Summary
Objectives
Skeletal myopathies are highly morbid, and in rare cases even fatal, immune-related adverse events (irAE) associated with immune checkpoint inhibitors (ICI). Skeletal myopathies are also a recognized statin-associated side effect. It is unknown whether concurrent use of statins and ICIs increases the risk of skeletal myopathies.
Methods
This was a retrospective cohort study of all patients who were treated with an ICI at a single academic institution (Massachusetts General Hospital, Boston, MA, USA). The primary outcome of interest was the development of a skeletal myopathy. The secondary outcome of interest was an elevated creatine kinase level (above the upper limit of normal).
Results
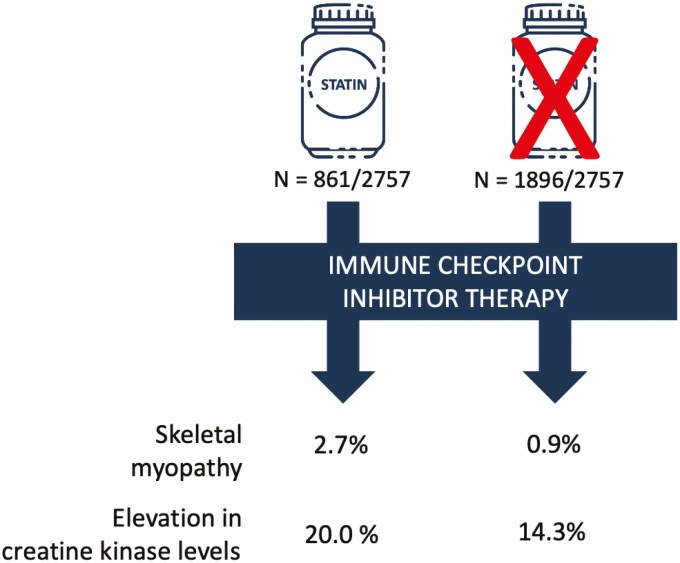
Among 2757 patients, 861 (31.2%) were treated with a statin at the time of ICI start. Statin users were older, more likely to be male and had a higher prevalence of cardiovascular and non-cardiovascular co-morbidities. During a median follow-up of 194 days (inter quartile range 65–410), a skeletal myopathy occurred in 33 patients (1.2%) and was more common among statin users (2.7 vs. 0.9%, P < 0.001). Creatine kinase (CK) elevation was present in 16.3% (114/699) and was higher among statin users (20.0 vs. 14.3%, P = 0.067). In a multivariable Cox model, statin therapy was associated with a >2-fold higher risk for skeletal myopathy (HR, 2.19; 95% confidence interval, 1.07–4.50; P = 0.033).
Conclusion
In this large cohort of ICI-treated patients, a higher risk was observed for skeletal myopathies and elevation in CK levels in patients undergoing concurrent statin therapy. Prospective observational studies are warranted to further elucidate the potential association between statin use and ICI-associated myopathies.
Keywords: skeletal myopathy, immune checkpoint inhibitors, statin therapy, immune-related adverse events
Graphical Abstract
Graphical Abstract.
Introduction
Immune checkpoint inhibitors (ICI) enhance T-cell-mediated anti-tumor activity and are being used in an expanding range of cancer types [1]. While ICIs were initially developed for use in advanced cancers refractory to other therapies, their use has expanded to adjuvant and neoadjuvant care [2–4]. In 2019, it was estimated that 36.1% of patients with cancer were eligible for ICI therapy and this number is anticipated to increase in the coming years [5].
Systemic inhibition of immune checkpoints may lead to ‘off-tumor’ immune-related adverse events (irAEs) that can affect any organ system and are reported to occur in up to 70% of patients treated with ICI in clinical trials [6]. Cancer and cardiovascular disease commonly co-exist,[7] and the use of ICIs is associated with progression of atherosclerosis,[8, 9] meaning many patients treated with ICIs are also likely to be concomitantly prescribed a statin [10]. Skeletal myopathies and muscle symptoms are well-recognized side-effects of statins and occur on a spectrum ranging in severity from myalgias (3%) to the occasional occurrence of rhabdomyolysis and immune-mediated necrotizing myopathy [7, 10]. In parallel, skeletal myopathies with ICIs are uncommon, accounting for 0.57% of reported irAEs, but are associated with a fatality rate up to 21% [11]. Whether concomitant statin use is associated with an increase in ICI-associated muscular adverse events including myositis and non-immune myopathy is unknown. Given that one in four US adults older than 40 years of age is currently prescribed a statin,[12] and the use of ICI is expected to increase over time, we sought to address this important evidence gap.
Methods
This was a retrospective cohort study of all patients who were treated with an ICI at a single academic institution (Massachusetts General Hospital, Boston, MA, USA) between July 2010 and March 2019. Patients treated with an ICI were identified using the hospital pharmacy database. Clinical data and laboratory parameters were derived from the Research Patient Data Registry. Only patients for whom prior medication data were available in the registry dataset were included in this study. The institutional review board approved the study, and the requirement for informed consent was waived.
Baseline statin therapy was defined as statin use prior to or at the time of first ICI initiation. Statin type and dose were recorded and the intensity of statin therapy was defined according to American Heart Association/American College of Cardiology guidelines (Supplementary Table 1) [13].
Study endpoints
The primary outcome of interest was the occurrence of any type of skeletal myopathy during ICI therapy, either of the inflammatory or non-inflammatory type. An inflammatory myopathy was defined based on the presence of muscle pain or weakness, accompanied by either a positive muscular biopsy, a diagnostic magnetic resonance or computed tomography imaging test or an abnormal electromyography study [14]. A non-inflammatory myopathy was defined as new muscle pain or weakness without a positive diagnostic test. We also tested the association between statin use and the occurrence of an elevated serum creatine kinase (CK) concentration (above the upper limit of normal), classified per the modified statin-related myotoxicity phenotype classification [15]. The association between statin intensity, lipophilicity and the occurrence of a skeletal myopathy or elevated serum CK level was also evaluated. Additional outcomes of interest were the occurrence of other irAEs including elevated liver function tests (LFTs) (transaminases at least three-fold over the upper limit of normal), pneumonitis, colitis, nephritis, dermatitis, and endocrinopathies, as per standard definitions [16].
Statistical analysis
Baseline characteristics are presented as continuous variables and summarized as either mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables are summarized as counts and percentages. Differences in categorical variables are assessed using either the chi-square test or Fisher’s exact test. Study outcomes in patients who were and were not taking statins at the time of initiation of ICI therapy were compared. Logistic regression was performed to calculate odds ratios (OR) with 95% CIs. Cox proportional hazard regression analysis was performed to calculate hazard ratios (HR) with 95% CIs, counting only the first event for each outcome. Clinically relevant unique predictor variables with a value of P < 0.10 in univariate analysis were entered into the final multivariable model; a forward stepwise selection was used. The incremental value between steps was measured by the likelihood-ratio test. As a second approach, parsimonious multivariable Cox proportional hazard models were also performed, that included age, hypertension or diabetes, and statin use. The proportional hazard assumption was tested with the use of log-log plots and examination of Schoenfeld residuals. All statistical tests were two-sided and 5% was set as the level of significance. Statistical analysis was performed using RStudio Version 1.1 (RStudio, Inc., Vienna, Austria) [17] and STATA software, version 15.1 (StataCorp, College Station, TX).
Results
Baseline characteristics
Of 2854 patients treated with an ICI during the study period, baseline statin prescription data was available in 2757 patients (96.6%), of whom 861 (31.2%) were prescribed a statin and 1896 (68.8%) were not. Baseline characteristics are shown in Table 1. Patients who were prescribed a statin were older, male and had a higher rate of cardiovascular risk factors, established cardiovascular and non-cardiovascular co-morbidities and the use of cardiovascular medications was more common.
Table 1.
Baseline characteristics of patients treated with immune checkpoint inhibitors
| On statin therapy | No statin therapy | ||||
|---|---|---|---|---|---|
| Demographic | P | ||||
| Number of Patients | 861 | 1896 | |||
| Sex – no. (%) | |||||
| Male | 571/861 | (66.3) | 1001/1896 | (52.8) | <0.001 |
| Female | 290/861 | (33.7) | 869/1896 | (47.2) | <0.001 |
| Age – years mean (SD) | 70 | (9.6) | 61 | (13.6) | <0.001 |
| Age – years, median (IQR) | 70 | (63–76) | 63 | (54–71) | <0.001 |
| Race or ethnic group – no. (%) | 0.07 | ||||
| White | 788/844 | (93.4) | 1673/1842 | (90.8) | |
| Asian | 19/844 | (2.3) | 77/1842 | (4.2) | |
| Black or African American | 18/844 | (2.1) | 39/1842 | (2.1) | |
| Hispanic | 7/844 | (0.8) | 22/1842 | (1.2) | |
| Other | 12/844 | (1.4) | 31/1842 | (1.7) | |
| Clinical parameters – mean. (SD) | |||||
| Body mass index - (kg/m2) | 28.1 | (5.8) | 26.4 | (5.6) | <0.001 |
| Systolic blood pressure (mmHg) | 129.3 | (18.9) | 126.7 | (18.3) | 0.002 |
| Diastolic blood pressure (mmHg) | 74.5 | (9.6) | 76.3 | (9.7) | <0.001 |
| Cardiovascular risk factors – no (%) | |||||
| Hypertension | 599/857 | (69.9) | 755/1892 | (39.9) | <0.001 |
| Diabetes | 245/857 | (28.6) | 187/1892 | (9.9) | <0.001 |
| Smoking current or prior | 163/857 | (19.0) | 266/1892 | (14.1) | 0.001 |
| Cardiovascular diagnoses – no (%) | |||||
| History of myocardial infarction | 91/862 | (10.6) | 41/1896 | (2.2) | <0.001 |
| History of ischemic stroke | 44/861 | (5.1) | 37/1896 | (2.0) | <0.001 |
| History of transient ischemic attack | 25/861 | (2.9) | 15/1896 | (0.8) | <0.001 |
| History of coronary revascularization | 123/861 | (14.2) | 60/1896 | (3.2) | <0.001 |
| History of atrial fibrillation or atrial flutter | 134/857 | (15.6) | 159/1892 | (8.4) | <0.001 |
| History of heart failure | 178/857 | (20.8) | 155/1892 | (8.2) | <0.001 |
| Cardiovascular medications – no. (%) | |||||
| Angiotensin converting enzyme inhibitor or angiotensin II receptor blocker | 350/848 | (41.3) | 262/1855 | (14.1) | <0.001 |
| Anti-arrhythmic | 57/848 | (6.7) | 57/1855 | (3.1) | <0.001 |
| Beta-blockers | 365/848 | (43.0) | 263/1855 | (14.2) | <0.001 |
| Calcium channel blockers | 201/848 | (23.7) | 195/1855 | (10.5) | <0.001 |
| Nitrates | 72/848 | (8.5) | 25/1855 | (1.3) | <0.001 |
| Loop diuretics | 322/848 | (38.0) | 349/1855 | (18.8) | <0.001 |
| Non-statin dyslipidemia therapies | 37/848 | (4.4) | 28/1855 | (1.5) | <0.001 |
| Aspirin | 370/848 | (43.6) | 208/1855 | (11.2) | <0.001 |
| Other anti-platelet therapies | 59/848 | (7.0) | 7/1855 | (0.4) | <0.001 |
| Low molecular weight heparin | 219/848 | (25.8) | 452/1855 | (24.4) | 0.44 |
| Direct oral anticoagulants | 37/848 | (4.4) | 41/1855 | (2.2) | 0.003 |
| Warfarin | 62/848 | (7.3) | 45/1855 | (2.4) | <0.001 |
| Statin intensity | |||||
| Low intensity | 111/856 | (13.0) | |||
| Moderate intensity | 510/856 | (59.6) | |||
| High intensity | 235/856 | (27.5) | |||
| Statin type | |||||
| Hydrophilic | 171/861 | (19.9) | |||
| Lipophilic | 690/861 | (80.1) | |||
| Other medical comorbidities – no (%) | |||||
| Chronic obstructive pulmonary disease | 130/857 | (15.2) | 155/1893 | (8.2) | <0.001 |
| Chronic kidney disease | 158/857 | (18.4) | 169/1893 | (8.9) | <0.001 |
| Cancer types – no. (%) | <0.001 | ||||
| Non-small cell lung | 300/859 | (34.9) | 480/1889 | (25.4) | <0.001 |
| Melanoma | 251/859 | (29.2) | 525/1889 | (27.8) | 0.47 |
| Head and neck | 103/859 | (12.0) | 229/1889 | (12.1) | 1.0 |
| Renal and genitourinary | 68/859 | (7.9) | 106/1889 | (5.6) | 0.027 |
| Breast | 31/859 | (3.6) | 88/1889 | (4.7) | 0.25 |
| Gastrointestinal | 18/859 | (2.1) | 90/1889 | (4.8) | 0.001 |
| Gynecologic | 15/859 | (1.7) | 92/1889 | (4.9) | <0.001 |
| Lymphoma | 18/859 | (2.1) | 80/1889 | (4.2) | 0.007 |
| Hepatocellular | 13/589 | (1.5) | 45/1889 | (2.4) | 0.19 |
| Cholangiocarcinoma | 9/859 | (1.0) | 30/1889 | (1.6) | 0.35 |
| Pancreatic | 4/859 | (0.5) | 33/1889 | (1.7) | 0.012 |
| Other | 29/859 | (3.4) | 91/1889 | (4.8) | 0.11 |
| Prior potentially cardiotoxic cancer therapies – no. (%) | |||||
| Radiation therapy | 187/857 | (21.8) | 384/1892 | (20.3) | 0.39 |
| Fluorouracil | 81/853 | (9.5) | 203/1866 | (10.9) | 0.30 |
| Anthracyclines | 35/853 | (4.1) | 116/1866 | (6.2) | 0.032 |
| Tyrosine kinase inhibitors | 19/853 | (2.2) | 42/1866 | (2.3) | 1.0 |
| Mitogen-activated protein kinase kinase 1 inhibitors | 13/853 | (1.5) | 31/1866 | (1.7) | 0.92 |
| Immune checkpoint inhibitor type – no. (%) | <0.001 | ||||
| Monotherapy | |||||
| Programmed death-ligand-1 | 71/861 | (8.2) | 208/1896 | (11.0) | |
| Cytotoxic-T-lymphocyte associated protein 4 | 82/861 | (9.5) | 136/1896 | (7.2) | |
| Programmed death-protein 1 | 669/861 | (77.7) | 1397/1896 | (73.6) | |
| Cytotoxic-T-lymphocyte associated protein 4 or programmed death protein 1 | 0/861 | (0) | 1/1896 | (0.1) | |
| Combination therapy | |||||
| Cytotoxic-T-lymphocyte associated protein 4/Programmed death protein 1 | 39/861 | (4.5) | 154/1895 | (8.1) | |
| Number of cycles of ICI – no, (IQR) | 5 | (2–11) | 5 | (2–11) | 0.56 |
| Steroid treatment at start of ICI | 126/741 | (17.0) | 291/1600 | (18.2) | 0.52 |
| Follow up time – days, (IQR) | 192 | (64–428) | 195 | (66–405) | 0.51 |
Statin use
Statin intensity data were available in 855 (99.3%) of the 861 patients who were prescribed statins. Most patients were on moderate intensity therapy (n = 509, 59.5%). Among those prescribed statins, the majority were on lipophilic statins (n = 690, 80.1%; Table 1).
Statins and skeletal myopathy
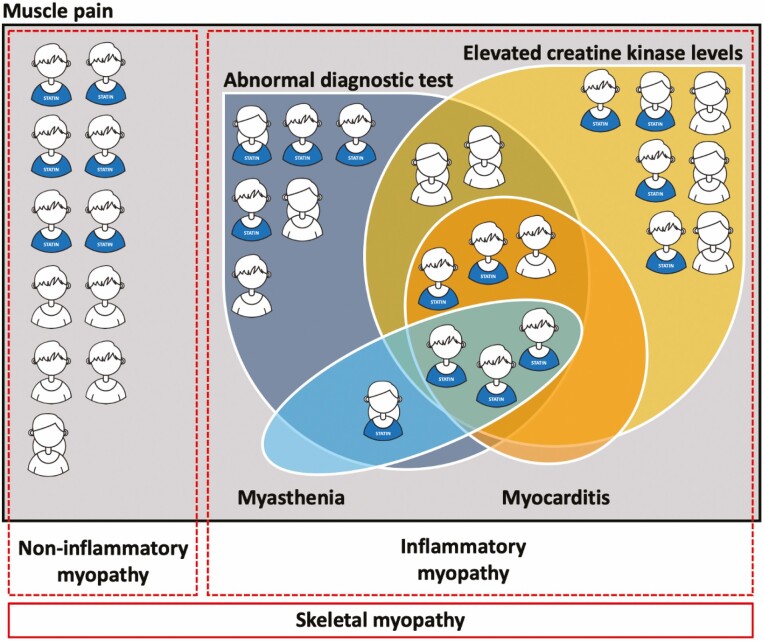
Any skeletal myopathy occurred in 33 patients (1.2%). The median time to development of skeletal myopathy was 119 (IQR, 35–217) days. In 22 patients, an inflammatory myopathy was diagnosed and in 11 patients the diagnosis was a non-inflammatory myopathy (Fig. 1). Out of the 22 patients with inflammatory myopathy, 11 cases were also confirmed by a neurologist.
Figure 1.
Venn diagram shows the overlap of muscle pain, abnormal diagnostic test, elevated creatine kinase levels (above the upper limit of normal), myocarditis, and myasthenia in patients with a skeletal myopathy.
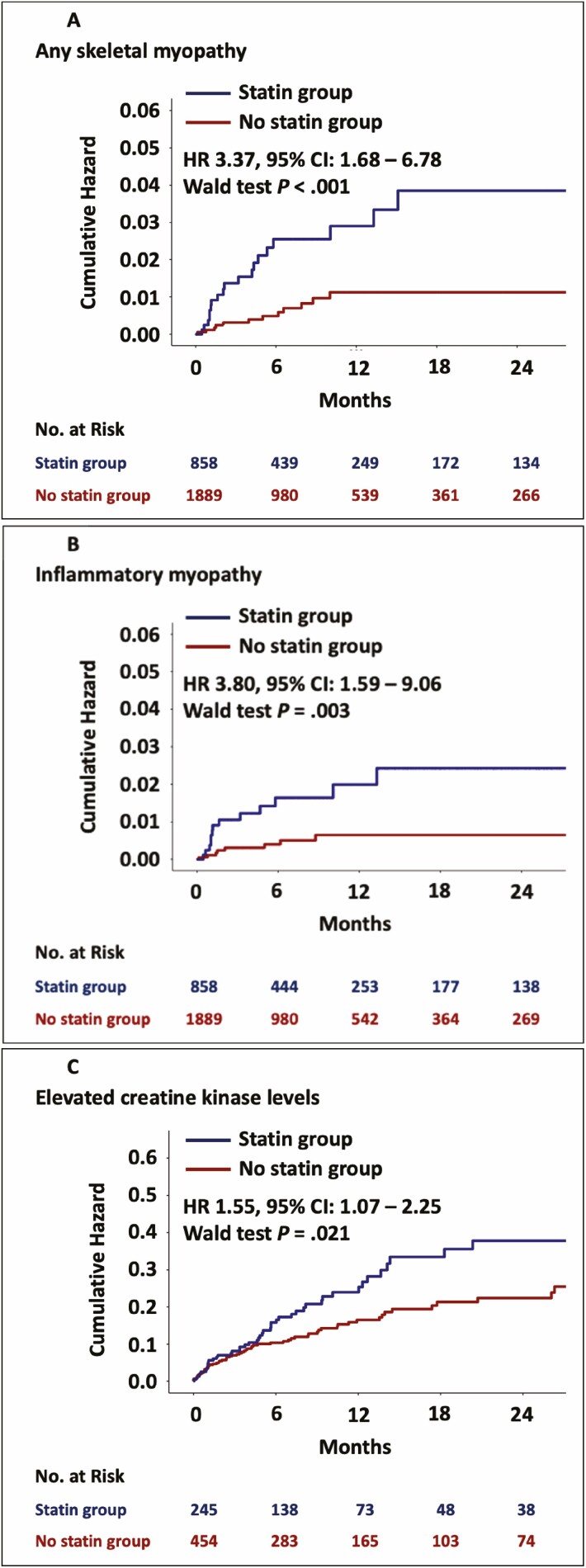
When patients with a skeletal myopathy were stratified by statin use, a skeletal myopathy was more common among patients prescribed a statin (2.3%, 20/858) as compared to those who were not (0.7%, 13/1889, P < 0.001; Table 2). There was no association between statin intensity and a myopathy. The occurrence of skeletal myopathy was also not different between lipophilic and hydrophilic statins (Table 2). In univariate Cox hazard analyses, statins were associated with a >3-fold increase in the risk for any skeletal myopathy (univariate HR, 3.37; 95% CI, 1.68–6.78; P < 0.001; Table 3, Fig. 2).
Table 2.
Myopathies and elevation in creatine kinase levels in patients treated with immune checkpoint inhibitors, based on statin therapy, statin intensity, and the type of statin (lipophilic or hydrophilic)
| On statin (n = 861) |
No statin (n = 1897) | Low intensity (n = 111/855) |
Moderate intensity (n = 509/855) |
High intensity (n = 235/855) |
Lipophilic (n = 690/861) |
Hydrophilic (n = 171/861) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any skeletal myopathy |
20/858 | (2.3) | 13/1889 | (0.7) | 3/111 | (2.7) | 12/507 | (2.4) | 5/235 | (2.1) | 17/687 | (2.5) | 3/171 | (1.8) |
| P < 0.001 | P = 0.95 | P = 0.78 | ||||||||||||
| Inflammatory myopathy | 14/858 | (1.6) | 8/1889 | (0.4) | 2/111 | (1.8) | 8/507 | (1.6) | 4/235 | (1.7) | 13/687 | (1.9) | 1/171 | (0.6) |
| P = 0.002 | P = 0.98 | P = 0.38 | ||||||||||||
| Elevation in creatine kinase levels | 49/245 | (20.0) | 65/454 | (14.3) | 7/38 | (18.4) | 30/134 | (22.4) | 11/72 | (15.3) | 43/199 | (21.6) | 6/46 | (13.0) |
| P = 0.067 | P = 0.46 | P = 0.26 | ||||||||||||
| Grade 1 | 33/245 | (13.5) | 48/454 | (10.6) | 4/38 | (10.5) | 21/134 | (15.7) | 7/72 | (9.7) | 30/199 | (15.1) | 3/46 | (6.5) |
| Grade 2 | 12/245 | (4.9) | 10/454 | (2.2) | 3/38 | (7.9) | 6/134 | (4.5) | 3/72 | (4.2) | 9/199 | (4.5) | 3/46 | (6.5) |
| Grade 3 | 4/245 | (1.6) | 7/454 | (1.5) | 3/134 | (2.2) | 1/72 | (1.4) | 4/199 | (2.0) | ||||
| P = 0.15 | P = 0.74 | P = 0.31 |
Table 3.
Univariate and stepwise forward selection multivariate Cox proportional hazard model results
| Univariate models | Multivariate model | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | Wald test P value | Hazard ratio | 95 % CI | Wald test P value | |||
| Outcome: Any skeletal myopathy | ||||||||
| Demographic variables | ||||||||
| Female sex | 0.56 | 0.27 | 1.18 | 0.13 | ||||
| Age | 1.03 | 1.00 | 1.06 | 0.030 | ||||
| Body mass index | 1.06 | 1.01 | 1.12 | 0.021 | ||||
| Systolic blood pressure | 1.02 | 1.00 | 1.04 | 0.035 | ||||
| Cardiovascular Risk Factors | ||||||||
| Hypertension | 6.45 | 2.49 | 16.72 | < 0.001 | 5.32 | 1.99 | 14.21 | 0.001 |
| Diabetes mellitus | 3.00 | 1.45 | 6.19 | 0.003 | ||||
| Smoking (current or prior) | 1.14 | 0.44 | 2.95 | 0.79 | ||||
| Medications | ||||||||
| Statin therapy | 3.37 | 1.68 | 6.78 | 0.001 | 2.19 | 1.07 | 4.50 | 0.033 |
| Cancer types | ||||||||
| Melanoma | 2.95 | 1.46 | 5.99 | 0.003 | 3.11 | 1.53 | 6.31 | 0.002 |
| Laboratory variables | ||||||||
| Glomerular filtration rate (mL/min/1.73m2) | 0.99 | 0.97 | 1.02 | 0.57 | ||||
| Outcome: Inflammatory myopathy | ||||||||
| Demographic variables | ||||||||
| Female sex | 0.90 | 0.38 | 2.10 | 0.80 | ||||
| Age | 1.05 | 1.01 | 1.09 | 0.020 | ||||
| Body mass index | 1.06 | 1.00 | 1.13 | 0.056 | ||||
| Systolic blood pressure | 1.02 | 1.00 | 1.05 | 0.038 | ||||
| Cardiovascular Risk Factors | ||||||||
| Hypertension | 7.14 | 2.11 | 24.15 | 0.002 | 5.41 | 1.55 | 18.90 | 0.008 |
| Diabetes mellitus | 3.36 | 1.41 | 8.01 | 0.006 | ||||
| Smoking current or prior | 1.36 | 0.46 | 4.03 | 0.58 | ||||
| Medications | ||||||||
| Statin therapy | 3.80 | 1.59 | 9.06 | 0.003 | 2.51 | 1.03 | 6.12 | 0.043 |
| Cancer types | ||||||||
| Melanoma | 2.91 | 1.23 | 6.89 | 0.015 | ||||
| Laboratory variables | ||||||||
| Glomerular filtration rate (ml/min/1.73 m2) | 0.99 | 0.97 | 1.02 | 0.61 | ||||
| Outcome: Elevation in creatine kinase levels | ||||||||
| Demographic variables | ||||||||
| Female sex | 1.51 | 1.04 | 2.18 | 0.031 | 1.67 | 1.15 | 2.43 | 0.007 |
| Age | 1.02 | 1.01 | 1.04 | 0.008 | 1.02 | 1.01 | 1.04 | 0.006 |
| Body mass index | 1.00 | 0.96 | 1.04 | 1.00 | ||||
| Systolic blood pressure | 1.00 | 0.99 | 1.02 | 0.37 | ||||
| Cardiovascular Risk Factors | ||||||||
| Hypertension | 1.46 | 1.00 | 2.13 | 0.048 | ||||
| Diabetes mellitus | 1.68 | 1.08 | 2.63 | 0.022 | 1.69 | 1.08 | 2.64 | 0.023 |
| Smoking current or prior | 1.08 | 0.66 | 1.77 | 0.76 | ||||
| Medications | ||||||||
| Statin therapy | 1.55 | 1.07 | 2.25 | 0.021 | ||||
| Cancer types | ||||||||
| Melanoma | 0.87 | 0.59 | 1.29 | 0.48 | ||||
| Laboratory variables | ||||||||
| Glomerular filtration rate (mL/min/1.73m2) | 1.00 | 0.99 | 1.01 | 0.66 |
Figure 2.
Kaplan–Meier curves of the cumulative hazard in statin users and non-users and number at risk tables. Panel A shows the cumulative hazard of any skeletal myopathy. Panel B shows the cumulative hazard of inflammatory myopathy and panel C shows the cumulative hazard of creatine kinase elevation.
Other predictors of a skeletal myopathy included older age, higher body mass index, higher systolic blood pressure, hypertension, diabetes mellitus, and melanoma. In multivariable Cox hazard models, statin therapy was associated with a >2-fold higher risk for skeletal myopathy (including hypertension and melanoma, HR, 2.19; 95% CI, 1.07–4.50; P = 0.033, Table 3), (including age and diabetes, HR, 2.50; 95% CI, 1.18–5.32; P = 0.017, Table 4), (including age and hypertension, HR, 2.18; 95% CI, 1.05–4.53; P = 0.037, Table 4).
Table 4.
Parsimonious multivariate Cox proportional hazard model results
| Model 1. | Model 2. | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | Wald test P value | Hazard ratio | 95% CI | Wald test P value | |||
| Outcome: Any skeletal myopathy | ||||||||
| Demographic variables | ||||||||
| Age | 1.01 | 0.97 | 1.04 | 0.678 | 1.02 | 0.99 | 1.05 | 0.273 |
| Cardiovascular Risk Factors | ||||||||
| Hypertension | 4.87 | 1.80 | 13.16 | 0.002 | ||||
| Diabetes mellitus | 2.10 | 0.99 | 4.45 | 0.054 | ||||
| Medications | ||||||||
| Statin therapy | 2.18 | 1.05 | 4.53 | 0.037 | 2.50 | 1.18 | 5.32 | 0.017 |
| Outcome: Inflammatory myopathy | ||||||||
| Demographic variables | ||||||||
| Age | 1.03 | 0.99 | 1.08 | 0.121 | ||||
| Cardiovascular Risk Factors | ||||||||
| Diabetes mellitus | 3.01 | 1.22 | 7.42 | 0.017 | 2.35 | 0.95 | 5.81 | 0.065 |
| Medications | ||||||||
| Statin therapy | 3.13 | 1.26 | 7.73 | 0.014 |
We also tested the association between statin use and the development of an inflammatory myopathy alone. In total, an inflammatory myopathy occurred in 22 (0.8%) patients and was more common among patients prescribed a statin (1.6 vs. 0.4%, P = 0.002) (Table 2). The median time to development of an inflammatory myopathy was 47 (IQR, 30–177) days. There was no association between the occurrence of an inflammatory myopathy and intensity of statin therapy nor with statin type (Table 2). In univariate Cox hazard analyses, statins were associated with a higher risk of an inflammatory myopathy (univariate HR, 3.80; 95% CI, 1.59–9.06; P = 0.003; Table 3, Fig. 2). Other predictors of an inflammatory myopathy included older age, higher systolic blood pressure, hypertension, diabetes mellitus, and melanoma. In multivariable Cox hazard models, statin therapy was associated with a >2.5-fold higher risk for inflammatory myopathy (including hypertension, HR, 2.51; 95% CI, 1.03–6.12; P = 0.043, Table 3), (including age, HR, 3.01; 95% CI, 1.22–7.42; P = 0.017, Table 4), (including diabetes mellitus, HR, 3.13; 95% CI, 1.26–7.73; P = 0.014, Table 4).
Statins and elevation in creatine kinase levels
During follow-up those patients who were on a statin therapy, were more likely to have at least one lab test with a CK level obtained (28.5%, 245/861) as compared to those who were not on a baseline statin (23.9%, 454/1897, P = 0.013). CK levels were available in 25.4% (699/2757) patients after ICI start. However, in the subset of patients with at least one CK drawn during follow-up, the number of CK results per patient was not different between the two groups (median 2 [IQR, 1–4] vs. median 1 [IQR, 1–3], P = 0.11). An elevated CK (above the upper limit of normal) occurred in 16.3% (114/699) patients. The median time to CK elevation was 124 (IQR, 32–281) days. The prevalence of elevated CK was highest in hepatocellular (27.3%) and gynecological (26.7%) cancers (Supplementary Table 2A). The median CK elevation was 1.6 (1.3–3.1) times the upper limit of normal. An elevation in CK after initiation of ICI therapy was more common among patients who were on a statin at the time of ICI initiation (20.0% vs. 14.3%, univariate HR, 1.55; 95% CI 1.07–2.25 P = 0.021, Table 3). In univariate Cox hazard analyses, statins were associated with a higher risk of an elevation in CK levels (univariate HR1.55; 95% CI, 1.07–2.25; P = 0.021; Table 3, Fig. 2). Other predictors of an elevation in CK levels included female sex, age, hypertension, and diabetes mellitus. In a multivariable Cox hazard model statin showed collinearity with age, therefore age was not included in the final multivariable model. There was no association between the occurrence of CK elevation and the intensity of statin therapy nor with statin type (Table 2). No significant difference was observed in the grades of CK elevation when compared between statin intensity or lipophilicity.
Statins and other irAEs
Immune-related adverse events
During a median follow-up of 194 days (IQR, 65–410), a total of 1178 (42.9%) patients developed any irAE (including skeletal myopathies) following treatment with ICI. The rate of any irAEs was marginally higher among patients who were prescribed a statin as compared to those who were not but this difference was not statistically significant (45.3% [389/858] vs. 41.8% [789/1889], P = 0.087) (univariate OR, 1.15; 95% CI 0.98–1.36 P = 0.080). Corticosteroids were required in 64.9% (734/1131) and the need for corticosteroids was more common among those prescribed a statin (71.2% [267/375] vs. 61.7% [467/756], P = 0.002) (Supplementary Table 3). The rate of individual irAEs was also compared between groups and was similar, apart from renal irAEs and the rate of elevation in liver function tests. Renal irAEs were more common in patients who were prescribed a statin (5.7 vs. 3.8%, P = 0.026, univariate OR, 1.55; 95% CI 1.06–2.25; P = 0.021) (Supplementary Table 3). However, those on a statin also had a higher prevalence of chronic kidney disease (18.4 vs. 8.9%, P < 0.001) and a lower baseline eGFR (61.9 ± 22.4 vs. 71.2±24.1 ml/min/1.73 m2, P < 0.001 (Supplementary Table 4).
In converse, elevation in liver function tests (transaminases at least 3-fold over the upper limit of normal), (22.5 vs. 26.5%, P = 0.033) were lower in patients who were prescribed a statin, and in whom there was a lower proportion of Grade 3 (5–20 times over the upper limit of normal) and Grade 4 (>20-fold over the upper limit of normal) LFT elevation (11.3 vs. 12.2% and 1.3 vs. 2.9%, P = 0.047, Supplementary Table 5A) However, when analyzed by cancer type, the elevation in LFTs was most common in patients with hepatocellular carcinoma (60.3%), pancreatic cancer (54.3%), and cholangiocarcinoma (47.4%) (Supplementary Table 2B). After excluding patients with these three cancer types, the occurrence of elevated LFTs (21.6 vs. 24.7%, P = 0.10) and the distribution of grades was similar between those who were and were not on a statin (Supplementary Table 5B).
Discussion
In this large retrospective cohort study, we observed that patients who were concomitantly prescribed a statin during ICI therapy had a higher risk of a skeletal myopathy as compared to patients who were not on statins. More specifically, statins were associated with a >2-fold higher risk of developing an inflammatory or non-inflammatory skeletal myopathy, a 2.5-fold higher risk of an inflammatory myopathy, and a higher risk of elevation in CK levels. These associations did not appear to be related to statin intensity and were similar between lipophilic and hydrophilic statins.
Data are evolving on the association between ICI use and the development of skeletal muscle toxicity. In large clinical trials, skeletal myopathies have been reported with a low incidence (≤1%) [18]. However, similar to other serious ICI-associated toxicities,[19–24] it is likely that skeletal myopathies with ICIs are underreported in clinical trials [25]. Furthermore, there are no standardized definitions for clinical myopathies in the setting of ICIs. Additionally, trials often do not provide any clinical descriptions of skeletal irAEs or may only report higher grade and frequent events (occurring in ≥10% of the patients). In contrast, retrospective and prospective observational data demonstrate a higher incidence of skeletal muscle and joint-related irAEs [25, 26]. Treatment with an ICI may be associated with an either inflammatory or a non-inflammatory myopathy [27]. The development of an inflammatory myopathy is an uncommon toxicity associated with ICI use that may present in isolation or with concomitant myocarditis or myasthenia-like ocular involvement, and is associated with significant morbidity and mortality [11, 28-30]. In our cohort, out of the 22 patients with inflammatory myopathy, 9 patients had either or both concomitant myocarditis or myasthenia, with 66.6% of them on statin therapy.
The risk factors for development of ICI-associated muscle pain and myositis remain poorly defined. Limited data suggest that ICI-associated muscle symptoms and an inflammatory myopathy is more common with combination ICIs [31]. However, beyond combination therapy, no other risk factors have been identified. In our study, we noted that the following were risk factors for the development of an ICI-associated skeletal myopathy; older age, male sex, hypertension, diabetes, and melanoma. Moreover, an increase in body mass index or systolic blood pressure was also associated with increased risk for an ICI-associated skeletal myopathy. In the largest report of ICI-associated myositis (n = 180), the mortality rate was 21.2% and significant morbidity including prolonged hospitalization, life-threatening illness, or disability affected a further 49.4% [11]. Precise timing to myositis onset was available in 61 patients, with a median time to onset of myositis of 26 days (IQR, 18–39 days) after initiation of ICI therapy, and a total of 25 (13.9%) patients had been treated with statins. We found that the median time to development of skeletal myopathy was 119 (IQR, 35–217) days and the difference may relate to the lower rates of late reporting in pharmacovigilance studies and our median time to onset is consistent with smaller case series [32, 33].
Our observation that incidental statin use was associated with a skeletal myopathy has potential implications. The most common reason that patients on an ICI are prescribed a statin is due to the overlapping presence of cardiovascular disease or risk factors [34, 35]. Thus, our finding of an association between statin use and muscle toxicities with an ICI,[11] may lead to discussions about statins among patients who develop a muscle toxicity on an ICI. In those, the indication for statin should be reviewed and alternative highly effective non-statin therapies (e.g. PCSK-9 inhibitors, ezetimibe, bempedoic acid, icosapent ethyl) could be considered. Additionally, in recent work, the use of an ICI was associated with a 3-fold increase in the progression of atherosclerosis and an increase in atherosclerotic cardiovascular events [8]. In that study, statin use was associated with a 50% lower rate of atherosclerosis progression as compared to non-statin patients [8]. This finding may lead to subsequent studies testing the value of statin use in statin naive patients being treated with an ICI.
Given that ICI-associated elevated transaminases occur in up to 17% of patients and accounts for up to 22% of all irAE-related deaths,[36, 37] our observation that statins do not appear to increase the rate of LFT elevation is important. We did observe that statins were associated with higher rates of renal irAEs, although chronic kidney disease was more prevalent and eGFR was lower at baseline in statin-treated patients and these are recognized risk factors for development of renal irAEs [38, 39]. Case studies have reported an association between statins and acute tubulointerstitial nephritis [40–42]. Whether there may be a synergistic mechanistic effect that increases the risk of renal irAEs in patients co-treated with statins and ICIs remains unclear. There was also a trend toward a higher rate of total irAEs in patients who were prescribed statins, driven by significant increases in muscular and renal irAEs and non-significant increases in gastrointestinal (19.6% vs. 17.6%), dermatologic (16.8% vs. 15.1%) and pulmonary (7.9% vs. 6.4%) irAEs, and irAEs among statin users more frequently required treatment with steroids.
This report needs to be interpreted in the context of the study design. This study was not designed to provide mechanistic insight. This was retrospective and single center in design; however, our cohort of patients on ICI therapy is significantly larger than any other publication. Patients on statins at the time of ICI therapy had more comorbidities, and due to collinearity, we were only able to adjust for a few comorbidities. Therefore, residual cardiovascular risk factors that determine statin use may also explain the increase in skeletal myopathy. The aim of the report was to evaluate the association between statin use at the time of ICI start and the development of muscle toxicity. Therefore, we did not evaluate subsequent treatment decisions and whether statins were discontinued after the diagnosis of an inflammatory or non-inflammatory myopathy. Additionally, there is a lack of standardized diagnostic criteria for ICI-associated muscle syndromes among published report. This lack of standardization has impacted both clinical trials reporting cancer outcomes on ICIs and clinical research focused on less common toxicities such as reported here. We used a broad definition for inflammatory myopathy, and we did not differentiate between presumptive, definite or highly probable myopathies. Therefore, some of the myopathies may have been misclassified. Moreover, not all the myopathy patients had their diagnosis confirmed by muscle biopsy. In future studies muscle biopsy may be routinely considered to confirm the diagnosis of myopathy and a detailed and standardized definition applied. Moreover, some of the patients with inflammatory myopathies may have paraneoplastic syndromes and distinguishing these syndromes from statin or ICI myopathy can be challenging. Moreover, LFTs are often elevated in myopathy, and we did not differentiate between skeletal and hepatobiliary origins of LFT elevations.
Conclusion
In this large retrospective cohort study of patients treated with an ICI, the risk for a skeletal myopathy and for an elevation in CK was higher in patients who were concurrently prescribed statins. These data support an increase in awareness of the potential association between statin use and ICI-associated muscle syndromes. Prospective observational studies are warranted to further elucidate the potential association between statin use and ICI-associated myopathies. Additionally, in patients who develop inflammatory and non-inflammatory myopathies while on a statin and an ICI, alternative and widely available secondary approaches for management of lipids could be considered.
Supplementary Material
Acknowledgements
The Editor-in-Chief, Tim Elliott, would like to thank Marianne Boes and Roberta Seidman for their contribution to the peer review of this article.
Glossary
Abbreviations
- CK
Creatine kinase
- HMGCR
HMG Co-A reductase
- ICI
Immune checkpoint inhibitor
- irAE
Immune-related adverse event
- LFT
Liver function test
Funding
This work was supported by National Institutes of Health/National Heart, Lung, Blood Institute [T32HL076136 to R.A., V.R., and A.Z.], [R01HL137562-, R01HL130539, and K24HL150238 to T.N.], and National Institutes of Health Mentored Clinical Scientist Development Award 1K08DK114563–01 [M.D.]. T.G.N. was also supported, in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg.
Author contributions
T.G.N. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: T.G.N., S.P.M. Acquisition, analysis, or interpretation of data: Z.D.D., S.P.M., Zafar Drafting of the manuscript: Z.D.D., S.P.M., T.G.N. Critical revision of the manuscript for important intellectual content: M.E.S., A.C.G., K.L.R. Statistical analysis: Z.D.D. Obtained funding: T.G.N. Supervision: T.G.N., M.E.S., A.C.G., K.L.R.
Conflict of interest
T.G.N. has been a consultant to and received fees from Intrinsic Imaging, H3-Biomedicine, AbbVie, Amgen, and Genentech, outside of the current work. T.G.N. also reports consultant fees from Bristol Myers Squibb for a Scientific Advisory Board focused on myocarditis related to immune checkpoint inhibitors and grant funding from AstraZeneca. M.D. has research funding from Novartis and Eli Lilly, has received consulting fees from Roche-Genentech, Tillotts Pharma, ORIC Pharmaceuticals, Partner Therapeutics, and Moderna, and is a member of the Scientific Advisory Board for Neoleukin Therapeutics, all outside of the current work. Dr. Sullivan has been a consultant to Asana, Bristol Myers Squibb, Merck, Replimune; and received research funding from Amgen and Merck, all outside of the current work. Other authors have no conflicts of interest or financial disclosures.
Data availability
The data, analytic methods, and study materials will be made available from the corresponding author on reasonable request after institutional approval and following institutional process.
References
- 1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018:378;158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 2. Moujaess E, Haddad FG, Eid Ret al. The emerging use of immune checkpoint blockade in the adjuvant setting for solid tumors: a review. Immunotherapy. 2019;11:1409–22. 10.2217/imt-2019-0087 [DOI] [PubMed] [Google Scholar]
- 3. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. 10.1126/science.aax0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews. Drug Discovery. 2014;13:883–4. 10.1038/nrd4476 [DOI] [PubMed] [Google Scholar]
- 5. Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3:e200423. 10.1001/jamanetworkopen.2020.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Connolly C, Bambhania K, Naidoo J. Immune-related adverse events: a case-based approach. Front Oncol. 2019;9:530. 10.3389/fonc.2019.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armenian SH, Xu L, Ky Bet al. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34:1122–30. 10.1200/JCO.2015.64.0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drobni ZD, Alvi RM, Taron Jet al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–311. https://doi.org/10.1161CIRCULATIONAHA.120.049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Reynolds KL, Lyon ARet al. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity. JACC: CardioOncology. 2021;3:35–47. 10.1016/j.jaccao.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newman Connie B, Preiss D, Tobert Jonathan Aet al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39:e38–81. 10.1161/ATV.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 11. Anquetil C, Salem J-E, Lebrun-Vignes Bet al. Immune checkpoint inhibitor–associated myositis. Circulation. 2018;138:743–745. 10.1161/CIRCULATIONAHA.118.035898 [DOI] [PubMed] [Google Scholar]
- 12. Salami JA, Warraich H, Valero-Elizondo Jet al. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the medical expenditure panel survey. JAMA Cardiol. 2017;2:56–65. 10.1001/jamacardio.2016.4700 [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Stone NJ, Bailey ALet al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143. 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109–29. 10.3233/JND-180308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alfirevic A, Neely D, Armitage Jet al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther. 2014;96:470–6. 10.1038/clpt.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department Of Health And Human Services. National Institutes Of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. [Google Scholar]
- 17. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 18. Hassel JC, Heinzerling L, Aberle Jet al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36–49. 10.1016/j.ctrv.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 19. Zhang L, Zlotoff DA, Awadalla Met al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031–4. 10.1161/CIRCULATIONAHA.119.044703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L, Awadalla M, Mahmood SSet al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 2020;41:1733–1743. 10.1093/eurheartj/ehaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Awadalla M, Mahmood SS, Groarke JDet al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75:467–78. 10.1016/j.jacc.2019.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Awadalla M, Golden DLA, Mahmood SSet al. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J Immunother Cancer. 2019;7:53. 10.1186/s40425-019-0535-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahmood SS, Fradley MG, Cohen JVet al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–64. 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thavendiranathan P, Zhang L, Zafar Aet al. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol. 2021;77:1503–16. 10.1016/j.jacc.2021.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cappelli LC, Gutierrez AK, Bingham CO 3rdet al. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res (Hoboken). 2017;69:1751–63. 10.1002/acr.23177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kostine M, Rouxel L, Barnetche Tet al. FHU ACRONIM . Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis. 2018;77:393–8. 10.1136/annrheumdis-2017-212257 [DOI] [PubMed] [Google Scholar]
- 27. Benfaremo D, Manfredi L, Luchetti MMet al. Musculoskeletal and rheumatic diseases induced by immune checkpoint inhibitors: a review of the literature. Curr Drug Saf. 2018;13:150–64. 10.2174/1574886313666180508122332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solimando AG, Crudele L, Leone Pet al. Immune checkpoint inhibitor-related myositis: from biology to bedside. Int J Mol Sci. 2020;21:3054. 10.3390/ijms21093054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Touat M, Maisonobe T, Knauss Set al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018; 91:e985–94. 10.1212/WNL.0000000000006124 [DOI] [PubMed] [Google Scholar]
- 30. Kadota H, Gono T, Shirai Yet al. Immune checkpoint inhibitor-induced myositis: a case report and literature review. Curr Rheumatol Rep. 2019;21:10. 10.1007/s11926-019-0811-3 [DOI] [PubMed] [Google Scholar]
- 31. Nguyễn T, Maria ATJ, Ladhari Cet al. Rheumatic disorders associated with immune checkpoint inhibitors: what about myositis? An analysis of the WHO’s adverse drug reactions database. Ann Rheum Dis 2020. annrheumdis-2020–217018. 10.1136/annrheumdis-2020-217018 [DOI] [PubMed] [Google Scholar]
- 32. Calabrese C, Kirchner E, Kontzias Aet al. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open. 2017;3:e000412. 10.1136/rmdopen-2016-000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah M, Tayar JH, Abdel-Wahab Net al. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Semin Arthritis Rheum. 2019;48:736–40. 10.1016/j.semarthrit.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 34. Koene RJ, Prizment AE, Blaes Aet al. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133: 1104–14. 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–97. 10.1093/eurheartj/ehz766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reynolds K, Thomas M, Dougan M. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23:991–7. 10.1634/theoncologist.2018-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cortazar FB, Marrone KA, Troxell MLet al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638–47. 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cortazar FB, Kibbelaar ZA, Glezerman IGet al. Clinical features and outcomes of immune checkpoint inhibitor–associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31:435–46. 10.1681/ASN.2019070676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Annigeri RA, Mani RM. Acute interstitial nephritis due to statin and its class effect. Indian J Nephrol. 2015;25:54–56. 10.4103/0971-4065.136883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Zyl-Smit R, Firth JC, Duffield Met al. Renal tubular toxicity of HMG-CoA reductase inhibitors. Nephrol Dial Transplant. 2004;19:3176–9. 10.1093/ndt/gfh474 [DOI] [PubMed] [Google Scholar]
- 42. Mamlouk O, Selamet U, Machado Set al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:2. 10.1186/s40425-018-0478-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytic methods, and study materials will be made available from the corresponding author on reasonable request after institutional approval and following institutional process.