Summary
Vaccine‐induced immune thrombotic thrombocytopenia (VITT) is a novel entity that emerged in March 2021 following reports of unusual thrombosis after ChAdOx1 nCoV‐19, (AstraZeneca) vaccination. Following the recognition of this syndrome, multiple consensus guidelines have been released to risk stratify patients presenting with possible symptoms after ChAdOx1 nCoV‐19 vaccination. All guidelines rapidly identify VITT in patients with the complete triad of thrombocytopenia, thrombosis and elevated D‐dimers after ChAdOx1 nCoV‐19 vaccination. However, with earlier recognition of the associated symptoms, the clinical manifestations are likely to be more heterogeneous and represent an evolving spectrum of disease. In this setting, current guidelines may lack the sensitivity to detect early cases of VITT and risk missed or delayed diagnoses. The broad clinical phenotype and challenges associated with diagnosis of VITT are highlighted in our present case series of four patients with confirmed VITT. Dependent on the guidance used, each patient could have been classified as a low probability of VITT at presentation. The present study highlights the issues associated with the recognition of VITT, the limitations of current guidance and the need for heightened clinical vigilance as our understanding of the pathophysiology of this novel condition evolves.
Keywords: vaccine, thrombosis, thrombocytopenia, COVID‐19, cerebral venous sinus thrombosis, splanchnic vein thrombosis
Introduction
In March 2021, European health regulators were alerted to case reports of thrombocytopenia and thrombosis, often in atypical sites, occurring after administration of the Vaxzevria vaccine (ChAdOx1 nCoV‐19, AstraZeneca). As of 4 April 2021, a total of 169 cases of cerebral venous sinus thrombosis (CVST) and 53 cases of splanchnic vein thrombosis (SVT) had been reported to the European drug safety database EudraVigilance. This thrombotic syndrome, since named vaccine‐induced immune thrombotic thrombocytopenia (VITT), has been reported to predominately affect females (80%) and those aged <55 years. In some instances, marked thrombocytopenia (<20 × 109/l) together with elevated D‐dimers and hypofibrinogenaemia were observed. On 13 April 2021 the European Medicines Agency (EMA) and AstraZeneca released a joint statement that a causal relationship between ChAdOx1 nCoV‐19 and VITT was plausible. 1 Six cases of a similar syndrome have recently been reported following AD26.COV2.S vaccination (Johnson & Johnson). 2 Nevertheless, European regulators have recommended that the benefits of vaccination with ChAdOx1 nCoV‐19 and AD26.COV2.S continue to outweigh the risks and recommended continued use of both vaccines, with increased public and physician awareness of the signs of VITT required.
Although VITT pathophysiology remains under investigation, recent studies have demonstrated high levels of platelet factor 4 (PF4) antibodies in affected individuals in the absence of previous heparin exposure. 3 , 4 , 5 , 6 , 7 As a result, the PF4 immunoglobulin G (IgG) enzyme‐linked immunosorbent assay (ELISA) has become central in VITT diagnostic algorithms. 8 , 9 , 10 , 11 Multiple national and international consensus guidelines regarding VITT diagnosis and management have been published in the last few weeks. 8 , 9 , 10 , 11 These guidelines have been developed based upon the laboratory and clinical findings observed in the initial VITT cases. Critically however, it is becoming increasingly clear that VITT may encompass a broader range of clinico‐pathological presentations. In the present study, we present four VITT cases developing after a first dose of ChAdOx1 nCoV‐19. Importantly, these cases highlight concerns with respect to the use of current VITT guidelines. In particular, these cases demonstrate that strict adherence to proposed diagnostic criteria may lead to delayed or even missed VITT diagnosis in some affected individuals. Given the fact that VITT requires specific treatment regimens and has been associated with significant mortality, we believe these cases serve as an important exemplar to focus attention on the potential clinical heterogeneity of this emerging VITT entity.
Clinical cases
Case 1
A 29‐year‐old healthcare worker developed a short‐lived bilateral visual disturbance followed by a headache, nausea, vomiting and leg cramps 7 days (D+7) after Vaxzevria (ChAdOx1 nCoV‐19, AstraZeneca) vaccination. She had no past medical history and was not on any regular medications. Clinical and neurological examinations were normal. Initial assessment demonstrated that she had a mild thrombocytopenia [136 × 109/l; normal reference range (NRR) 140–450 × 109/l] and markedly raised D‐dimers (4.8 mg/l; NRR <0.5 mg/ml) (Table I). A previous platelet count from the year 2020 was within the normal range. Doppler ultrasonography (US) of her lower limb was normal and she was discharged. She re‐presented at D+10 due to worsening headache, with progressive thrombocytopenia (61 × 109/l), elevated D‐dimers (5.2 mg/l) and a normal fibrinogen (2.6 g/l; NRR 1·9–3.5 g/l) (Fig 1A). Computed tomography (CT) Brain and venogram were normal, but she was admitted and haematology consulted (D+12). Despite the lack of thrombosis, VITT diagnosis was considered and testing demonstrated strongly positive anti‐PF4 IgG ELISA, with a strongly positive result [optical density (OD) 2·242 units (u), D+12]. Apixaban 5 mg twice daily (BD) orally (PO) was commenced (platelets 71 × 109/l, normal fibrinogen). Subsequent magnetic resonance (MR) Brain and venogram (D+13) and repeat Doppler US of the lower limbs (D+14) demonstrated no thrombosis. Abdominal examination and liver function tests were normal. As splanchnic vein thrombosis has been associated with VITT, a CT abdomen and pelvis was performed on D+14 and identified occult right portal vein and hepatic vein thromboses. Later that day the modified heparin‐induced platelet activation (HIPA) and PF4‐induced platelet activation (PIPA) assays were confirmed as positive (D+14). Consequently she was treated with intravenous (IV) Ig (1 g/kg for 2 days) and argatroban (2 μg/kg/min, IV) was commenced until the platelet count normalised (D+16, Fig 1A), when she switched back onto apixaban (10 mg BD PO).
Table I.
Baseline demographics, laboratory and imaging results.
| Age/ gender | Presenting symptoms | At admission |
Anti‐PF4 IgG ELISA OD, u (NRR) HIPA/PIPA (where available) |
Imaging during inpatient stay | Site of thrombosis (days after vaccine) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Day after vaccine | Platelets, ×109/l (local NRR) | D‐Dimer, mg/l (local NRR) | Fibrinogen, g/l (local NRR) | ||||||
| Case 1 | 29/F | D+7 severe headache, N&V, left leg cramps | D+10 | 61 (140–450) | 5·2 (≤0·5) | 2·6 (1·9–3·5) |
2·242 (<0·4) on D+12 HIPA positive PIPA positive both reported D+14 |
CT brain and venogram, MR brain/venogram US Doppler US left lower limb, CT Abdomen/pelvis | Portal vein and hepatic branch vein (D+15) |
| Case 2 | 38/M | D+14 bruising and petechiae | D+16 | 24 (140–450) | >4 (≤0·5) | 2·4 (1·9–3·5) |
2·48 (<0·4) on D+17 HIPA negative PIPA positive both reported D+24 |
CT pulmonary angiogram | Two proximal pulmonary emboli (D+17) |
| Case 3 | 50/F | D+20 thunderclap headache, persistent, N&V | D+23 | 110 (150–450) | 0·37 (≤0·5) | 2·82 (1·5–4·5) | 2·20 (<0·4) on D+26 | CT angiogram to exclude SAH; CT venogram cerebral | Dural venous sinus thrombosis (D+24) |
| Case 4 | 35/F | D+10 petechiae, bruising, headache | D+14 | 50 (140–450) | 9·83 (<0·42) | 1·16 (1·5–4) | 2·88 (<0·4) on D+14 | MR venogram (brain) | No thrombosis identified |
CT, computed tomography; ELISA, enzyme‐linked immunosorbent assay; F, female; HIPA, heparin induced platelet aggregation assay; IgG, immunoglobulin G; M, male; MR, magnetic resonance; N&V, nausea and vomiting; NRR, normal reference range; OD, optical density; PF4, platelet factor 4; PIPA, PF4‐induced platelet activation assay; SAH, subarachnoid haemorrhage; u, units; US, ultrasonography.
All days after vaccination relate to ChAdOx1 nCoV‐19 (AstraZeneca) vaccination.
Fig 1.
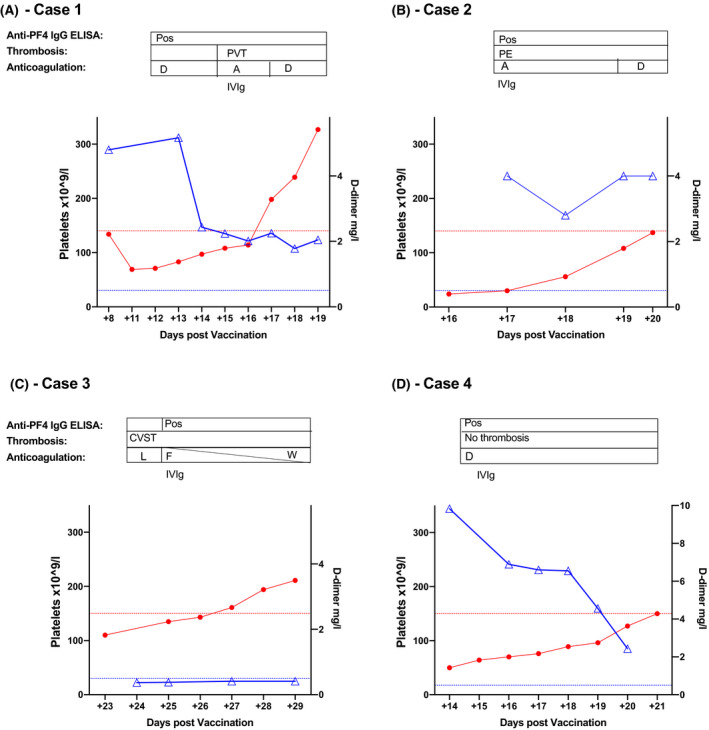
Clinical timeline following admission. For each patient the platelet count (red closed circles) is represented on the left y‐axis with the lower limit of normal indicated by the dashed red line. D‐dimers (blue open triangles) are graphed on the right y‐axis, with the upper limit of normal for D‐dimers indicated by the blue dashed line. The timing of thrombosis, positive anti‐PF4 IgG ELISA, use of anticoagulation and IVIg are indicated over each graph. A, Argatroban; CVST, cerebral venous sinus thrombosis; D, Direct oral anticoagulant use; ELISA, enzyme‐linked immunosorbent assay; F, Fondaparinux; IgG, immunoglobulin G; IV, inravenous; L, therapeutic low‐molecular‐weight heparin; PE, pulmonary embolism; PF4, platelet factor 4; Pos, positive anti‐PF4 IgG ELISA; PVT, portal vein thrombosis; W, Warfarin. [Colour figure can be viewed at wileyonlinelibrary.com]
Case 2
A 38‐year‐old man with cerebral palsy (non‐verbal) presented at D+16 after Vaxzevria (ChAdOx1 nCoV‐19, AstraZeneca) vaccination with a 2‐day history of bruising and petechiae; otherwise clinical assessment was unremarkable. Investigations demonstrated thrombocytopenia (24 × 109/l) and elevated D‐dimers (>4 mg/l; NRR <0.5 mg/l, Table I). On D+17 a haematology assessment was requested after a family member remarked on a subtle tachypnoea. CT pulmonary angiogram identified two proximal pulmonary emboli (PE) and anti‐PF4 IgG ELISA testing was positive (OD 2·48 u; NRR <0·4 u, D+17). The patient was treated with IVIg (1 g/kg for 2 days). As the platelet count increased to >30× 109/l on D+17, therapeutic anticoagulation with argatroban (2 μg/kg/min, IV) was initiated (Fig 1B). This was transitioned to apixaban 10 mg BD PO on D+19 due to difficulty maintaining IV access and nausea with further platelet recovery (84 × 109/l). Platelet activation assays taken on D+17 were reported on D+24, with a negative HIPA, but positive PIPA assay.
Case 3
A 50‐year‐old female with no previous medical history or risks for thrombosis developed a severe thunderclap headache, nausea and vomiting at D+20 after Vaxzevria (ChAdOx1 nCoV‐19, AstraZeneca) vaccination. She attended the local acute ambulatory care unit 3 days later (D+23) and was mildly thrombocytopenic (110 × 109/l; NRR 150–450 × 109/l) (Table I). CT angiogram excluded a subarachnoid haemorrhage, but a possible filling defect was noted in the right transverse and sigmoid sinus. This was confirmed as a dural venous sinus thrombosis on CT venogram (D+24). At that point her platelet count was recovering to 135 × 109/l with normal D‐dimers (0·37 mg/l; NRR <0.5 mg/l) and fibrinogen (2·82 g/l; NRR 2–4.5 g/l) (Fig 1C). She was commenced on therapeutic low‐molecular‐weight heparin [LMWH; enoxaparin 1 mg/kg BD subcutaneous (SC)]. A VITT diagnosis was considered and heparin‐PF4 IgG ELISA on D+26 was strongly positive (OD 2·2 u; NRR <0·4 u). Anticoagulation was changed to fondaparinux (7.5 mg once daily SC) and IVIg (0.5 g/kg for 2 days) and prednisolone (30 mg PO) were given. Throughout her inpatient stay, both fibrinogen and D‐dimer levels remained within the NRR with only a mild transient thrombocytopenia (nadir 110 × 109/l, Fig 1C). She was transitioned to warfarin and discharged on D+33 on tapering prednisolone.
Case 4
A 35‐year‐old woman presented at D+14 after ChAdOx1 nCoV‐19 vaccination due to headache, persistent bruising and petechiae, first noted at D+10. Initial blood tests demonstrated thrombocytopenia (50 × 109/l), a mildly prolonged prothrombin time (PT, 12.7 s; NRR 9·6–11.8 s) and activated partial thromboplastin time (APTT, 31·3 s; NRR 20·8–30.8 s), as well as hypofibrinogenaemia (1·16 g/l; NRR 1·5–4 g/l) (Table I). Her D‐dimer was markedly elevated at 9·83 g/l (NRR <0·42 g/l), as reported previously. 12 A MR venogram did not identify any intracranial thrombosis. An anti‐PF4 IgG ELISA was strongly positive (OD 2·876 u; NRR < 0·4 u, D+14). Given emerging reports of VITT at that time, the patient was anticoagulated pre‐emptively with apixaban (2.5 mg BD, increased to 5 mg BD on normalisation of platelets and fibrinogen), with gradual normalisation of thrombocytopenia, D‐dimer and fibrinogen over the following days (Fig 1D).
Discussion
Over recent weeks, the concept of VITT has emerged as an entirely novel clinical entity that can be associated with significant morbidity and mortality, even in young and otherwise healthy recipients. The limited clinical data regarding this rare disorder associated with use of coronavirus disease 2019 (COVID‐19) adenoviral vaccines has posed significant clinical challenges. To address this issue, a variety of guidelines have been rapidly developed with the aim of assisting physicians in VITT diagnosis and management. Perhaps unsurprisingly given the limited data available, there are significant differences between different VITT guideline recommendations (Table II). The guidelines all consistently identify patients with VITT who present with a ‘classical’ clinical triad of thrombosis, thrombocytopenia and elevated D‐dimers after vaccination. However, we believe that the clinico‐pathological spectrum associated with VITT may be much wider than first envisaged. This hypothesis is supported by the cases presented in the present study.
Table II.
Classification of each patient at admission according to current guidance.
| Guideline | Definite case | Probable case | Suspected case | Unlikely case | Patient assignation at presentation: |
|---|---|---|---|---|---|
| UK EHP 10 |
D+5 to +28 Acute thrombosis Plts <150 × 109/l Raised DD ± low Fib Positive HIT ELISA |
DD > 4000 Or DD > 2000 with strong clinical suspicion |
‘Possible’ cases: D+5 to +28 Acute thrombosis AND/OR Plts <150 × 109/l |
D+5 to +28 Acute thrombosis or low platelet count with DD <2000 and normal Fib |
Case 1: No thrombosis on initial screening but low plts and DD >4000 = probable VITT Case 2: Low plts, DD >4000, no immediate signs of thrombosis = probable VITT Case 3: CVST with low plts but normal DD = possible VITT Case 4: No thrombosis but low plts and DD >4000 = probable VITT |
| Thrombosis Canada (Thrombosis) 11 |
D+4 to +20 Acute thrombosis AND Plts <150 × 109/l Normal blood film |
If following criteria met: Presenting at D+1–3 or after D+20 or plts >150 × 109/l or DD normal or No thrombosis |
Case 1: No thrombosis on initial screening = unlikely VITT Case 2: Low plts but no signs of thrombosis at first admission = unlikely VITT Case 3: CVST with low plts but normal DD/Fib = unlikely VITT Case 4: No thrombosis on initial screening = unlikely VITT |
||
| ISTH 9 |
D+4 to +28 Acute thrombosis Plts <150 × 109/l DD >× 4ULN* Positive HIT ELISA |
D+4 to +28 Acute thrombosis AND Plts <150 × 109/l |
Outside D+4 to +28 or no signs of thrombosis or no thrombosis on imaging AND Plts >150 × 109/l |
Case 1: No acute thrombosis on imaging at presentation; low plts, high DD = not VITT Case 2: Low plts but no signs of thrombosis at first admission = unlikely VITT Case 3: CVST with low plts but normal DD = suspected VITT Case 4: No acute thrombosis on imaging at presentation; low plts, high DD = not VITT |
|
| GTH 8 |
D+4 to +16 Thrombosis and/or thrombocytopenia Positive HIT ELISA Positive modified HIPA assay |
D+4 to +16 Thrombosis and/or thrombocytopenia |
Case 1: No acute thrombosis on imaging at presentation; low plts, = suspected VITT Case 2: Low plts but no signs of thrombosis at first admission = suspected VITT Case 3: CVST with low plts but normal DD, D+23 at presentation = outside suggested timeframe, unlikely VITT Case 4: No acute thrombosis on imaging at presentation; low plts, = suspected VITT |
CVST, cerebral venous sinus thrombosis; D, day; DD, D‐dimer; EHP, Expert Haematology Panel; ELISA, enzyme‐linked immunosorbent assay; Fib, fibrinogen; GTH, Gesellschaft für Thrombose‐ und Hämostaseforschung; HIPA, heparin‐induced platelet activation assay; HIT ELISA, anti‐PF4 IgG ELISA, previously used in Heparin Induced Thrombocytopenia (HIT) testing; IgG, immunoglobulin G; ISTH, International Society on Thrombosis and Haemostasis; PF4, platelet factor 4; Plts, platelets; ULN, upper limit of normal; VITT, vaccine‐induced immune thrombotic thrombocytopenia.
Where HIT ELISA testing not available.
For Case 1, despite symptoms of concern for VITT (thrombocytopenia with high D‐dimers) she was discharged home on her first presentation due to a negative Doppler US. She re‐presented with worsening headache and was found to have increased thrombocytopenia, but symptom‐directed radiological assessment still failed to detect objective evidence of thrombosis. Based on these data, the patient would be classified as ‘probable VITT’ using the UK Expert Haematology Panel (EHP) guidance, ‘suspected VITT’ with the German Gesellschaft für Thrombose‐ und Hämostaseforschung (GTH) criteria, ‘unlikely VITT’ according to the Thrombosis Canada guidelines and ‘not VITT’ by the International Society on Thrombosis and Haemostasis (ISTH) criteria (Table II). Critically, depending on which guidance was followed, anti‐PF4 IgG testing may thus not necessarily have been performed and VITT diagnosis may have been entirely missed. Ultimately, portal vein thrombosis in this patient was only detected because the positive anti‐PF4 IgG ELISA result triggered imaging for possible splanchnic vein thrombosis.
Although Case 2 had more marked thrombocytopenia, history was limited due to communication difficulties and clinical examination was significant only for bruising and petechiae. Accordingly, this patient would be classified as ‘unlikely VITT’ using the Canadian and ISTH guidance (Table II). Conversely, the elevated D‐dimer would result in ‘probable VITT’ according to the UK EHP guidance. Although isolated thrombocytopenia would prompt further investigations as per the German GTH recommendations, it is notable that this patient first presented at D+16. Any further delay in presentation would have placed this patient beyond the GTH timeframe (symptoms 4–16 days after vaccination, Table II). Critical to accurate diagnosis in this patient was a high clinical suspicion coupled with a low threshold for performing radiological investigations, which ultimately enabled prompt PE identification. In this patient the standard HIPA assay was negative. However, the novel PIPA assay, involving the addition of exogenous PF4 to enhance the antibody mediated platelet activation, was positive confirming the diagnosis of VITT (Table III). 4 In a series of 24 patients with VITT, HIPA testing was positive in only five of 24 patients, with inhibition of platelet activation by LMWH in 19/24 patients. In contrast, 23/24 exhibited a positive PIPA assay, highlighting the role of PF4 in this condition. 4 Variation in functional platelet laboratory assessments was seen in our present cases, with a positive HIPA and PIPA in Case 1, but a negative HIPA with positive PIPA for Case 2. This underscores the need for carefully directed specialist testing when functional platelet assays are used in the assessment of VITT.
Table III.
Assays used in the assessment of VITT.
| Test name | Assay type | Mechanism | Notes |
|---|---|---|---|
| Anti‐PF4 ELISA | Immunological assay | ELISA based approach to detect the presence of anti‐PF4/heparin antibodies | Used in both HIT and VITT assessment |
| Heparin‐induced platelet activation (HIPA) | Functional assay |
Investigates if patient antibodies activate platelets in the presence of heparin. Donor platelets are incubated with patient serum/plasma and heparin. HIPA positive implies formation of heparin‐antibody‐PF4 complex that binds to platelet receptors and induces donor platelet activation and aggregation. |
In contrast to HIT, patients with VITT may show inhibition rather than enhancement of platelet aggregation in the presence of low dose heparin. |
| PF4‐induced platelet activation (PIPA) | Functional assay |
Modified HIPA, first described by Greinacher et al. 4 Investigates if patient antibodies activate platelets in the presence of increased PF4. Donor platelets are incubated with patient serum/plasma but instead of heparin, additional PF4 is added. In PIPA‐positive cases, the presence of PF4 with patient serum/plasma enhances platelet activation, independent of heparin. |
Unclear if positive PIPA is due to anti‐PF4 autoantibody production due to vaccine or vaccine‐related antibodies that cross‐react with PF4 and platelets. |
ELISA, enzyme‐linked immunosorbent assay; HIT, heparin induced thrombocytopenia; PF4, platelet factor 4; VITT, vaccine‐induced immune thrombotic thrombocytopenia.
Case 3 raises particular clinical concerns as she had normal D‐dimer and fibrinogen levels throughout. In addition, her initial presentation at D+23 is beyond the ‘window’ proposed for VITT consideration in the Thrombosis Canada and German GTH guidelines. Based on the Thrombosis Canada guidance, this patient would be classified as ‘unlikely VITT’. In contrast, the ISTH guidelines would classify as ‘suspected VITT’ and the UK EHP as ‘possible VITT’ due to the combination of thrombocytopenia and thrombosis despite normal D‐dimers.
Case 4 contrasts starkly to the other cases, as no objectively confirmed thrombosis was identified. However, this patient presented with hypofibrinogenaemia and mild prolongations of both PT and APTT. In our other three cases, fibrinogen, PT and APTT remained entirely normal throughout their clinical course. Due to the presence of thrombocytopenia but lack of thrombosis, this patient would be classified as ‘probable VITT’ using the UK EHP guidelines, ‘suspected VITT’ with the German GTH criteria, ‘unlikely VITT’ with the Thrombosis Canada guidance, ‘not VITT’ using the ISTH criteria (Table II) and may not have been referred for anti‐PF4 IgG ELISA testing.
Initial reports outlined similarities between VITT and autoimmune heparin‐induced thrombocytopenia (HIT), a condition associated with unusually severe thrombocytopenia, thrombosis and disseminated intravascular coagulation (DIC). 4 , 7 In the case series from Greinacher et al. 4 five of 11 patients had evidence of DIC all with markedly elevated D‐dimers (>10 mg/l). Although hypofibrinogenaemia was common in the UK series (14/23 patients) only three had associated prolongations of both PT and APTT, and D‐dimer levels in these patients varied. 5 In our present series, only Case 4 had hypofibrinogenaemia with a mild prolongation of PT and APTT. This patient had the most significant derangement of D‐dimers (9·83 mg/l), but no detected thrombosis. These interindividual variations may reflect differing manifestations of the induced immune response in VITT, varying from a predominantly hyperfibrinolytic picture to overtly thrombotic phenotype.
Current guidelines serve to improve awareness of VITT, but classification is based on clinical and laboratory data at a single time‐point. These cases highlight that the cases may vary dynamically over time with both frequent reassessment and consideration of screening for occult thrombosis required. With improved awareness of this condition it is more likely that patients may present earlier, while the disorder is still in evolution. Physicians should therefore not discount the possibility of VITT in patients with two of the three key features, as this may represent an evolving phenotype. In this instance, we would advocate to proceed to anti‐PF4 IgG ELISA testing with close monitoring of clinical parameters and screening for thrombosis at typical sites, even in the absence of symptoms.
Author contributions
All authors (Michelle Lavin, Patrick T. Elder, Denis O’Keeffe, Helen Enright, Eileen Ryan, Anna Kelly, Ezzat El Hassadi, Feargal P. McNicholl, Gary Benson, Giao N. Le, Mary Byrne, Kevin Ryan, Niamh M. O’Connell and James S. O’Donnell) collected the data. Michelle Lavin, Kevin Ryan, Niamh M. O’Connell and James S. O’Donnell analysed the data, Mary Byrne and Gary Benson directed laboratory analysis. All authors (Michelle Lavin, Patrick T. Elder, Denis O’Keeffe, Helen Enright, Eileen Ryan, Anna Kelly Ezzat El Hassadi, Feargal P. McNicholl, Gary Benson, Giao N. Le, Mary Byrne, Kevin Ryan, Niamh M. O’Connell and James S. O’Donnell) were involved in writing and reviewing the papers.
Conflict of interest
Michelle Lavin has received indirect funding from Takeda to support an educational initiative and received consultant fees from Sobi and Tremeau Pharmaceuticals. Niamh M. O’Connell has received research support from or acts as PI in studies by Freeline, Sobi, Takeda, Roche and Uniqure, has received speaker’s fees from Bayer, Bristol‐Myers Squibb, Novo Nordisk, Pfizer, Roche and Sobi and served on scientific advisory boards for Bristol‐Myers Squibb, Freeline, Pfizer, Roche, Sobi, Takeda and Uniqure. James S. O’Donnell has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Sobi, Boehringer Ingelheim, Leo Pharma, Takeda and Octapharma. He has also served on the advisory boards of Baxter, Sobi, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda and Pfizer. James S. O’Donnell has also received research grant funding awards from 3M, Baxter, Bayer, Pfizer, Shire, Takeda, 3M and Novo Nordisk.
Acknowledgements
James S. O’Donnell and the Irish COVID‐19 Vasculopathy Study (ICVS) are supported by a Health Research Board COVID‐19 Rapid Response award (COV19‐2020‐086) and also by a philanthropic grant from the 3M Foundation to RCSI University of Medicine and Health Sciences in support of COVID‐19 research. HIPA and PIPA testing were performed in the Universitatsmedizin Greifswald laboratory, Germany. We would like to thank the patients involved for providing their consent to share information regarding their clinical journey and outcomes. Open access funding provided by IReL.
References
- 1. EMA & AstraZeneca . VAXZEVRIA/COVID‐19 Vaccine AstraZeneca : link between the vaccine and the occurrence of thrombosis in combination with thrombocytopenia [cited 2021 April 21]. Available at: https://www.ema.europa.eu/en/documents/dhpc/direct‐healthcare‐professional‐communication‐dhpc‐vaxzevria‐previously‐covid‐19‐vaccine‐astrazeneca_en‐0.pdf.
- 2. Centers for Disease Control and Prevention . Joint CDC and FDA Statement on Johnson & Johnson COVID‐19 Vaccine [cited 2021 April 21]. Available at: https://www.cdc.gov/media/releases/2021/s0413‐JJ‐vaccine.html.
- 3. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund‐Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021. (Online ahead of print). 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021. (Online ahead of print). 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021. (Online ahead of print). 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CC, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector‐based COVID‐19 vaccine. J Thromb Haemost. 2021. (Online ahead of print). 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thaler J, Ay C, Gleixner KV, Hauswirth AW, Cacioppo F, Jürgen G, et al. Successful treatment of vaccine‐induced prothrombotic immune thrombocytopenia (VIPIT). J Thromb Haemost. 2021. (Online ahead of print). 10.1111/jth.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C, et al. Diagnosis and management of vaccine‐related thrombosis following Astrazeneca COVID‐19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021. (Online ahead of print). 10.1055/a-1469-7481. [DOI] [PubMed] [Google Scholar]
- 9. International Society on Thrombosis and Haemostasis (ISTH) . ISTH guidance on vaccine‐induced immune thrombotic thrombocytopenia. 2021. [cited 2021 April 20]. Available at: https://cdn.ymaws.com/www.isth.org/resource/resmgr/news/ISTH_VITT_Flow_Chart_Final.pdf. [Google Scholar]
- 10. UK EHP . Guidance produced from the Expert Haematology Panel (EHP) focused on Covid‐19 vaccine induced thrombosis and thrombocytopenia. 2021. 2–5. [cited 2021 April 20]. Available at: https://b‐s‐h.org.uk/about‐us/news/guidance‐produced‐by‐the‐expert‐haematology‐panel‐ehp‐focussed‐on‐vaccine‐induced‐thrombosis‐and‐thrombocytopenia‐vitt/.
- 11. Thrombosis Canada . Vaccine‐induced prothrombotic immune thrombocytopenia (Vipit). 2021:1–3 [cited 2021 April 20]. Available at: https://thrombosiscanada.ca/wp‐uploads/uploads/2021/04/51.‐Vaccine‐induced‐prothrobotic‐immune‐thrombcytopenia_02April2021‐1.pdf.
- 12. Ryan E, Benjamin D, McDonald I, Barrett A, Ryan K, McHugh J, et al. AZD1222 vaccine‐related thrombocytopenia without thrombosis in a young female. Br J Haematol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


