Abstract
This is a case of symmetrical drug‐related intertriginous and flexural exanthema‐like eruption following ChAdOx1 nCoV‐19 (AstraZeneca‐Oxford) vaccination. Investigations, including repeated skin swabs, ruled out an infectious cause. He was subsequently treated with oral prednisolone, which led to a resolution of his symptoms.
Dear Editor,
A 61‐year‐old patient presented with a 4‐week history of tender rash on the bilateral groins, with no associated rash elsewhere. The rash had appeared 1 day after he had received the second ChAdOx1 nCoV‐19 (AstraZeneca‐Oxford) vaccine. His medical history included type 2 diabetes, which had been well‐controlled with oral antidiabetic medications.
Physical examination revealed a florid, symmetrical rash with a well‐demarcated inflammatory border on both groins (Fig. 1a) and well‐defined patches of erythema involving the gluteal area (Fig. 1b). This was associated with superficial erosions, skin desquamation and crusting on the scrotal skin. There was no mucosal involvement. Skin swabs and scrapings were negative for bacterial and fungal growth, respectively, and viral PCR for herpes and varicella zoster viruses was also negative. Blood tests showed normal levels of inflammatory markers but slightly raised white cell and neutrophil counts. Zinc level was normal. The patient declined a diagnostic skin biopsy.
Figure 1.
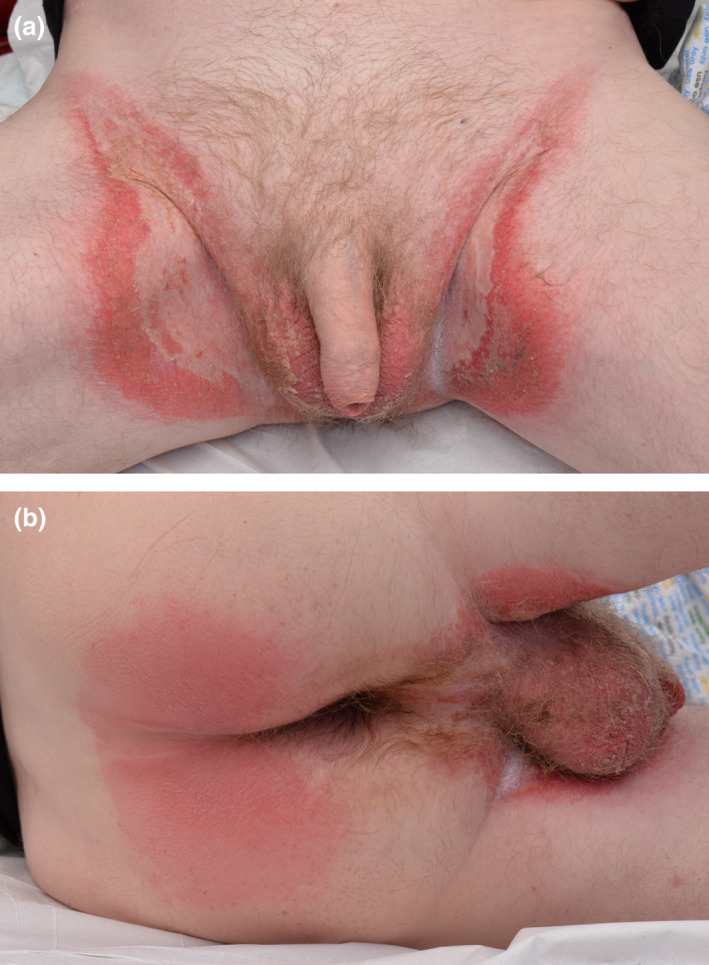
(a) Symmetrical rash with well‐demarcated inflammatory border on both groins; (b) well‐defined patches of erythema on the gluteal area.
In view of the clinical history and examination finding, a diagnosis of symmetrical drug‐related intertriginous and flexural exanthema (SDRIFE)‐like eruption was considered. The patient was subsequently treated with oral prednisolone 30 mg for 2 weeks, then 20 mg for 2 weeks, followed by a taper of 5 mg/week. He was also prescribed betamethasone/clotrimazole cream (Lotriderm; Organon Pharma, Hoddesdon, Hertfordshire, UK) and potassium permanganate soaks to weeping areas. This led to a significant improvement after 1 month of treatment.
The AstraZeneca‐Oxford vaccine is a recombinant, replication‐deficient chimpanzee adenoviral vector vaccine that contains the genetic material to encode the S glycoprotein of SARS‐CoV‐2. The vaccine was approved in late December 2020 by the Medicines and Healthcare products Regulatory Agency after being shown to be safe and effective following an interim analysis of phase III trials. 1 Cutaneous reactions are less commonly reported with adenoviral vaccines such as AstraZeneca‐Oxford compared with messenger RNA (mRNA) vaccines such as BNT162b2 (Pfizer‐BioNTech) and mRNA‐1273 (ModernaTX). Vaccine‐associated cutaneous reactions commonly include delayed large local reactions, local injection‐site reactions, urticaria and maculopapular reactions. 2 AstraZeneca‐Oxford has been associated with a rare risk of thromboembolism and vaccine‐induced prothrombotic immune thrombocytopenia, which may appear as purpura, erythema and oedema of the extremity. 3 Other cutaneous reactions include injection‐site reactions and delayed local reactions. 3 , 4 AstraZeneca‐Oxford contains the excipient polysorbate 80 (E433), which is thought to induce hypersensitivity reactions such as dermatitis, urticaria and anaphylactoid eruptions. 3 This is different from mRNA vaccines in which the excipient polyethylene glycol is thought to be the cause. 2 SDRIFE is a type IV delayed hypersensitivity drug reaction characterized by a symmetrical eruption affecting the inguinal/genital, gluteal/perianal areas and other intertriginous areas such as the axillae, elbows and knees, and typically occurs hours to days following exposure to a systemic agent without systemic involvement. Common drug causes are β‐lactam antibiotics, terbinafine, iodine radio‐contrast media and monoclonal antibodies such as infliximab. 5
To our knowledge, this is only the second case of SDRIFE‐like eruption secondary to COVID‐19 vaccines. The first case was reported following administration of CoronoVac vaccine, which contains an inactivated form of the COVID‐19 virus. This case report adds to the collection of cutaneous reactions assocated with AstraZeneca‐Oxford.
Conflict of interest: the authors declare that they have no conflicts of interest.
References
- 1. Voysey M, Clemens SAC, Madhi SA et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bogdanov G, Bogdanov I, Kazandjieva J, Tsankov N. Cutaneous adverse effects of the available COVID‐19 vaccines. Clin Dermatol 2021. 10.1016/j.clindermatol.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim JE, Lee H, Paik SS et al. Delayed cutaneous reaction to ChAdOx1 nCoV‐19 vaccine: is it an ‘AstraZeneca arm’? J Eur Acad Dermatol Venereol 2021. 10.1111/jdv.17476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug‐related intertriginous and flexural exanthema (SDRIFE). Am J Clin Dermatol 2011; 12: 171–80. [DOI] [PubMed] [Google Scholar]


