Abstract
We describe a case of a pityriasis rubra pilaris (PRP)‐like eruption occurring following administration of the Pfizer–Biontech mRNA COVID‐19 vaccine, with worsening of the condition following the second dose. To our knowledge, this is the first reported case of a PRP‐like eruption as a cutaneous adverse event of the Pfizer–Biontech mRNA COVID‐19 vaccine.
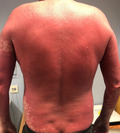
Since the approval of the novel mRNA vaccines for SARS‐CoV‐2, the dermatology community has sought to characterize the adverse cutaneous effects associated with administration of the vaccine. In the BNT162b2 (Pfizer–BioNTech) mRNA vaccine Phase III study, no participants reported cutaneous adverse events (AE) aside from injection‐site reactions. 1 We report a case of pityriasis rubra pilaris (PRP)‐like eruption following administration of the BNT163b2 COVID‐19 vaccine.
An otherwise fit and well 51‐year‐old man presented with a widespread, scaly, erythematous rash following administration of the BNT163b2 COVID‐19 vaccine. He had developed an erythematous scaly rash in his groin and over his knees 3 days following the first dose of the vaccine, and he had been treated by his general practitioner for psoriasis with Enstilar™ foam and emollients, which had achieved partial success. A few days following the second vaccine dose at 12 weeks, the patient noticed the rash worsening, with the plaques becoming more confluent and affecting 60% of his body surface area. Despite continued treatment with topical therapies, his skin continued to worsen and subsequently presented to the acute medical unit where he was found to be mildly hypotensive and tachycardic. He denied taking any medication preceding the skin eruption.
On physical examination, the patient was found to have a confluent, mildly scaly, erythematous skin eruption extending from his scalp to both arms and the proximal thighs with sparing of the periumbilical area (Fig. 1). There were scattered erythematous plaques over his lower legs. His nails were normal and clinically there was no evidence of palmoplantar hyperkeratosis. The differential diagnosis included a drug‐induced psorasisform rash and PRP.
Figure 1.
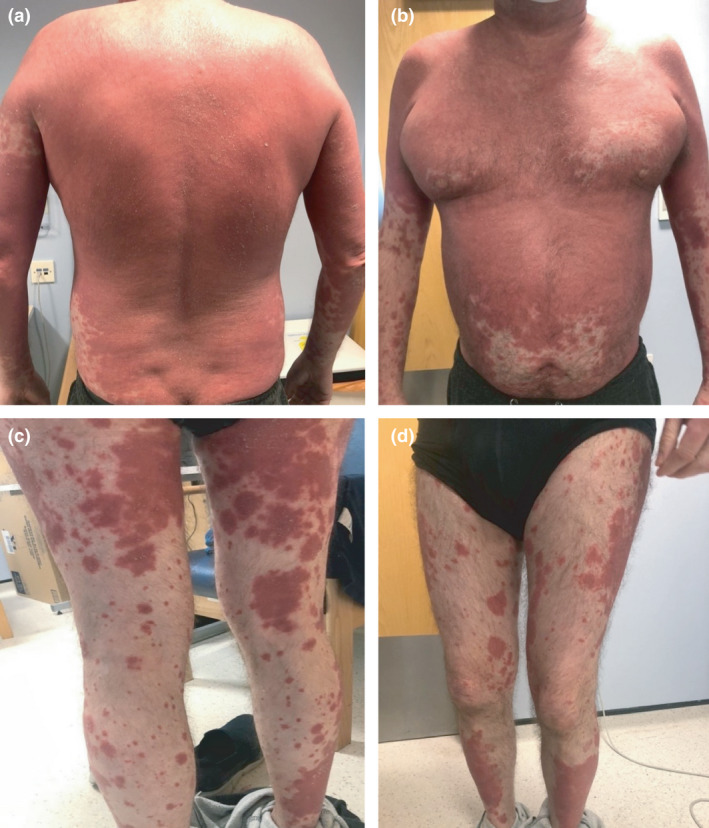
Confluent, mildly scaly, erythematous skin eruption extending from the patient’s scalp to both arms and the proximal thighs with sparing of the periumbilical area. Scattered erythematous plaques over this lower legs were also evident.
Histological examination of a skin biopsy showed prominent alternating orthokeratosis and parakeratosis in horizontal and vertical directions. The epidermis showed mild irregular acanthosis with broader rete ridges than expected in a psoriasiform reaction. There was mild and focal spongiosis with slight lymphocytic exocytosis. There was mild perivascular and perifollicular lymphocytic inflammation within the papillary dermis with neutrophils and occasional eosinophils seen focally (Fig. 2). Overall, the histological features were consistent with a diagnosis of PRP.
Figure 2.
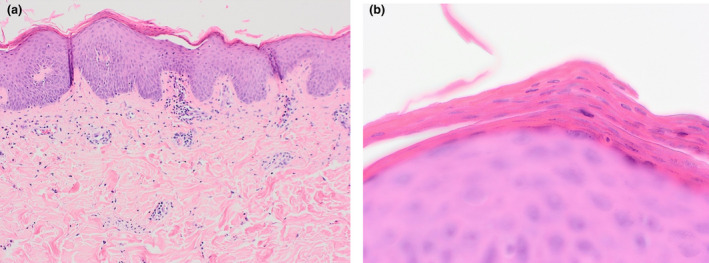
(a) Prominent alternating orthokeratosis and parakeratosis in horizontal and vertical directions. The epidermis showed mild irregular acanthosis with broader rete ridges than expected in a psoriasiform reaction. There was mild and focal spongiosis with slight lymphocytic exocytosis. There was also mild perivascular and perifollicular lymphocytic inflammation within the papillary dermis with neutrophils and occasional eosinophils seen focally. (b) Prominent alternating orthokeratosis and parakeratosis in the distinct ‘checkerboard’ pattern. Haematoxylin and eosin, original magnification (a) × 20; (b) × 600.
Blood tests showed raised level of C‐reactive protein, but white cell and eosinophil counts were normal. Serological testing for blood‐borne viruses and SARS‐CoV‐2 PCR was negative. Chest radiography results were normal and there was no suggestion of occult malignancy based on the history or physical examination.
A diagnosis of PRP‐like eruption was made and the probable trigger thought to be the BNT163b2 COVID‐19 vaccine. The patient was treated with acitretin 20 mg once daily and topical mometasone 0.1% ointment, resulting in improvement of his condition; at his most recent follow‐up, 4 months after starting acitretin, he was still continuing with the treatment.
A recent registry of 414 patients who received either of the two mRNA COVID‐19 vaccines described cutaneous reactions, including local injection‐site reactions, urticarial and morbilliform eruptions, but PRP‐like eruptions have yet to be described. 2 As seen in our case, worsening or recurrence was seen in up to 43% of patients following administration of the second dose. 2
The aetiology of PRP is not known, but it has been suggested that mutations in the gene for caspase recruitment domain‐containing protein 14 (CARD14) could be implicated in the pathophysiology. 3 Reported associations include COVID‐19 infection, other viral illness and various drugs such as imantinib. There have been cases of postvaccination PRP following inoculation with traditional viral vector vaccines such as the diphtheria and influenza vaccines. 4
To our knowledge, this is the first reported case of PRP following administration of the BNT163b2 COVID‐19 vaccine. If PRP occurs after the first dose, then a discussion with the patient, weighing the risks and benefits including worsening of disease compared with the possibility of severe disease/death from COVID‐19 should be undertaken. Although the efficacy and safety profiles of mixing vaccine types has not yet been established, studies such as Com‐COV indicated that changing the type of vaccine given as the second dose may help mitigate the severity or recurrence of AE such as PRP. 5
This case adds an important potential AE of the BNT163b2 mRNA COVID‐19 vaccine, and dermatologists need to be made aware of the potential severe cutaneous AEs of the different COVID‐19 vaccines, as early recognition in the future may aid management.
Conflict of interest: the authors declare that they have no conflicts of interest.
References
- 1. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383: 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Craiglow BG, Boyden LM, Hu R et al. CARD14‐associated papulosquamous eruption: a spectrum including features of psoriasis and pityriasis rubra pilaris. J Am Acad Dermatol 2018; 79: 487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohamed M, Belhadjali H, Hammedi F et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria‐pertussis‐tetanus and oral poliovirus vaccines. Indian J Dermatol Venereol Leprol 2015; 81: 618–20. [DOI] [PubMed] [Google Scholar]
- 5. Shaw RH, Stuart A, Greenland M et al. Heterologous prime‐boost COVID‐19 vaccination: initial reactogenicity data. Lancet 2021; 397: 2043–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


