Abstract
The deterioration of tissue structure and decline in physiological function during aging are accompanied by alterations to the gut microbiota. The elderly has higher risks of various diseases and chronic diseases. However, inter‐individual differences are more apparent in elderly than younger, and a proportion of individuals have a delayed onset or even avoid developing chronic diseases. This difference in health status is influenced by both heredity and Lifestyle and environmental factors. During the process of aging, the gut microbiota is also affected by the external environment, and provides a buffer to external challenge, and thus the gut microbiota reflects an individual's personal experience. Moreover, the immune system undergoes a series of changes with age, which are related to chronic inflammation in the elderly. The formation, maturation and senescence of the intestinal immune system is closely related to the gut microbiota. Additionally, changes in the gut microbiota of elderly individuals may modulate the immune system, which may in turn affect health status. Herein, we summarize the correlations between the gut microbiota with individual health status in the elderly and explore the related mechanisms, which may provide a basis to maintain or enhance the health of the elderly though interventions targeting the gut microbiota.
Keywords: aging, chronic disease, chronic inflammation, gut microbiota, immunity
The composition of the gut microbiota changes with the increasing of the age. The changes are various in different people. The gut microbiota of some people turns into a combination of fewer beneficial bacteria and more pro‐inflammatory bacteria, which affects immune system and causes a low‐grade inflammatory state. As a result, the body becomes more susceptible to certain diseases and this is closely related to the more apparent inter‐individual differences in health status in the elderly.
1. INTRODUCTION
Aging is a major concern in modern society. An aging population places a larger burden on socio‐economic and healthcare systems. Aging people may develop various age‐related chronic diseases, with inter‐individual differences mainly due to lifestyle and living environment, genetic background, and accidents. Elderly people with poor health status have a poorer quality of life than healthy individuals. However, the major factors that determine the differences in the health status of the elderly remain unknown. Furthermore, whether these differences can be evaluated, or even modulated, via convenient and effective approaches is unclear.
Healthy aging involves reducing and delaying age‐related morbidity. Fortunately, not all elderly individuals are fragile, and some have the ability to delay or even avoid chronic disease. The incidence of multiple chronic diseases increases with age, which can lead to the accumulation and coexistence of multiple diseases and elevate the risk of all types of diseases among elderly individuals.1 The deterioration of tissue structure and the decrease of physiological function with aging play an essential role in age‐related morbidity.2 Moreover, aging is related to the accumulation of genetic and epigenetic modifications that limit the body's ability to maintain homeostasis and respond to external changes and challenges.3 An individual's current health status is not only genetically determined, but also a reflection of their past experiences, such as exposure to various stresses, good or bad lifestyle habits, mental and physical challenges and environmental factors. Thus, the health status of elderly individuals can vary widely.
Aging and health status affect a number of biological indices that could potentially be evaluated as indicators of individual health status. For example, the human microbiota is a promising indictor of health status that correlates with both internal and external effects on the body. The composition of the gut microbiota is not fixed, and can be affected by several factors, including diet, lifestyle, infection, activation of the immune response and the level of IgA produced by B cells.4 The taxonomic composition of the gut microbiota varies among elderly individuals, and reflects current and previous health status.
However, the microbiota can also act as a buffer against external damage and coordinate the health of the body.5 Therefore, the human microbiota may reflect the health status of the organism during the aging process, and also may defend against damage. Additionally, the microbiota may affect the immune system function of the elderly, consequently modulating body biological regulatory network. Herein, we attempt to find answers to age‐related disease states in terms of the microbiome and the resulting immune status. The correlation between them could give us a series of potential biomarkers for the early diagnosis, and for the prediction of the prognosis of the aging‐related diseases. Meanwhile, the causation between them might be valuable targets for the interventions to the diseases (Figure 1).
FIGURE 1.
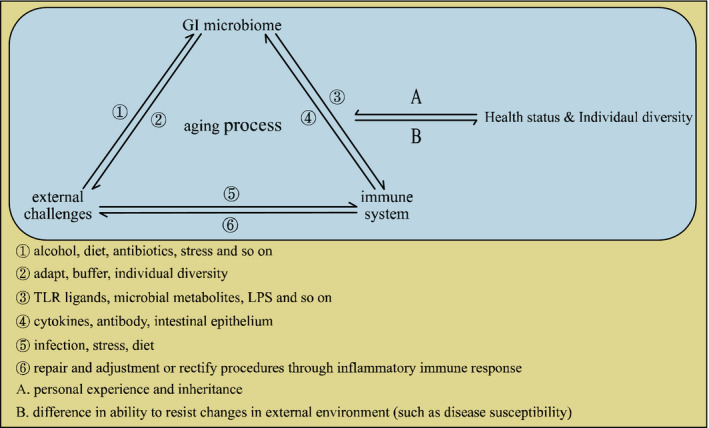
A possible network that connects external challenges, the immune system and diverse health status diversity to the GI microbiome during the aging process
2. AGING AND HUMAN MICROBIOTA
Early studies by Jeffery et al revealed that the gut microbiota change with age.4 Aging‐associated changes in the gut microbiota were reported to manifest as an increase in pro‐inflammatory symbionts and a decrease in beneficial microorganisms,6 while another study did not find significant differences in the microbiota composition of healthy young adults and healthy aged adults from the same population.7 However, a recent analysis of the microbiota cohort of healthy individuals using GWAS provided a statistical model. This model could predict the age of the human host from their gut microbiota and clearly showed that the microbiota changes with age.8 In general, changes in the structure of the gut microbiota reflect changes in intestinal function and host metabolism.9
2.1. The structure of the microbiota changes with age and external factors
Thirty‐nine of 95 species of gut microbiota have been shown to play a crucial role in predicting age. A study in Ireland showed that Bacteroidetes species and the Firmicutes phylum predominate in the gut microbiota of elderly individuals.4 Compared to younger individuals, the relative proportion of Bacteroides spp. was higher and the relative abundance of Clostridium groups was increased in the gut microbiota of elderly individuals. Older individuals also showed a reduction in the abundance of Ruminococcus, Prevotella and related genera.4 Bacteroidetes account for more than half (53%) of the core microbiota in elderly individuals, compared with 8%‐27% in healthy young adults. The gut microbiota of elderly individuals contained a wide array of Bacteroides, Alistipes and Parabacteroides species, while that of healthy young adults also contained a unique Bacteroides species, most similar to Bacteroides vulgatus. Moreover, when the aggregated microbiota was compared, cluster IV was most predominant cluster among the elderly group, whereas cluster XIVa was more prevalent in the healthy young cohort. Gut microbiota senescence has been related to external factors, such as alcohol, antibiotics, probiotics, or diet.8 Thus, the accumulation of external effects may possibly explain the altered gut microbiota diversity in the elderly.
The microbiota is also affected by the outside environment at various stages of life. Before delivery, vaginal infections can enable bacteria to invade the uterine environment of pregnant women. The microbiota of the mother's intestines is transmitted to the fetus through the blood. The mode of delivery (vaginal delivery and caesarean section) affects the initial bacterial inoculum of the newborn. In the early stages of life, acquired factors such as diet (breastfeeding versus milk powder feeding, supplementary food supplementation), genetic factors, environmental exposure and antibiotic use, affect the formation of the microbiota.10 Such inter‐individual variation in factors may explain for the large differences in the gut microbiota of infants and young children.11 By the age of three, the diet becomes more diverse, and the microbiota gradually transform into an adult‐like form.10 A core microbiota is shared between puberty and adulthood.12 The gut microbiota of adults is quite stable, and also more flexible and complex, and can buffer exogenous invasions such as stress. However, the gut microbiota of adults can still be altered by environmental disturbances.13 A recent study showed that even short‐term exposure to diets consisting entirely of animal or plant products significantly change the structure of the adult gut microbial community.13 Therefore, inter‐individual variations in numerous factors can lead to differences in the gut microbiota both during early life and also in adults. Gut microbiota that can offset exogenous damage may accumulate the influence of these external factors through specific alternation, which may partially explain the greater diversity of the gut microbiota in the elderly.
2.2. Age‐related diseases are accompanied by significant changes in the gut microbiota
A recent study showed that the gut microbiota composition of 5XFAD transgenic (Tg) mice, which are widely used in Alzheimer's disease research, undergoes significant changes during the progression of AD, whereas the gut microbiota composition of wild‐type (WT) mice exhibited little change. At the phylum level, Bacteroides, Firmicutes and Verrucomicrobia were most abundant in 2‐month‐old Tg mice (47.3%, 33.0%, 12.2%, respectively). By seven months of age, Firmicutes had become the predominant phylum (62.8%), while the relative abundance of Bacteroidetes and Verrucomicrobia decreased significantly, indicating a change in bacterial type.14
Evidence indicates that members of the Firmicutes and Bacteroidetes phyla stimulate the inflammasome pathway in the intestine and lead to an inflammatory response.15 Based on the changes in the gut microbiota of the elderly, an inflammatory response in the gut is likely to develop during ageing This age‐related imbalance in the gut microbiota could reduce the integrity of the intestinal barrier.6 Subsequent leakage of microbial products may upregulate the circulating levels of pro‐inflammatory factors (such as interferons, TNFα, interleukin‐6 and interleukin‐1) and promote a persistent low‐grade inflammatory state, which has been related to cognitive decline and dementia.1 The persistent low‐grade inflammatory state has also been observed in other chronic diseases, such as IBD,16, 17 diabetes type I,18 CVD19 and metabolic disorders.20 The pro‐inflammatory state caused by dysbiosis of the gut microbiota is considered to play key roles in these common diseases of the elderly.
3. AGING‐RELATED CHANGES IN THE IMMUNE SYSTEM
The effects of aging on the immune system are associated with significant inter‐individual differences.21 The gradual decline in immune function caused by aging leads to a reduction in adaptability and increase in vulnerability.22 For example, disruptions to homeostasis of the host lead to increased morbidity and mortality in the elderly.2, 23 Moreover, aging is commonly thought to have a distinct effect on adaptive immunity in elderly individuals.
3.1. Aging and chronic inflammation
In the innate immune system, neutrophils exhibit a sharp decline in bactericidal and phagocytic function as the cells age.24 Macrophage function also changes, with increased secretion of IL‐6 and IL‐8 induced by mitogens and lipopolysaccharides. Dendritic cells also significantly reduce in number, distribution, and likelihood of production and development from hematopoietic precursors.25 Natural killer cells also exhibit reduced cytotoxicity as the cells age.26 In terms of the adaptive immune system, the thymus atrophies and the number of naive T cells decrease, while the proportion of memory T cells increase during aging. The number and proliferation of Th1 cells also decrease. Additionally, the number and proliferation of memory Th2 cells increases; which leads to production of higher levels of IL‐10, which further inhibits the function of Th1 cells. The consequence is a decrease in Th1‐type immunity mediated by cells. Moreover, the decrease in the production of antibodies by B cells in response to antigens is thought to be primarily due to the decline in helper T cell activates for B cells.21 These alternations increase susceptibility to immune‐mediated diseases. In addition, the increase in pro‐inflammatory factors is characteristic of chronic inflammation,27 which has been related to degenerative diseases such as diabetes and osteoporosis.28
Neurons are active participants in the interaction between the central nervous system and the immune system, and regulate the activation of microglia to maintain the integrity of the central nervous system.29 Aging can influence the interaction between microglia and neurons by altering the nervous environment, and results in increased microglial reactivity.30 Compared with healthy microglial cells, aged microglia a more rapid induction and release the higher levels of cytokines upon activation. Primed microglia appear to be in an intermediate activation state with deamidation and upregulation of MHCII.30 After further stimulation induced by activation of the peripheral or central innate immune system, primed or sensitized microglia releases pro‐inflammatory cytokines significantly and for a long time.31 It also shows significant enhancement of the pro‐inflammatory cytokines IL‐1β, IL‐6, and TNF‐α after immune stimulation with the bacterial endotoxin lipopolysaccharide in vitro.32 Also, the levels of the anti‐inflammatory cytokines IL‐10 and IL‐4 decrease with age‐related increases in the levels of the pro‐inflammatory cytokines.33 These changes have been linked to cognitive decline and depressive behavior.34
Chronic inflammation and chronic diseases, such as diabetes and cancer are associated with aging.35 The underlying inflammatory response increases with age, which in turn leads to chronic, low‐grade inflammation that accelerates aging.35 This process is known as "inflammaging", and is also a process of immunosenescence.36 Various mechanisms are involved in inflammaging, such as the redox stress hypothesis, mitochondrial dysfunction, immunosenescence, chronic infection and homeostasis reconstruction.37
3.2. Aging and intestinal immune
Induction and maintenance of the intestinal mucosal immune response is a multi‐step process. First, intestinal cavity antigens are transported across the intestinal epithelium.38 In aging mice, the specific expression of E26 transformation‐specific family transcription factor Spi‐B in M cells is impaired 38 and expression of CCL20 in the follicle‐associated epithelium (FAE) is reduced,39 which could affect the functional maturation of M cells in FAE.38 The density of mature M cells in Peyer's patches is significantly decreased in aged mice, which prevented the granuloantigen from transcytosis of FAE. In addition, the expression of zonula occludens‐1 (ZO‐1), junctional adhesion molecules (JAMs), and occludins are decreased in the intestinal epithelium of aged rats and baboons.40 The resulting destruction of the tight junctions in the intestinal epithelium may lead to increased paracellular permeability to intraluminal antigens and pro‐inflammatory stimuli.
Mononuclear phagocytes (MNPs), consisting of macrophages and typical dendritic cells, engulf the pathogen and present antigens to T cells located in gut‐associated lymphoid tissue (GALT) and mesenteric lymph nodes (MLN).38, 41 T cells then polarize along the effector or tolerant pathway. In contrast, antigen‐specific IgA+ B cell immunoblasts undergo antibody isotype switching, differentiate, and subsequently migrate to the lamina propria. Finally, plasma cells in the lamina propria produce IgA locally, which is subsequently transported across the intestinal epithelium and secreted into the lumen. Aging affects these intestinal immune responses to varying degrees61. Immunohistochemical staining indicated that the numbers of CD11c+ MNP were reduced in Peyer's patch in one‐year‐old mice,42 and the number of macrophages was significantly decreased in the lamina propria of aged dogs.43 However, the high‐sensitivity immunohistochemical analysis of Peyer's knots in aged mice revealed that the apparent M‐cellinducingCD11c + CD45R + (B220) cells in FAE was significantly reduced,44 while the density of CD11c+ MNP in the sub‐epithelium dome (SED) was not adversely affected61. The ability of dendritic cells to initiate T cell responses appears to be impaired in old mice.45 Another study also reported that aging dendritic cells are characterized by a significant decrease in IL‐12p70 and IL‐15 production, accompanied by a decrease in the expression of the CD80/CD86 co‐stimulatory signal, and that the reduced IL‐15 appears to reduce the ability to initiate T cell response of aging dendritic cells.45 Moreover, aging intestinal‐derived dendritic cells cannot induce the differentiation and secretion of TGF‐β in CD4 + CD25 + LAP + T cells, and enable to up‐regulated the amount of IFN‐γ produced by CD4 + CD25 + LAP + T cells. In contrast, gut‐derived dendritic cells directly activate T cells by producing TGF‐β, or produces lower levels of IFN‐γ, or indirectly via TGF‐β producing T cells to activate T cells.46 On the other hand, TGF‐β also determines the fate of B cells in Peyer's patches (PPs) and affects their homing to the lamina propria of distant mucosal sites.47 How the levels of intestinal IgA change with aging are still unclear. Some studies have found intestinal IgA decreased,48 whereas others found intestinal IgA was unchanged or even increased during aging.46 However, the heterogeneity of the VH gene pool of IgA antibodies is increased in aged mice, and the affinity of IgA to antigen decreases.49
4. THE GUT MICROBIOTA AFFECTS THE IMMUNE SYSTEM IN ELDERLY INDIVIDUALS
4.1. The gut microbiota regulates the immune system
A study published in 2019 showed that patients treated with broad‐spectrum antibiotics before receiving immune checkpoint inhibitors (ICIs) for cancer treatment were nearly twice as likely to respond poorly to ICI than patient who were not treated with broad‐spectrum antibiotics. Broad‐spectrum antibiotics may lead to an imbalance in the gut microbiota that affects the treatment of cancer with ICIs.50 Moreover, oral administration of broad‐spectrum antibiotics in mice reduced the severity of experimental autoimmune encephalomyelitis (EAE).51 Single colonization of germ‐free mice with segmented filamentous bacteria (SFB) enhanced the ability to recruit neutrophils and other immune cells of T helper 17 (Th17) cells in the intestinal lamina propria and the central nervous system, and caused severe EAE. Therefore, enhancement of Th17 cell‐mediated inflammation by SFB may exacerbate EAE.52 These studies indicate a possible mechanism by which the gut microbiota can promote the progression of aging related diseases by affecting the immune system.
Comparison of germ‐free and colonized mice revealed that colony colonization had a profound effect on the formation of lymphoid tissue and subsequent development of the immune system.53 Thus, the microbiota affects the immune system. On one hand, the microbiota affects the development of myeloid cells.54 The level of myeloid production is related to the complexity of the gut microbiota and mediated by the level of TLR ligands present in the serum.55 Microbial‐derived short‐chain fatty acids (SCFA) can promote hematopoiesis in the bone marrow.54 However, the signals produced by the microbiota do not seem to affect the lymphopoiesis of innate lymphoid cells (ILCs), as these cells develop normally in the absence of microbiota.56 On the other hand, the microbiota affects the maturation and function of myeloid cells56, 57 and innate lymphoid cells.56, 57 The persistence of microbiota‐derived TLR ligands drives neutrophil senescence57 and affects the number of basophils in the circulation.58 SCFAs such as acetate and propionate produced by Bacteroides fermented polysaccharides and butyrate produced by thick‐walled fungi can promote the integrity and function of intestinal epithelium.15, 59 SCFAs can also inhibit the production of proinflammatory cytokines by macrophages and neutrophils,60 promote the function of microglia,61 inhibit the maturation of dendritic cells and macrophages, promote the production of antibodies by B cells,62 and promote the differentiation and expansion of T regulatory cells (Tregs).63 The gut microbiota decomposes dietary tryptophan to produce indole derivatives, which in turn activate the promiscuous nuclear receptor subfamily, 1 group, I member 2 (NR1I2) and the aryl hydrocarbon receptor (AhR).64 Activation of ANR promotes the maintenance of ILC3 cells, which enhance the integrity of the intestinal mucosa by secreting IL‐22.65 Activation of NR1I2 also enhances epithelial barrier function.64, 66 Polyamine, a derivative of arginine produced and secreted by gut bacteria inhibits expression of pro‐inflammatory cytokines by LPS‐induced monocytes and macrophages. Primary bile acids and secondary bile acids reduce the expression of proinflammatory cytokines in monocytes, macrophages, dendritic cells and liver macrophages.67 Taurine produced decomposition of primary bile acids by the gut microbiota maintains and enhances epithelial barrier function by promoting IL‐18 production.68, 69 ATP secreted by the gut microbiota limits the number of follicular helper T cell (Tfh) cells in Peyer's patches, and thereby reduces B‐cell secretion of bacteria‐specific IgA across the intestinal epithelium.70 ATP also promotes the differentiation of TH17 cells in the intestinal mucosa70 and may promote epithelial barrier function by activating NLRP3 inflammasomes and subsequently inducing secretion of IL‐18 by macrophages.70 Polysaccharide A (PSA) secreted by Bacteroides fragilis exerts a strong anti‐inflammatory effect by inducing secretion of IL‐10 from CD4+ T cells and changing the TH1: TH2 ratio to favour TH1.71
4.2. Immune changes regulated by the gut microbiota in elderly individuals
Changes in the gut microbiota may increase the expression of pro‐inflammatory factors and lead to chronic age‐related inflammation. For example, the permeability of the intestinal mucosa increases in the absence of sufficient SCFA production, which allows intestinal bacteria to pass through and enter the bloodstream. The pathogen‐associated molecular patterns (PAMP) of invading bacteria, such as lipopolysaccharide (LPS), bind to pattern recognition receptors (PRR) expressed by immune cells and adipocytes, and trigger the production of pro‐inflammatory cytokines to lead to widespread persistent, low‐grade inflammation.72 Moreover, changes in gut microbiota metabolites may also alter the function of immune cells in the immune system. For example, the reduction in B cell antibody production caused by a decrease in TH1 cells in the elderly may be related to reduced PSA secretion by B fragilis. Related immune changes in the elderly can lead to susceptibility and vulnerability to disease, and chronic inflammation can also accelerate aging. Notably, one study found that the weakened gut germinal center responses observed in aged mice could be improved by fecal transplantation from the same stain young mice.73 In addition, inter‐individual differences in aging of the immune system21 may explain why some elderly people have the ability to delay the occurrence of or avoid chronic diseases. The gut microbiota acts as a buffer to individual external environmental and lifestyle factors. The corresponding changes induced by external stimulus occur to accumulate the influences of these factors, thus the gut microbiota of the elderly display greater inter‐individual variations than that of younger individuals.4 The greater inter‐individual variations could be one mechanism underlying the inter‐individual differences in immunosenescence. Thus, the gut microbiota may lead to the emergence of health diversity in the elderly. The relationship between gut microbiota and immune system in the elderly is illustrated in Figure 2.
FIGURE 2.
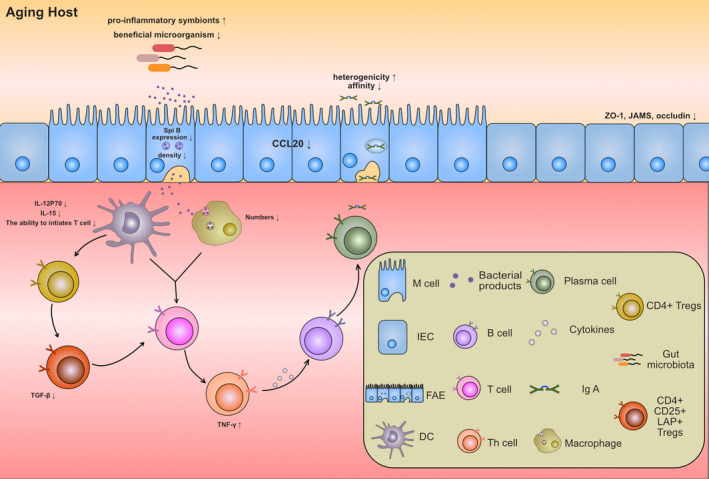
Changes of the gut microbiota and intestinal mucosal immunity in aging hosts. Proposed mechanism by which aging of the intestinal mucosal immune system leads to gut microbiota dysregulation and immune cell dysfunction. The density and antigen cross‐presentation of intestinal epithelial M cells decrease during aging, which leads to downregulation of antigen intake by dendritic cells and macrophages. Moreover, downregulation of antigen‐presenting cell activated associated cytokines and the quantity of antigen‐presenting cell restrained T cell activation, which reduces the secretion of cytokines, such as IFN‐γ. In turn, these changes reduce the affinity of IgA for antigen and increased the heterogenicity of IgA. In addition, gut microbiota dysregulation characterized by a decrease in beneficial microorganisms and increase in pro‐inflammatory symbionts also occur in the aging host
Based on the premise that the health diversity of the elderly is related to changes and differences in the gut microbiota, a recent study showed that drug treatment inhibited the gut microbiota dysbiosis and improve neuroinflammation and reduced cognitive disorders in a mouse model of Alzheimer's disease.14 In addition, transplantation of the microbiota from patients with Parkinson's disease increased motor dysfunction in a mouse model of Parkinson's mouse compared to transplantation of microbiota from healthy controls.74 Thus, the gut microbiota may influence the aging process of the immune related network. Therefore, we believe that the alterations to the gut microbiota caused by the internal and external environment are key factor in age‐related diseases. The alterations to the gut microbiota have shaped the differences in the health status of the elderly by affecting the human immune system for a long time. Thus, it is feasible that regulation of immune changes by modulating the gut microbiota may delay aging and improve the quality of life of the elderly.
5. SUMMARY AND PERSPECTIVE
Elderly individuals are susceptible to a variety of chronic diseases, such as cognitive impairment, that represent a significant socioeconomic burden. Aging‐related diseases are frequently associated with immunosenescence caused by chronic inflammation. The development of several age‐related diseases is accompanied by age‐related changes in the gut microbiota changes. The immune system also undergoes a series of changes during aging, which contribute to a chronic inflammatory state in the elderly. Aging has a significant effect on the intestinal immune system. The formation and maturation of the intestinal immune system is closely related to the gut microbiota. Therefore, changes in the gut microbiota in the elderly can affect the state of the immune system, and even accelerate immunosenescence. The occurrence of gut microbiota dysbiosis in parallel to immunosenescence may induce rapid progression of aging‐related diseases. In conclusion, as a buffered medium of internal and external factors interaction, the gut microbiota provides a reflection of the internal state of the host, especially the immune state. Thus, the gut microbiota may represent an effective biomarker of health status. As proof of causality, the gut microbiota is involved in the mechanisms underlying aging‐related diseases. Therefore, the gut microbiota can be explored as a target for intervention to improve the quality of life of the elderly and achieve the goal of healthy aging.
CONFLICTS OF INTEREST
Authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Wu Yong‐Lin completed the collection and analysis of relevant literature and drafted the manuscript as the main writer of the review. Xu Jun, Rong Xing‐Yu, Wang Feifei and Wang Hui‐Jing participate in the analysis and sorting of literature materials. Zhao Chao is the architect of the concept, and the person in charge of the project and guides the writing of the paper. All authors have read and approved the content of the manuscript.
Wu Y‐L, Xu J, Rong X‐Y, Wang F, Wang H‐J, Zhao C. Gut microbiota alterations and health status in aging adults: From correlation to causation. Aging Med. 2021;4:206–213. 10.1002/agm2.12167
Wu and Xu equally contributed to this study.
Funding information
This study was supported by the National Key Research and Development Program of China (2018YFC2000500/03, 2018ZX10101003‐005‐010). Chao Zhao is the recipient of a grant (#142016) from Fok Ying Tung Education Foundation from the Chinese Ministry of Education and an outstanding talent granted from Fudan University (2015).
REFERENCES
- 1.Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of "healthy" aging of elderly people. Immun Ageing. 2021;18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troen BR. The biology of aging. Mt Sinai J Med. 2003;70:3. [PubMed] [Google Scholar]
- 3.Burton DG. Cellular senescence, ageing and disease. Age (Dordr). 2009;31:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffery IB, Lynch DB, O'Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly JPR, Slater SL, O’Boyle N, et al. Host‐associated niche metabolism controls enteric infection through fine‐tuning the regulation of type 3 secretion. Nat Commun. 2018;9:4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullender T, Chassaing B, Janzon A, et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bian G, Gloor GB, Gong A, et al. The gut microbiota of healthy aged Chinese is similar to that of the healthy young. mSphere. 2017;2:e00327–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galkin F, Aliper A, Putin E, Kuznetsov I, Gladyshev VN, Zhavoronkov A et al. Human microbiome aging clocks based on deep learning and tandem of permutation feature importance and accumulated local effects. bioRxiv 507780 (2018).
- 9.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat med. 2016;22:713–722. [DOI] [PubMed] [Google Scholar]
- 11.Lim ES, Wang D, Holtz LR. The bacterial microbiome and virome milestones of infant development. Trends Microbiol. 2016;24:801–810. [DOI] [PubMed] [Google Scholar]
- 12.Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8:343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Sun G, Feng T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids‐shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res. 2019;29:787–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macia L, Tan J, Vieira AT, et al. Metabolite‐sensing receptors GPR43 and GPR109A facilitate dietary fibre‐induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. [DOI] [PubMed] [Google Scholar]
- 16.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. [DOI] [PubMed] [Google Scholar]
- 17.Hopkin S, Lord JM, Chimen M. Dysregulation of leukocyte trafficking in ageing: Causal factors and possible corrective therapies. Pharmacol res. 2021;163:105323. [DOI] [PubMed] [Google Scholar]
- 18.Malik A, Morya RK, Bhadada SK, Rana S. Type 1 diabetes mellitus: Complex interplay of oxidative stress, cytokines, gastrointestinal motility and small intestinal bacterial overgrowth. Eur J Clin Invest. 2018;48:e13021. [DOI] [PubMed] [Google Scholar]
- 19.Moore KJ. Targeting inflammation in CVD: advances and challenges. Nat Rev Cardiol. 2019;16:74–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Effoe VS, Correa A, Chen H, Lacy ME, Bertoni AG. High‐sensitivity C‐reactive protein is associated with incident type 2 diabetes among African Americans: The Jackson heart study. Diabetes Care. 2015;38:1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ter Horst R, Jaeger M, Smeekens SP, et al. Host and environmental factors influencing individual human cytokine responses. Cell. 2016;167:1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolich‐Zugich J. Author Correction: The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19:1146. [DOI] [PubMed] [Google Scholar]
- 23.Nikolich‐Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19:10–19. [DOI] [PubMed] [Google Scholar]
- 24.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122:128–130. [DOI] [PubMed] [Google Scholar]
- 26.Ogata K, An E, Shioi Y, et al. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol. 2001;124:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune‐metabolic viewpoint for age‐related diseases. Nat Rev Endocrinol. 2018;14:576–590. [DOI] [PubMed] [Google Scholar]
- 28.Li JY, Chassaing B, Tyagi AM, et al. Sex steroid deficiency‐associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126:2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skelly DT, Griffin ÉW, Murray CL, et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL‐1‐dependent mechanisms. Mol Psychiatry. 2019;24:1533–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye SM, Johnson RW. An age‐related decline in interleukin‐10 may contribute to the increased expression of interleukin‐6 in brain of aged mice. NeuroImmunoModulation. 2001;9:183–192. [DOI] [PubMed] [Google Scholar]
- 34.D'Avila JC, Siqueira LD, Mazeraud A, et al. Age‐related cognitive impairment is associated with long‐term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation. J Neuroinflammation. 2018;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebastiani P, Thyagarajan B, Sun F, et al. Biomarker signatures of aging. Aging Cell. 2017;16:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72:1218–1225. [DOI] [PubMed] [Google Scholar]
- 38.Mabbott NA, Kobayashi A, Sehgal A, Bradford BM, Pattison M, Donaldson DS. Aging and the mucosal immune system in the intestine. Biogerontology. 2015;16:133–145. [DOI] [PubMed] [Google Scholar]
- 39.Ren W, Wu K‐F, Li XI, et al. Age‐related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin Exp Res. 2014;26:183–191. [DOI] [PubMed] [Google Scholar]
- 40.Chan JF, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hume DA, Mabbott N, Raza S, Freeman TC. Can DCs be distinguished from macrophages by molecular signatures? Nat Immunol. 2013;14:187–189. [DOI] [PubMed] [Google Scholar]
- 42.Kato H. Lack of oral tolerance in aging is due to sequential loss of Peyer's patch cell interactions. Int Immunol. 2003;15:145–158. [DOI] [PubMed] [Google Scholar]
- 43.Kleinschmidt S, Meneses F, Nolte I, Hewicker‐Trautwein M. Distribution of mast cell subtypes and immune cell populations in canine intestines: evidence for age‐related decline in T cells and macrophages and increase of IgA‐positive plasma cells. Res Vet Sci. 2008;84:41–48. [DOI] [PubMed] [Google Scholar]
- 44.Ebisawa M, Hase K, Takahashi D, et al. CCR6hiCD11c(int) B cells promote M‐cell differentiation in Peyer's patch. Int Immunol. 2011;23:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol. 2008;181:7977–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santiago AF, Alves AC, Oliveira RP, et al. Aging correlates with reduction in regulatory‐type cytokines and T cells in the gut mucosa. Immunobiology. 2011;216:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cazac BB, Roes J. TGF‐beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. [DOI] [PubMed] [Google Scholar]
- 48.Thoreux K, Owen RL, Schmucker DL. Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology. 2000;101:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicoletti C, Yang X, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150:543–549. [PubMed] [Google Scholar]
- 50.Pinato DJ, Howlett S, Ottaviani D, et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. 2019;5:1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duc D, Vigne S, Bernier‐Latmani J, et al. Disrupting myelin‐specific Th17 cell gut homing confers protection in an adoptive transfer experimental autoimmune encephalomyelitis. Cell Rep. 2019;29:378–390. [DOI] [PubMed] [Google Scholar]
- 52.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. [DOI] [PubMed] [Google Scholar]
- 53.Torres J, Hu J, Seki A, et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ‐free mice. Gut. 2020;69:42–51. [DOI] [PubMed] [Google Scholar]
- 54.Khosravi A, Yáñez A, Price J, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balmer ML, Schürch CM, Saito Y, et al. Microbiota‐derived compounds drive steady‐state granulopoiesis via MyD88/TICAM signaling. J Immunol. 2014;193:5273–5283. [DOI] [PubMed] [Google Scholar]
- 56.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. [DOI] [PubMed] [Google Scholar]
- 57.Zhang D, Chen G, Manwani D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanagawa M, Uchida K, Ando Y, et al. Basophils activated via TLR signaling may contribute to pathophysiology of type 1 autoimmune pancreatitis. J Gastroenterol. 2018;53:449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun M, Wu W, Chen L, et al. Microbiota‐derived short‐chain fatty acids promote Th1 cell IL‐10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colombo AV, Sadler RK, Llovera G, et al. Microbiota‐derived short chain fatty acids modulate microglia and promote Abeta plaque deposition. ELIFE. 2021;10:e59826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez HN, Moroney JB, Gan H, et al. B cell‐intrinsic epigenetic modulation of antibody responses by dietary fiber‐derived short‐chain fatty acids. Nat Commun. 2020;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. [DOI] [PubMed] [Google Scholar]
- 64.Bachem A, Makhlouf C, Binger KJ, et al. Microbiota‐derived short‐chain fatty acids promote the memory potential of antigen‐activated CD8+ T cells. Immunity. 2019;51:285–297.e5. [DOI] [PubMed] [Google Scholar]
- 65.Melo‐Gonzalez F, Hepworth MR. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology. 2017;150:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garg A, Zhao A, Erickson SL, et al. Pregnane X receptor activation attenuates inflammation‐associated intestinal epithelial barrier dysfunction by inhibiting cytokine‐induced myosin light‐chain kinase expression and c‐Jun N‐terminal kinase 1/2 activation. J Pharmacol Exp Ther. 2016;359:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinot E, Sèdes L, Baptissart M, et al. Bile acids and their receptors. Mol Aspects Med. 2017;56:2–9. [DOI] [PubMed] [Google Scholar]
- 68.Kaplanski G. Interleukin‐18: Biological properties and role in disease pathogenesis. Immunol Rev. 2018;281:138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen ML, Takeda K, Sundrud MS. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol. 2019;12:851–861. [DOI] [PubMed] [Google Scholar]
- 70.Faas MM, Saez T, de Vos P . Extracellular ATP and adenosine: The Yin and Yang in immune responses? Mol Aspects Med. 2017;55:9–19. [DOI] [PubMed] [Google Scholar]
- 71.Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26:110–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonnenburg JL, Backhed F. Diet‐microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stebegg M, Silva‐Cayetano A, Innocentin S, Jenkins TP, Cantacessi C, Gilbert C. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat Commun. 2019;10:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota regulate motor deficits and neuroinflammation in a model of parkinson disease. Cell. 2016;167:1469–1480.e.12. [DOI] [PMC free article] [PubMed] [Google Scholar]


