The timepoint of administering the monoclonal antibodies bamlanivimab and etesevimab is indicated by the red arrow. The timeframe of administering the anti‐leukaemia treatment (steroids and chemotherapy) is shown in purple and orange, respectively. Middle panel: Anti‐S1 antibodies were quantified using an ELISA kit: Anti‐SARS‐CoV‐2 QuantiVac (IgG) [Euroimmun, Lübeck, Germany (binding antibody units per ml)]. Anti‐RBD antibodies were quantified using an Access SARS‐CoV‐2 IgG II Reagent Kit [Beckman Coulter Brea, CA, USA (arbitrary units per ml)]. Viral neutralizing titers were quantified as previously described. 6 Bottom panel: SARS‐CoV‐2 RT‐PCR results based on nasopharyngeal samples (multiplex TaqPath COVID‐19; ThermoFisher Scientific, Waltham, MA, USA). The red and green dots indicate positive and negative tests, respectively. Ct, cycle threshold; ELISA, enzyme‐linked immunosorbent assay; mAbs, monoclonal antibodies; RDB, receptor‐binding domain; RT‐PCR, real‐time polymerase chain reaction; VNT, viral neutralizing titer.
Induction chemotherapy was administered according to standard guidelines for acute lymphoblastic leukaemia, including corticosteroids, vincristine, daunorubicin, pegaspargase and triple intrathecal chemotherapy. The patient also received a single dose of rasburicase to treat mild tumour lysis syndrome with increased serum uric acid levels. Aplasia occurred from day 15 to day 42 (neutrophil count nadir of 50/mm3). No severe complications occurred. Complete remission was observed at the end of induction chemotherapy. The patient received a consolidation course.
Patients with a haematological malignancy are at an increased risk of rapid viral evolution, impaired viral clearance, altered humoral response, exhausted T‐cell phenotype and prolonged virus shedding. 4 , 5 Without effective anti‐SARS‐CoV‐2 drugs to treat acute leukaemia patients with COVID‐19, the clinician must decide to either delay/interrupt the anti‐leukaemic treatment or continue the leukaemia treatment and thereby render the patient at an even greater risk of developing a severe form of COVID‐19.
Overall, these observations suggest that mAbs may be well tolerated for the treatment of SARS‐CoV‐2 infection in a patient undergoing therapy for high‐risk leukaemia. The intensive anti‐leukaemic treatment was administered without delay or interruption, and complete remission was observed at the end of induction therapy. Co‐occurrence of COVID‐19 and high‐risk haematological malignancy may occur in regions with a high surge of COVID‐19. Additional studies should be conducted to evaluate the safety and efficacy of mAbs in adult and paediatric patients with both an aggressive haematological malignancy and SARS‐CoV‐2 infection.
Author contributions
PS wrote the paper; LN, PMSV, AA and ML performed the biological analyses; SS, MV, SV and VB acquired clinical data; XdL and HC coordinated the study; All authors edited and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflicts of interest
The authors have no competing interests to disclose.
Acknowledgements
The authors thank Sandra Moore for revising the paper, and Elif Nurtop (PhD‐UVE), Toscane Fourié (PhD‐UVE), technicians of the UVE serological group, as well as the patient and her family for participation in the study.
References
- 1. Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136:2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meena JP, Kumar Gupta A, Tanwar P, Ram Jat K, Mohan Pandey R, Seth R. Clinical presentations and outcomes of children with cancer and COVID‐19: a systematic review. Pediatr Blood Cancer. 2021;68:e29005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurt AC, Wheatley AK. Neutralizing antibody therapeutics for COVID‐19. Viruses. 2021;13:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, et al. Case study: prolonged infectious SARS‐CoV‐2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdul‐Jawad S, Baù L, Alaguthurai T, del Molino del Barrio I, Laing AG, Hayday TS, et al. Acute immune signatures and their legacies in severe acute respiratory syndrome coronavirus‐2 infected cancer patients. Cancer Cell. 2021;39:257–275.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gallian P, Pastorino B, Morel P, Chiaroni J, Ninove L, de Lamballerie X. Lower prevalence of antibodies neutralizing SARS‐CoV‐2 in group O French blood donors. Antiviral Res. 2020;181:104880. [DOI] [PMC free article] [PubMed] [Google Scholar]


