Abstract
Xrn1p of Saccharomyces cerevisiae is a major cytoplasmic RNA turnover exonuclease which is evolutionarily conserved from yeasts to mammals. Deletion of the XRN1 gene causes pleiotropic phenotypes, which have been interpreted as indirect consequences of the RNA turnover defect. By sequence comparisons, we have identified three loosely defined, common 5′-3′ exonuclease motifs. The significance of motif II has been confirmed by mutant analysis with Xrn1p. The amino acid changes D206A and D208A abolish singly or in combination the exonuclease activity in vivo. These mutations show separation of function. They cause identical phenotypes to that of xrn1Δ in vegetative cells but do not exhibit the severe meiotic arrest and the spore lethality phenotype typical for the deletion. In addition, xrn1-D208A does not cause the severe reduction in meiotic popout recombination in a double mutant with dmc1 as does xrn1Δ. Biochemical analysis of the DNA binding, exonuclease, and homologous pairing activity of purified mutant enzyme demonstrated the specific loss of exonuclease activity. However, the mutant enzyme is competent to promote in vitro assembly of tubulin into microtubules. These results define a separable and specific function of Xrn1p in meiosis which appears unrelated to its RNA turnover function in vegetative cells.
Many mature cellular RNA species arise by processing of precursors. In these maturation processes, unwanted RNA fragments are removed from pre-RNA molecules and degraded. The turnover of RNA, especially of mRNA, plays a crucial role in the regulation of gene expression (reviewed in references 12 and 68). Important insights into general mRNA turnover processes were reached for the yeast Saccharomyces cerevisiae, where deadenylation-dependent and -independent degradation pathways were identified (12). Several enzymatic activities like decapping, endonuclease, poly(A) nuclease, and 3′-5′ and 5′-3′ exoribonuclease are required for these processes (17, 58, 59; reviewed in reference 12).
Xrn1p is the major cytoplasmic 5′-3′ exoribonuclease involved in RNA turnover (12, 79). Mutations in XRN1 are viable and result in defects in the turnover of pre-rRNA (25, 81) and mRNA (reviewed in reference 12). The majority of Xrn1p in vegetative and meiotic cells is located in the cytoplasm, consistent with the major role of this protein in cytoplasmic RNA turnover (28). Besides the defects in RNA metabolism, xrn1 mutants exhibit pleiotropic phenotypes including slow growth, hypersensitivity to the microtubule-depolymerizing drug benomyl, loss of viability upon nitrogen starvation, meiotic arrest, reduced spore viability, defects in microtubule-related processes (for a review, see reference 26), and meiotic recombination defects and synergistic interactions with meiotic recombination mutants (86). Xrn1p is evolutionarily conserved, and homologs have been identified in Schizosaccharomyces pombe (84) and mammals (6). The pleiotropic defects of xrn1 mutations and the diverse biochemical activities of the protein (see below) have led to the isolation of this gene in several independent approaches. Therefore, XRN1 (46, 79) is also known as SEP1 (43), DST2 (Stpβp) (18), KEM1 (39), RAR5 (41), and SKI1 (35).
The 5′-3′ exonuclease activity of Xrn1p is capable of degrading a variety of substrates including RNA (79, 80), single-stranded DNA (ssDNA), and double-stranded DNA (dsDNA) (34). For DNA substrates, Xrn1p was found to have a preference for G4 tetraplex-containing DNAs, a structure that may form at telomeres (47). Xrn1p has also been identified as a homologous pairing protein (Sep1 [43]), a potentially important activity for homologous recombination. This and genetic data led Tishkoff et al. (86) to propose a role for Xrn1p in a meiotic recombination pathway independent of Rad51p and Dmc1p.
S. cerevisiae cells lacking Xrn1p show a number of phenotypes in cellular processes related to microtubule function such as increased sensitivity to the microtubule-destabilizing drug benomyl, increased chromosome loss, a karyogamy defect (hence the name KEM1, for karyogamy defect-enhancing mutation [39]), impaired spindle pole body separation, and a defect in nuclear migration (32, 39). Moreover, purified Xrn1p promoted the in vitro polymerization of porcine brain as well as S. cerevisiae tubulin into microtubules and bound to these microtubules in a cosedimentation assay. Genetic analysis of double mutants with mutations in XRN1 and tubulin genes (TUB1 and TUB2) also suggested interaction between Xrn1p and microtubules (32). A possible association of Xrn1p with the microtubular cytoskeleton is supported by immunofluorescence data of the homologous protein in mammalian cells (6).
We have identified three 5′-3′ exonuclease motifs shared by a number of 5′-3′ exonucleases including the Xrn1p family. By mutational analysis of two critical residues in motif II, we demonstrate the functional significance of the sequence alignments. Interestingly, the mutations in Xrn1p caused separation of function. All mitotic defects in such mutants were indistinguishable from the defects caused by a gene deletion. However, the separation-of-function alleles did not cause the severe meiotic phenotypes typical for the gene deletion. In vivo analysis of the mutants demonstrated a complete absence of exonuclease activity measured in rRNA and mRNA turnover assays. Biochemical analysis of a purified mutant Xrn1p demonstrated a severe reduction, if not a complete loss, of exonuclease activity. Nucleic acid binding and homologous pairing activity were indistinguishable from those of the wild-type enzyme. Moreover, the mutant was still able to promote in vitro assembly of microtubules. These data indicate that Xrn1p has two separable functions in S. cerevisiae, one in RNA turnover and a specific function in meiosis which is possibly related to its interaction with tubulin.
MATERIALS AND METHODS
S. cerevisiae strains and media.
Standard media and methods for S. cerevisiae strains have been described previously (74). All the strains used are listed in Table 1 and were grown in standard media unless otherwise indicated. Xrn1p mutations at amino acids 206 and 208 were introduced by site-directed mutagenesis (45). Primer mut1 [5′-CATAATCAAA(T/G)CTGCG(T/G)CAAGACCG-3′] with two degenerated positions resulted in the xrn1-D206A mutation, while primer mut2 [5′-CATAATCAAAGCTGCG(T/G)CAAGACCG-3′] with one mutated and one degenerate site produced xrn1-D208A and xrn1-D206A,D208A. The mutations were verified by DNA sequencing of the relevant region and cloned into a CEN-ARS complementation vector (7). Since all three mutants behaved identically in all assays, it is highly unlikely that unrelated mutations were introduced spuriously. Moreover, we used native T4 DNA polymerase, which has a very high fidelity, in the in vitro mutagenesis. The engineered SalI site just in front of the ATG was abolished by ligation to an XhoI site. As exact isogenic controls, the wild-type XRN1 gene was cloned in the same way to exclude any effects of the slight sequence change resulting from this step. The resulting isogenic strains are designated wt* for wild type or dmc1* for the dmc1 strain. The mutated variants as well as the wt* strains were introduced into the chromosome by genomic replacement of the xrn1Δ::URA3 allele. After selection on plates containing 5-fluoroorotic acid, the correct integrations were verified by Southern blot analysis. No systematic or reproducible difference between wild-type and wt* strains could be detected (data not shown).
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| DBY1399a | MATα ade2 ura3-52 | D. Botstein (Stanford) |
| WDHY548a | MATα ade2 ura3-52 XRN1b | This study |
| WDHY448a | MATα ade2 ura3-52 xrn1Δ::URA3-1 | This study |
| WDHY492a | MATα ade2 ura3-52 xrn1-D206A | This study |
| WDHY493a | MATα ade2 ura3-52 xrn1-D208A | This study |
| WDHY494a | MATα ade2 ura3-52 xrn1-D206A,D208A | This study |
| WDHY549c | MATα ho::LYS2 lys2 ura3 leu2::hisG his4-X XRN1b | This study |
| WDHY550c | MATa ho::hisG lys2 ura3 his4-B can1rXRN1b | This study |
| WDHY143c | MATα ho::LYS2 lys2 ura3 leu2::hisG his4-X xrn1Δ::URA3-1 | This study |
| WDHY551c | MATa ho::hisG lys2 ura3 his4-B can1rxrn1Δ::URA3-1 | This study |
| WDHY489c | MATα ho::LYS2 lys2 ura3 leu2::hisG his4-X xrn1-D206A | This study |
| WDHY552c | MATa ho::hisG lys2 ura3 his4-B can1rxrn1-D206A | This study |
| WDHY490c | MATα ho::LYS2 lys2 ura3 leu2::hisG his4-X xrn1-D208A | This study |
| WDHY553c | MATa ho::hisG lys2 ura3 his4-B can1rxrn1-D208A | This study |
| WDHY491c | MATα ho::LYS2 lys2 ura3 leu2::hisG his4-X xrn1-D206A,D208A | This study |
| WDHY554c | MATa ho::hisG lys2 ura3 his4-B can1rxrn1-D206A,D208A | This study |
| WDHY1450c | XRN1/XRN1d | This study (RKY1672)e |
| WDHY1451c | XRN1b/XRN1b,d | This studye |
| WDHY1452c | dmc1Δ::LEU2/dmc1Δ::LEU2d | This study (RKY1953)e |
| WDHY1453c | dmc1Δ::LEU2/dmc1Δ::LEU2 XRN1b/XRN1b,d | This studye |
| WDHY1454c | xrn1Δ::URA3/xrn1Δ::URA3d | This study (RKY1952)e |
| WDHY1455c | xrn1-D208A/xrn1-D208Ad | This studye |
| WDHY1456c | dmc1Δ::LEU2/dmc1Δ::LEU2 xrn1Δ::URA3/xrn1Δ::URA3d | This study (RKY1954)e |
| WDHY1457c | dmc1Δ::LEU2/dmc1Δ::LEU2 xrn1-D208A/xrn1-D208Ad | This studye |
Isogenic S288c strains.
Wild-type (wt) constructed as described in Materials and Methods and denoted as wt* or dmc1*.
Isogenic SK-1 strains (36) useful for genetic analysis were kindly supplied by N. Kleckner (Harvard University).
Strains have the basic genotype MATa/MATα leu2::hisG/leu2::hisG his4-B::ADE2::his4-X/his4-B ura3/ura3 lys2/lys2 ho::LYS2/ho::LYS2 ade2/ade2.
Constructed for this study with haploid strains kindly supplied by R. Kolodner (University of California San Diego). The equivalent diploid RKY strain number of Tishkoff et al. (86) is given in parentheses.
Methods for in vivo characterization.
For growth tests, overnight cultures were diluted to an optical density at 600 nm of 1.0. Then 2-μl volumes of 100, 10−1, 10−2, and 10−3 serial dilutions containing approximately equal cell numbers for all strains were spotted on yeast extract-peptone-dextrose (YPD) plates with or without 15 μg of benomyl (Carbendazim; NEN DuPont) per ml. Photographs were taken after 2 to 4 days of incubation depending on the temperature (25, 30, or 37°C).
The nitrogen starvation test was carried out exactly as described previously (39). Briefly, cells from an overnight culture were diluted in medium lacking nitrogen to a concentration of 106 cells/ml and incubated at different temperatures (30 and 37°C). Aliquots were plated on YPD plates at various time points, and colonies were counted after the plates were incubated for 3 days at 30°C.
For meiotic analysis, diploid SK-1 strains were selected on SD-Lys-Leu plates and five independent colonies were stored at −70°C. After a selection step on YPG plates to avoid petite mutants, the strains were immediately sporulated by a method optimized for SK-1 (62) and asci were counted after 24 h. Spore viability was determined by tetrad analysis of asci after incubation of spore clones for 3 days at 30°C. Meiotic recombination analysis was performed exactly as described previously (86).
Isolation of total RNA and separation of RNA on agarose gels were performed as described previously (4). Poly(A)+ RNA was obtained by binding to Oligotex beads (Qiagen AG, Basel, Switzerland) as specified by the manufacturer.
Biochemical methods.
The cellular levels of Xrn1p were determined by immunoblotting as described previously (28). The mutated xrn1-D208A gene was cloned as a BamHI-HindIII fragment into the BamHI-HindIII-cut overexpression vector pRDK249 (34). More than 95% pure Xrn1p and Xrn1-D208Ap were obtained by the standard purification method (34) as modified by Holler et al. (29).
Exonuclease assays (30-μl reaction volumes) were carried out as described previously (37, 79, 80) in buffer containing 13 mM MgCl2. The amount of substrate was 2 nmol in all experiments, while the amount of both enzymes was 286 fmol for T7 ssDNA, 28.6 pmol for T7 dsDNA, and 100 fmol for mouse β-actin ssRNA. For turnover number determinations, every experiment was also done with 1 nmol of substrate to show that saturating substrate concentrations were present. Binding of mouse β-actin ssRNA was determined by filter binding assays (20-μl reaction volumes) with KOH-treated nitrocellulose filters as described previously (27) in buffer not containing Mg2+.
Strand exchange assays were performed as described previously (34, 37) with phage M13. Resection of the dsDNA substrates linearized with SmaI at the 3′ end was achieved by digestion with Escherichia coli exonuclease III, and resection of substrates linearized at the 5′ end was achieved with T7 gene 6 exonuclease as described previously (34, 38). The 30-μl reaction mixtures contained 20 μM for dsDNA, 10 μM for ssDNA, and 13 mM Mg2+ or Ca2+, as indicated. Reaction products were analyzed on TAE agarose gels as described previously (34), and images of the ethidium bromide-stained gel were captured on a gel documentation system. Molar concentrations and amounts of nucleic acids refer to mononucleotides.
Purification of brain tubulin and microtubule assembly.
Freshly excised porcine brains were obtained from a local slaughterhouse. Tubulin and microtubule-associated proteins (MAPs) were purified as described previously (9) by two cycles of polymerization-depolymerization (71) followed by phosphocellulose chromatography (P11; Whatman) by the method of Sloboda and Rosenbaum (77). In vitro microtubule assembly experiments were performed with purified brain tubulin in the presence of Xrn1p or Xrn1-D208Ap. The total reaction volume was 25 μl, and the reaction mixtures were incubated at 37°C for 45 min. The final protein concentrations were 0.5 mg of tubulin per ml, 0.3 mg of Xrn1 protein per ml, and 0.3 or 0.5 mg of Xrn1-D208A protein per ml in buffer containing 50 mM morpholineethanesulfonic acid (MES; pH 6.4), 2 mM EGTA, 1 mM MgCl2, 1 mM GTP (all from Sigma), 10% glycerol, 50 μg of DNase per ml, and 100 μg of leupeptin per ml (all from Fluka). Experiments with Xrn1p and Xrn1-D208Ap were always carried out in parallel with the same solutions of purified tubulin and reagents. Control experiments consisted of purified tubulin (0.5 mg/ml) in the presence or absence of MAPs (0.3 mg/ml). Following assembly, the samples were placed on 200-mesh copper carbon-coated grids and stained with 0.5% uranyl acetate as described previously (54). Electron micrographs were taken on a Philips EM410 electron microscope operated at 80 kV.
RESULTS
Xrn1p shares conserved sequence motifs with other 5′-3′ exonucleases.
A sequence comparison of several Mg2+-dependent 5′-3′ exonucleases from bacteriophages, prokaryotes, and eukaryotes revealed some highly conserved amino acid residues (Fig. 1) (30, 57, 66, 70). In the crystal structure of phage T4 RNase H, these residues are clustered in the proposed active site. In particular, some conserved aspartic (D) and glutamic (E) acid residues coordinate two Mg2+ ions in the reactive center of the exonuclease and are believed to play a crucial role in catalysis (57). Besides the Fen1 protein (DNaseIV, MF-1) (23, 66, 87) and the Rad2p/Xpg (24) related proteins, the Xrn1p subfamily forms a new branch of 5′-3′ exonucleases in eukaryotes. Xrn1p and the related proteins (Rat1p in S. cerevisiae, Exo2p and Dhp1p in S. pombe, mXrn1p and Dhm1p from mice [see the legend to Fig. 1]) share the highly conserved amino acids considered to be important for 5′-3′ exonuclease activity (Fig. 1). The crucial residues can be divided into three motifs (I to III in Fig. 1). Within the motifs, the spacing between highly similar amino acids and the occurrence of hydrophobic or hydrophilic residues are conserved. Outside the motifs, there is no recognizable similarity between the respective subfamilies. The existence of 5′-3′ exonuclease motifs in Xrn1p suggests that the conserved amino acids might also play a role in the exonuclease function of this protein.
FIG. 1.
Xrn1p shares sequence motifs with other Mg2+-dependent 5′-3′ exonucleases. Amino acid residues that are similar (●), hydrophobic (○), and charged or polar (±) are marked. These residues are conserved in more than 75% of the proteins. Highly conserved amino acids that are clustered in the proposed active site of T4 RNase H are highlighted with asterisks (57). Abbreviations: Sc, S. cerevisiae; Sp, S. pombe; Mm, Mus musculus; Hs, Homo sapiens; Ec, E. coli; Hi, Haemophilus influenzae; Taq, Thermus aquaticus; Tf, Thermus flavus; Ml, Mycobacterium leprae; Mt, Mycobacterium tuberculosis; Bc, Bacillus caldotenax; Spn, Streptococcus pneumoniae. The numbers refer to the first or last amino acid in the alignment. The three 5′-3′ exonuclease motifs (I to III) are indicated. The references for sequences are MmXpg (75), SpRad13 (13), T3Exo (8), HiPol1 (20), MlPol1 (21), SpnPol1 (52), EcSdab (11), ScXrn1p (39), SpExo2 (84), MmXrn1 (6), ScRat1 (2), SpDhp1 (82), MmDhm1 (76). All others are found in reference 57. Note that exonuclease activity is not shown for all the proteins listed here.
Residues in the conserved 5′-3′ exonuclease motif II of Xrn1p were mutated by site-directed mutagenesis. This motif features two aspartic acid residues (D206 and D208 in Xrn1 [Fig. 1]) that are separated by one amino acid (DXD motif). Both aspartic acids (D) were changed individually and together to the small neutral amino acid alanine (A) to avoid, as far as possible, effects of the mutations on the protein structure. Three mutations were obtained: two single-point mutations, xrn1-D206A and xrn1-D208A, and the double mutation, xrn1-D206A,D208A. After the chromosomal XRN1 gene was replaced with the specific XRN1 mutations and an identical wild-type construct, the resulting mutants and wild type were tested for the defects observed in xrn1Δ cells. The cellular levels of all mutant proteins were very similar to the wild-type level as visualized in immunoblots of whole-cell extracts from the relevant strains (see Fig. 5A).
FIG. 5.
(A) Levels of wild-type and mutant Xrn1p. Whole-cell extract (40 μg) from the diploid strains WDHY143 × WDHY551 (lane 1, xrn1Δ), WDHY549 × WDHY550 (lane 2, wt*), WDHY490 × WDHY553 (lane 3, xrn1-D208A), WDHY489 × WDHY552 (lane 4, xrn1-D206A), and WDHY491 × WDHY554 (lane 5, xrn1-D206A,D208A) was analyzed by immunoblotting with anti-Xrn1p antibodies. The arrow indicates the wild-type and mutant Xrn1p. Equal amounts of protein were loaded in all lanes as verified by Coomassie blue staining of an independent gel. (B) Purification of Xrn1 and Xrn1-D208A proteins. The proteins were analyzed by Coomassie blue staining after electrophoresis on a 10% acrylamide gel. Lanes: 1, high-molecular-mass markers (Bio-Rad); 2, 1.5 μg of Xrn1 protein; 3, 1.5 μg of Xrn1-D208A protein. The sizes of the molecular mass standard are given on the left in kilodaltons.
Mutations in the exonuclease motifs abolish in vivo exonuclease activity.
To ascertain that the introduced mutations in the DXD motif did indeed abolish the exonuclease activity of Xrn1p, two in vivo tests were performed. Cells lacking Xrn1p accumulate ITS1 (an internal transcribed spacer) fragment that results from the processing of pre-rRNA. This by-product of rRNA maturation can be visualized by Northern hybridization (25). Whereas in wild-type cells no ITS1 fragment can be detected, in xrn1Δ cells a prominent band of the expected length appeared (Fig. 2). The same band was also present at identical intensity in the strains bearing point mutations in the DXD motif.
FIG. 2.
Accumulation of an ITS1 fragment in strains with mutations in XRN1. Portions (60 μg) of total RNA were separated on a 1.5% denaturing agarose gel and blotted to a nylon membrane. Lanes contain (from left to right) WDHY492 (xrn1-D206A), WDHY493 (xrn1-D208A), WDHY494 (xrn1-D206A,D208A), DBY1399 (wt), WDHY548 (wt*), and WDHY448 (xrn1Δ). The filter was probed with the oligonucleotide ITS-51 (25). The arrow indicates the accumulated ITS1 fragment.
An accumulation of deadenylated transcripts in cells lacking Xrn1p has been reported (31). As a second test for the exonuclease defect of strains carrying mutations in the DXD motif, the accumulation of deadenylated mRNAs was examined by Northern blot analysis of poly(A)+ and poly(A)− RNA with actin and MFα1 sequences as probes. As shown in Fig. 3, most of the actin and MFα1 transcripts were polyadenylated in wild-type cells. Only 11 to 12% of the mRNAs were lacking a poly(A) tail. In contrast, in all xrn1 mutants, 59 to 87% of the transcripts were present in the deadenylated form. The effect in the strains mutated in the DXD motif was the same as if not stronger than in xrn1Δ mutants. The results with wild-type and xrn1Δ cells are consistent with previous results obtained by Hsu and Stevens (31). From these data we conclude that cells with the mutations xrn1-D206A, xrn1-D208A, and xrn1-D206A,D208A exhibit no detectable in vivo exonuclease activity of Xrn1p in the two RNA turnover assays used.
FIG. 3.
Degradation of deadenylated mRNAs is defective in strains with point mutations in XRN1. A Northern blot shows the accumulation of deadenylated mRNA species. Poly(A)+ and poly(A)− RNA was prepared from the same strains used in the experiment in Fig. 2. Poly(A)+ RNA (0.5 μg) and poly(A)− RNA (15 μg) were separated on a 1.2% denaturing formaldehyde agarose gel and transferred to a membrane. The hybridization probes were actin and MFα1 (31). Bands were quantified on a PhosphorImager, and the results were normalized to the amount of total RNA. Shown are images from the PhosporImager.
Separation-of-function mutations in XRN1.
Mutations in XRN1 cause slow growth and hypersensitivity to the microtubule-depolymerizing drug benomyl (32, 39). To test the effect of the mutations in the DXD motif, a growth test on media with and without addition of benomyl was performed (Fig. 4A). To investigate a possible influence of temperature, the plates were incubated at 25, 30, and 37°C. The xrn1 null mutant is known to exhibit a slow-growth phenotype (85), which is visible in Fig. 4A on the plate lacking benomyl. All three xrn1 mutations showed a slow-growth phenotype identical to the xrn1Δ control strain independent of the incubation temperature (Fig. 4A and data not shown). The benomyl hypersensitivity of the strains bearing mutations in the DXD motif was also as severe as in the deletion strain. Here a temperature effect could be observed, as expected because of the cold sensitivity of microtubules. At low temperatures (25°C), the addition of benomyl affected even the wild-type cells while the mutants hardly grew at all (Fig. 4A). At the highest temperature (37°C), the wild-type strains grew normally and the mutated strains performed poorly. At 30°C, an intermediate behavior was observed (data not shown). The drop dilutions assays, as shown in Fig. 4A, are typically sensitive enough to detect even minor differences in sensitivities to drugs, and within the limits of this assay the XRN1 point mutants did not show any difference from the XRN1 deletion mutant.
FIG. 4.
Strains carrying point mutations in XRN1 show slow growth, benomyl hypersensitivity, and loss of viability upon nitrogen starvation. (A) Slow growth and benomyl sensitivity. Serial dilutions of strains WDHY448 (xrn1Δ), WDHY548 (wt* [Fig. 2]), DBY1399 (wt), WDHY492 (xrn1-D206A), WDHY493 (xrn1-D208A), and WDHY494 (xrn1-D206A,D208A) were spotted on YPD plates with and without benomyl (ben) and incubated at 25°C. The results of one of five experiments are shown. The difference between the wt (DBY1399) and wt* (WDHY548) strains is not significant and was not seen in other experiments performed at this temperature or the other temperatures. (B) Loss of viability upon nitrogen starvation. The same strains as in panel A were incubated in medium without nitrogen, and viability was determined after the times indicated (■, xrn1Δ; □, wt*; ⧫, xrn1-D206A; ◊, xrn1-D208A; ▴, xrn1-D206A,D208A). Shown are the relative numbers of CFU after the cells were plated on YPD. The data represent one typical experiment of five.
Cells with a deletion of XRN1 lose viability upon prolonged incubation in medium lacking nitrogen. In contrast, wild-type cells arrest uniformly as unbudded cells and remain viable (39). A nitrogen starvation test was carried out with cells carrying mutations in the DXD motif (Fig. 4B). Strains bearing the xrn1-D206A and/or the xrn1-D208A mutations lost viability in a similar manner to the deletion strain (xrn1Δ). The effect was more pronounced at the high incubation temperature of 37°C. In a parallel experiment at 30°C, the phenotype of all mutant cells was less extreme (data not shown). In each case, the wild-type cells were still fully viable at the end of the experiment.
In all three tests for mitotic phenotypes (slow growth, hypersensitivity to benomyl, and N2 starvation) the xrn1 point mutations had the same effect as a deletion of the XRN1 gene. There was no distinguishable difference between the three DXD motif mutants. The xrn1-D208A mutation was fully recessive when a heterozygous diploid strain was tested for slow growth, benomyl hypersensitivity, and loss of viability upon nitrogen starvation (data not shown).
Sporulation is severely defective in xrn1Δ strains. Cells arrest after premeiotic DNA synthesis at pachytene, and only ∼10% form asci (3, 86). The viability of the resulting spores is reduced to ∼50% (85). To examine the meiotic phenotypes of the DXD motif mutations, a set of isogenic SK-1 strains was constructed (Materials and Methods; Table 1). SK-1 strains are characterized by very efficient sporulation and high spore viability (36). This strain background had been previously used in the characterization of the xrn1Δ mutant (3, 85, 86). The wild-type and xrn1Δ strains sporulated as expected (79.4 and 7.8%, respectively [Table 2]). The strains carrying the point mutations showed significantly improved sporulation (24.7 to 39.7%; Table 2) over the gene deletion.
TABLE 2.
Increased sporulation and spore viability in strains with mutations in the exonuclease motif of Xrn1p compared to the deletion strain
| Relevant genotype | % Sporulationa | % Spore viabilityb |
|---|---|---|
| wt* | 79.4 ± 4.6 | 96.8 ± 2.1 |
| xrn1Δ | 7.8 ± 1.6 | 57.5 ± 9.5 |
| xrn1-D206A | 26.4 ± 2.7 | 87.7 ± 8.3 |
| xrn1-D208A | 39.7 ± 4.7 | 88.4 ± 4.8 |
| xrn1-D206A,D208A | 24.7 ± 7.2 | 84.3 ± 3.1 |
Sporulation was quantified in isogenic SK-1 strains WDHY549 × WDHY550 (wt* denotes the exact isogenic control [see Materials and Methods]), WDHY143 × WDHY551 (xrn1Δ), WDHY489 × WDHY552 (xrn1-D206A), WDHY490 × WDHY553 (xrn1-D208A), and WDHY491 × WDHY554 (xrn1-D206A,D208A), counting at least 200 cells for each determination. Numbers are means ± standard deviations from five independent sporulation experiments.
The viability of spores derived from the same strains was determined by dissecting a total of 204 tetrads. Means ± standard deviations for the five independent strains are shown.
For each strain, about 200 tetrads were dissected and the viability of the spores was examined (Table 2). Again, the values for the wild type (96.8%) and the deletion strain (57.5%) were consistent with the previous report (85). The strains with the mutations in the DXD motif had significantly higher spore viability (84.3 to 88.4%) than the xrn1Δ strain did.
In combination with a deletion in DMC1, which encodes a meiosis-specific RecA-like protein (10), xrn1Δ causes a severe reduction in intrachromosomal popout recombination and convertants (86). Using this meiotic recombination assay, we demonstrated that the xrn1-D208A dmc1 double mutant was significantly improved over the xrn1Δ dmc1 double mutant in commitment to meiotic intrachromosomal recombination (Table 3). Commitment to meiotic intrachromosomal popout recombination was severely reduced in the double mutant with the gene deletion (17-fold compared to wild type at 24 h [Table 3]), whereas with the point mutation the reduction was only minor (3-fold). For commitment to conversion, the effect of the double mutant containing the gene deletion was less extreme (3-fold at 7 h, and 5-fold at 24 h [Table 3]), consistent with previous observations (86). Also this reduction was less pronounced in the double mutant with xrn1-D208A (no reduction at 7 h, 3-fold reduction 24 h [Table 3]).
TABLE 3.
Increased commitment to meiotic intrachromosomal recombination in xrn1-D208A dmc1 double mutants compared to xrn1Δ dmc1 double mutants
| Relevant genotypea | Frequency of His+ Ade− popouts (10−4) atb:
|
Frequency of His+ Ade+ convertants (10−4) atb:
|
||||
|---|---|---|---|---|---|---|
| 0 h | 7 h | 24 h | 0 h | 7 h | 24 h | |
| wt | 5.4 | 103 (100%) | 550 (100%) | 11.4 | 105 (100%) | 350 (100%) |
| wt* | 5.8 | 84 (82%) | 459 (83%) | 8.7 | 118 (112%) | 367 (105%) |
| dmc1 | 19.4 | 502 (487%) | 588 (107%) | 12.6 | 122 (116%) | 307 (88%) |
| dmc1* | 13.4 | 552 (536%) | 741 (135%) | 10.3 | 157 (150%) | 374 (107%) |
| xrn1Δ | 3.1 | 46 (45%) | 211 (38%) | 9.8 | 133 (126%) | 237 (68%) |
| xrn1-D208A | 3.9 | 158 (153%) | 298 (54%) | 15.6 | 121 (115%) | 224 (64%) |
| dmc1 xrn1Δ | 3.5 | 29 (28%) | 34 (6%) | 4.2 | 37 (35%) | 66 (19%) |
| dmc1 xrn1-D208A | 1.7 | 130 (126%) | 164 (30%) | 2.9 | 111 (106%) | 121 (35%) |
All strains carry his4-B::ADE2::his4-X on one homolog of chromosome III and his4-B on the other. They are isogenic derivatives of SK-1 strains: WDHY1450 (wt), WDHY1451 (wt*), WDHY1452 (dmc1), WDHY1543 (dmc1*), WDHY1454 (xrn1Δ), WDHY1455 (xrn1-D208A), WDHY1456 (dmc1 xrn1Δ), and WDHY1457 (dmc1 xrn1-D208A).
Four independent diploids for each genotype were sporulated, and the means are given. The differences between the values discussed in the text are statistically significant, since their standard deviations do not overlap. Commitment to meiotic recombination levels was measured as described in the text, and each meiotic value represents the meiotic frequency minus the mitotic (0-h) frequency (86). Numbers in parentheses indicate the frequency of recombination in the mutant as a percent of the wild-type frequency.
These results suggest that the mutations xrn1-D206A, xrn1-D208A, and xrn1-D206A,D208A are separation-of-function alleles. The mitotic defects are indistinguishable from those in the deletion mutant, while the meiotic phenotypes are significantly less severe.
Purified Xrn1-D208Ap protein has no exonuclease activity in vitro.
Xrn1p exhibits Mg2+-dependent 5′-3′ exonuclease activity on RNA (79, 80) and DNA substrates (34). To determine the in vitro activities of an Xrn1p with a mutation in the DXD motif, a mutant protein was purified. Since all three available mutants showed exactly the same behavior in all tests up to this point, only one, xrn1-D208A, was chosen for biochemical analysis. The preparations of wild-type Xrn1p and Xrn1-D208Ap contained more than 95% pure proteins (Fig. 5B). Scanning of an overloaded gel did not identify any contaminating bands (data not shown).
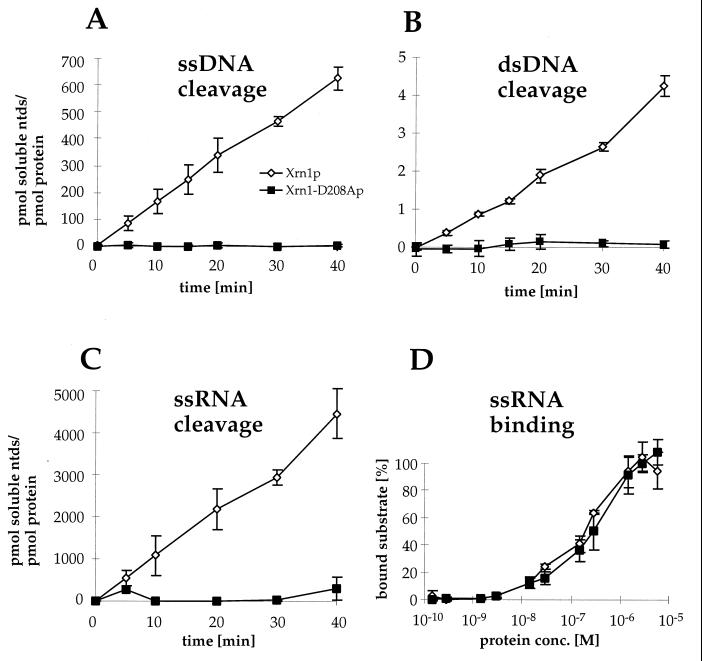
Exonuclease activity was tested on three different substrates: ssRNA transcripts of the mouse β-actin gene and HaeIII-digested ssDNA and dsDNA from phage T7. The graphs in Fig. 6A to C represent data from time course nuclease experiments for the three substrates. The turnover numbers for Xrn1p were calculated from experiments done at two substrate concentrations and are 108 mol/mol/min for ssRNA, 15.7 mol/mol/min for ssDNA, and 0.10 mol/mol/min for dsDNA. The exoribonuclease activity of Xrn1p on ssRNA was very similar to that reported for a poly(A) substrate (79). Xrn1p requires a 5′-phosphate-ending substrate (79), and all substrates used here had a 5′-phosphate group. The intact structure of the substrates was also indicated by the robust activity of the wild-type Xrn1 protein on these substrates. Dephosphorylation of the ssRNA made this substrate refractory to degradation by wild-type Xrn1p (data not shown). Also, degradation of ssDNA was comparable to previously described values (34, 37). The turnover number for dsDNA was reported previously to be higher (34, 37), which might be due to differences in the substrate preparation. The same low exonuclease activity on dsDNA was measured for another preparation of Xrn1p (data not shown). It should be noted that any partially single-stranded substrate will be degraded with at least a 150-fold-higher activity and therefore will yield an apparently enhanced activity on dsDNA.
FIG. 6.
In vitro exonuclease activity of Xrn1-D208A protein is abolished, but substrate binding is retained. (A to C) Time course reactions with Xrn1p and Xrn1-D208Ap were performed on homogeneously labeled substrates: nuclease assay with HaeIII digested bacteriophage T7 ssDNA (A), nuclease assay with HaeIII digested bacteriophage T7 dsDNA (B), and nuclease assay with ssRNA transcript from the mouse actin gene (C). (D) Binding of Xrn1p and Xrn1-D208Ap to actin RNA (nuclease substrate of panel C) was determined by filter binding. Each graph represents the mean and standard deviations of three experiments.
Reaction mixtures containing Xrn1-D208Ap showed no detectable exonuclease activity compared to the background. These observations corresponded exactly to the expectations from the in vivo analysis. These results demonstrate that Xrn1-D208Ap has very low or undetectable exonuclease activity on the three standard substrates used. To ascertain that the absence of nuclease activity was not a result of impaired substrate binding by Xrn1-D208Ap we performed quantitative filter binding studies with the substrate used for Fig. 6C. The data show that Xrn1-D208Ap binds this substrate as well as wild-type protein does (Fig. 6D). This is consistent with the purification data for the mutant protein, which involves affinity chromatography on a ssDNA cellulose column. The elution profile of Xrn1-D208Ap from this column was identical to that of the wild-type protein (data not shown). Collectively, these data show that Xrn1-D208Ap is specifically deficient in the nuclease activity.
Xrn1-D208Ap is proficient for homologous pairing of resected DNA substrates.
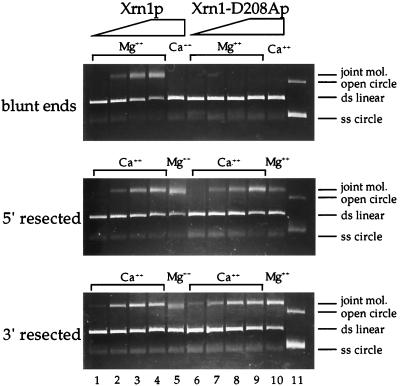
One of the initial discoveries of Xrn1p was in an in vitro homologous pairing assay involving linear dsDNA and circular ssDNA (43). Based on these in vitro properties and on recombination defects found in the xrn1Δ mutant, Xrn1p was proposed to play a direct role during homologous recombination (19, 85; reviewed in reference 44). The exonuclease activity of Xrn1p is essential for its activity in this in vitro assay, since the enzyme is inactive in the presence of Ca2+ (34) (Fig. 7, top, lanes 1 to 5). Ca2+ does not serve as a cofactor for the Xrn1p exonuclease activity. If, however, the linear duplex is pretreated with an exonuclease resulting in 5′ or 3′ resected ends, Xrn1p is active in the presence of Ca2+, eliminating the need for the intrinsic exonuclease activity (34) (Fig. 7, middle and bottom, lanes 1 to 5). Performing identical assays with Xrn1-D208A protein demonstrated that the in vitro homologous pairing activity was entirely absent on blunt-ended duplex DNA (Fig. 7, top, lanes 6 to 10). This is in agreement with the data obtained with the wild-type protein by using Ca2+ and confirmed the loss of exonuclease activity in the mutant enzyme. Xrn1-D208Ap was fully proficient in the homologous pairing assay when the linear duplex substrate had been pretreated with exonuclease, leaving either 5′ or 3′ resected ends (Fig. 7, middle and bottom, lanes 6 to 10). Therefore, we conclude that the mutant enzyme is still proficient in this homologous pairing assay without any evidence for associated dsDNA exonuclease activity.
FIG. 7.
Xrn1-D208Ap is proficient in homologous pairing of resected DNA substrates. In vitro homologous pairing reactions with circular ssDNA and linear dsDNA with blunt ends (top), 5′ resected ends (middle), or 3′ resected ends (bottom). Reaction mixtures contained either no protein (lanes 1 and 6), 95 nM Xrn1p (lane 2) or Xrn1-D208Ap (lane 7), 285 nM Xrn1p (lane 3) or Xrn1-D208Ap (lane 8), and 952 nM Xrn1p (lanes 4 and 5) or Xrn1-D208Ap (lanes 7 and 8). The presence of the relevant divalent cation (Mg2+ or Ca2+) is indicated. Lane 11 contains supercoiled and open circular M13mp19 DNA. The positions of the reaction products (joint mol.) and of open circular DNA are indicated on the right of each gel. Lanes 1 and 6 with 3′ resected dsDNA substrates (bottom gel) show a small amount of protein-independent joint-molecule formation.
Microtubule assembly by Xrn1-D208Ap.
Xrn1p promotes in vitro microtubule assembly from tubulin with high efficiency. The microtubules obtained from porcine brain or S. cerevisiae tubulin in the presence of Xrn1p were longer and more flexible and formed large bundles in comparison to microtubules obtained in the presence of porcine brain MAPs (32). Since no significant difference between porcine brain and S. cerevisiae tubulins was noted in that study, we have restricted our analysis here to porcine brain tubulin, which is more easily prepared. This assay measures in a qualitative fashion whether a protein can induce the assembly of tubulin into microtubules (9, 32). In the absence of Xrn1p or MAPs, no microtubules were formed under these experimental conditions and only amorphic tubulin aggregates were visible under the electron microscope (references 9 and 32 and data not shown).
To determine whether the xrn1-D208A mutation affects the ability of the protein to promote microtubule assembly, we have performed assembly assays with Xrn1-D208Ap in parallel with Xrn1p at the protein concentrations which proved optimal for Xrn1. As shown in Fig. 8A, B, E, and F, tubulin assembled into microtubules in the presence of Xrn1-D208Ap as in the presence of the wild-type protein. The microtubules, which appeared intact and with protofilaments readily visible (Fig. 8B), showed the typical long, flexible morphology observed with Xrn1p (Fig. 8C and D). In the absence of Xrn1p or Xrn1-D206Ap no microtubules could be observed and only amorphic tubulin aggregates were visible (data not shown).
FIG. 8.
Xrn1-D208Ap induces microtubule formation. Microtubules were assembled in vitro with purified porcine brain tubulin (0.7 mg/ml) in the presence of 0.3 mg of Xrn1-D208Ap per ml (A and B), 0.3 mg of Xrn1p per ml (C and D), or 0.5 mg of Xrn1-D208Ap per ml (E and F). Two micrographs are shown for each experiment, at magnifications of ×4,092 (A, C, and E) and ×28,830 (B, D, and F). The bars represent 2 μm (A, C, and E) and 200 nm (B, D, and F).
DISCUSSION
The Xrn1p subfamily constitutes a new branch of the 5′-3′ exonuclease family.
The family of Mg2+-dependent 5′-3′ exonucleases includes enzymes from bacteriophages, bacteria, yeast, and mammals. Regions of homology were described for enzymes with RNase H activity (30), which coincided with conserved sites in the 5′-3′ exonuclease domains of prokaryotic DNA polymerases and bacteriophage 5′-3′ exonucleases (22). Two families of eukaryotic 5′-3′ exonucleases that also show structure-specific endonuclease activity have been identified (23): Fen1 (DNaseIV, MF-1) (23, 66, 87) and Rad2p/Xpg (24, 61). The regions of significant homology in the 5′-3′ exonucleases suggest structural and functional conservation (66), and its predictive power to identify 5′-3′ exonucleases from gene sequences has been pointed out (70). The Xrn1p subfamily of 5′-3′ exonucleases consists of the cytoplasmic Xrn1p and its nuclear counterpart Rat1p (6; reviewed in reference 78). Both enzymes are evolutionarily conserved from yeasts to mammals (6) and share the distinctive amino acid sequence features of the 5′-3′ exonuclease family. They exhibit 5′-3′ exonuclease activity as far as has been determined.
The sequence similarity between these 5′-3′ exonucleases contrasts with their different functions. Differences in substrate preference are probably due to other regions of the proteins which contribute to the specific recognition of a variety of structures. Coupling of the exonuclease activity to other functions within the same protein (as in the prokaryotic DNA polymerases) or to other proteins through protein-protein interactions (as for Xrn1p that interacts with the microtubular cytoskeleton [see below]) can result in a wide variety of functions of these exonucleases in the cell.
The mutagenesis of the DXD motif of Xrn1p resulted in a protein with no detectable exoribonuclease activity in vivo (Fig. 2 and 3) and no significant exonuclease activity in vitro (Fig. 6 and 7). These observations show that the DXD motif is crucial for the exonuclease function of Xrn1p and underlines the relevance of the exonuclease motifs found in the sequence comparison (Fig. 1). Seven acidic residues, corresponding to amino acids D35, D86, E176, E178, D206, D208, and D291 of Xrn1p, are conserved in all proteins (Fig. 1). Mutational analysis showed the importance of some of the equivalent residues for the activity of HsFen1 (73), T4 RNase H (57), EcPol1 (88), and MtPol1 (56). According to the available structural data, these residues are all directly involved in coordinating two Mg2+ ions in the active site of the enzymes. D206 and D208 of Xrn1p each appear to interact with a different Mg2+ ions in the active site as shown from the position of the equivalent residues in the crystal structures of T4 RNase H (57), T5Exo (14), and TaqPol1 (40). If this extrapolation of the interaction of the two aspartic acids with different Mg2+ ions is correct, this suggests that both ions are equally important for the exonuclease activity of Xrn1p.
Mutations in motif II abolish the RNA turnover function of Xrn1p in vivo in vegetative cells.
Mutations in the XRN1 gene lead to pleiotropic phenotypes. A simple and therefore appealing explanation of these findings is that Xrn1p is involved in RNA turnover and that all xrn1 mutant phenotypes are a result of the disturbed mRNA and protein levels in the cell. However, the interaction of Xrn1p with microtubules (32) is not easily matched with a role in RNA turnover. We have speculated before that the two observations could be reconciled by proposing a role of Xrn1p in RNA transport and degradation along microtubules (discussed in reference 6). Mutations in Xrn1 that abolish specifically the exonuclease activity of the protein are ideally suited to examine the effect of disturbed RNA turnover without affecting other possible functions of the protein.
All defects in vegetative cells with point mutations in the DXD motif of Xrn1p were as severe as in a strain with a gene deletion. Based on the data of the microtubule assembly assay (Fig. 8), the interaction with the microtubular cytoskeleton was not significantly affected by the mutation. Thus, the observed defects in the point mutant appear to be a consequence of the inability of the mutated proteins to degrade RNA. This indicates that the mitotic phenotypes observed in xrn1Δ strains are likely to be indirect effects of the lack of exonuclease activity. It is interesting that cells with an Xrn1 protein that shows normal interactions with microtubules were still hypersensitive to the microtubule-destabilizing drug benomyl. This may suggest an indirect effect of the exonuclease defect on a structural component of the microtubular cytoskeleton (e.g., changed levels of tubulin). However, the benomyl-sensitivity associated with the mutant Xrn1 protein may also be a consequence of a slightly altered morphology of microtubules or a small quantitative effect in microtubule assembly that cannot be fully appreciated in the qualitative microtubule assembly assay.
Mutations in motif II cause separation of function.
The xrn1Δ mutant exhibits an almost quantitative pachytene arrest in meiotic prophase (3, 86). In the few cells that do sporulate, an additional phenotype of reduced spore viability can be recognized (86). The xrn1-D206A and xrn1-D208A point mutants exhibit significantly improved (three- to fivefold) sporulation in comparison to the isogenic xrn1Δ cells. This level is still two- to threefold below the typical wild-type level (Table 2). Similarly, the motif II mutations significantly improved the spore viability in comparison to the xrn1Δ cells, so that it almost reached wild-type levels (see Table 2). The severe reduction of meiotic intrachromosomal popout recombination and to a lesser degree convertants found in the dmc1 xrn1Δ double mutant (86) is greatly alleviated in the dmc1 xrn1-D208A strain (up to fivefold [Table 3]). We interpret this data to mean that the xrn1-D206A and xrn1-D208A mutations cause separation of function, revealing a specific meiotic function of Xrn1p that is less affected in the point mutants. The separation of function between the vegetative and meiotic function in these mutants is not complete. This is typical for separation-of-function mutations and has been noted previously (1, 51, 53, 69). A specific role of Xrn1p in meiosis is also indicated by the isolation of a dominant extragenic suppressor of the xrn1Δ mutation which specifically suppresses the meiotic arrest phenotype and the spore inviability phenotype but none of the vegetative phenotypes (77a).
What is the meiosis-specific role of Xrn1p?
Several models may account for a possible meiosis-specific role of Xrn1p. A trivial explanation would be that the Xrn1-D208A mutant protein retained some residual exonuclease activity that becomes relevant under sporulation conditions. While we consider this possibility highly unlikely because there is no evidence in vivo or in vitro for such a residual activity, it cannot formally be ruled out. It has been shown previously that targeting Rat1p, a strictly nuclear 5′-3′ exoribonuclease that is highly homologous to Xrn1p (2), to the cytoplasm allowed the restoration of efficient sporulation (33). This had been interpreted to mean that only the cytoplasmic exoribonuclease activity of Xrn1p was required for meiosis (33). Unless the Xrn1-D208A mutant protein described here exhibits an exonuclease activity that has not been detected in two in vivo assays (Fig. 2 and 3) and four in vitro assays (Fig. 6 and 7) with single-stranded and double-stranded nucleic acid substrates, this interpretation is not consistent with the present data. We note that Rat1p and Xrn1p exhibit extensive sequence homology that extends well beyond the sequence similarities between the 5′-3′ exonucleases shown in Fig. 1. In particular, the regions from amino acids 621 to 730 in Rat1p and 528 to 637 in Xrn1p are highly homologous (49% identical and 62% similar) (2), but this homology is not shared with other 5′-3′ exonucleases that are not Xrn1p or Rat1p homologs (6). Thus, the two proteins may share more than the 5′-3′ exonuclease function, suggesting that the substitution of the Xrn1p function by cytoplasmic Rat1p may not be as specific to the exoribonuclease function as previously suggested.
A first possible model for a meiosis-specific function of Xrn1p with a direct role in homologous recombination has been proposed on the basis of the in vitro homologous pairing activity of Xrn1p and the meiotic recombination phenotypes of the XRN1 gene deletion (44, 86). The original in vitro homologous pairing assay used was not entirely specific for recombination proteins (38), but Xrn1p also catalyzes paranemic joints in a more specific pairing reaction (15). The yeast protein localization data indicated a largely cytoplasmic localization (28) but left open the possibility of a direct role in the nucleus. Fractionation showed a modest amount of Xrn1p in the nuclear fraction in comparison to controls, while no evidence for a nuclear localization was obtained from in situ immunofluorescence studies in yeast (28) and mammals (6). Targeting Xrn1p to the nucleus by addition of a nuclear localization signal resulted in complementation of a defect in the essential, nuclear Rat1p exonuclease, strongly suggesting that under normal conditions no Xrn1p is nuclear (33). While the model for a direct role in recombination critically depends on some Xrn1p being localized in the nucleus, the second model (see below), suggesting that the interaction of Xrn1p with microtubules is the meiosis-specific function, is consistent with a cytoplasmic localization.
A second possible model proposes that the meiosis-specific function of Xrn1p is related to its interaction with the microtubular cytoskeleton, where it may act as a microtubule-nucleic acid interface (6). Based on the phenotypes of the XRN1 mutant in vegetative cells and on the in vitro property of the Xrn1 protein in inducing tubulin to assemble to microtubules, it was suggested that Xrn1p may have a function in the microtubular cytoskeleton besides its role in RNA turnover and that the two roles may be related (32, 39). Since the cytology in budding yeast did not provide detailed resolution of the cytoplasmic sublocalization of Xrn1p (28), a more detailed study with the mammalian homolog was undertaken (6). In mammalian cells with well-developed cytoplasm, a significant fraction of Xrn1p was associated with the cytoplasmic microtubular cytoskeleton based on immunocolocalization experiments (6). This colocalization was abolished when the microtubules were disrupted by drugs or low temperatures, further corroborating the significance of the immunofluorescence results (6). Although the sensitivity of xrn1 cells to the microtubule-destabilizing drug benomyl may be an indirect consequence of the RNA turnover defect, the cytological results in mammalian cells have significance for the situation in yeast, since the mammalian homolog was found to fully complement all phenotypes of the xrn1 deletion mutant including the meiotic defects (6). Further evidence for a possible role of Xrn1p in other processes than RNA turnover comes from a study with XRN1 point mutants that were exonuclease deficient (63). Overexpression of exonuclease-deficient Xrn1 proteins exerted the same negative effect on vegetative growth as did overexpression of wild-type protein, leading the authors of that study to speculate that Xrn1p is involved in an as yet unidentified function (63). The effect on meiosis of these mutants was not examined in that study (63).
Based on the studies in vegetative yeast and mammalian cells, a possible model for the meiosis-specific role of Xrn1p lies in its interaction with the microtubular cytoskeleton. Several findings suggest the involvement of microtubules in chromosome metabolism during meiotic prophase (reviewed in references 49 and 90). Chromosomal motions in meiotic prophase are inhibited by the microtubule inhibitor colchicine but not by a non-microtubule-binding derivative of colchicine (reviewed in references 49 and 90). In the fission yeast S. pombe, meiosis-specific functions of cytoplasmic microtubules in nuclear movement during meiotic prophase are particularly well documented (16, 83; reviewed in reference 42). Similar nuclear movements in meiotic prophase have been identified in budding yeast, and it is possible that cytoplasmic microtubules play a role in this as well (reviewed in reference 90). The meiotic prophase arrest of xrn1Δ cells leads to structural aberrations in the synaptonemal complex (SC) (3) that are reminiscent of SC aberrations induced by microtubule drugs added during prophase (50). Deletion of the XRN1 homolog in S. pombe also results in meiotic defects, similar to those of xrn1Δ (84), with a highly reduced spore yield (5% of wild type) and reduced spore viability (50 to 60%). Moreover, mutations in the kinesin-like motor protein Kar3 cause meiotic defects (5) that are reminiscent of those caused by an xrn1Δ mutation in particular the SC phenotype (3). Both XRN1 (as KEM1) (39) and KAR3 (65) were genetically identified as karyogamy-defective mutants. Karyogamy is a process involving cytoplasmic microtubules (67), and Kar3p colocalizes in the cytoplasm with microtubules and the spindle pole body (55, 64). The localization of Kar3p during meiosis is not known. In addition, SPO15/VPS1, a gene required for sporulation in budding yeast, has been identified as a dynamin-related GTPase that associates with microtubules in vitro (60, 89). Thus, XRN1, SPO15, and KAR3 may be involved in microtubule-directed chromosome movement during meiotic prophase, but their exact role in meiosis remains to be elucidated (reviewed in reference 90). Such a role of Xrn1p in meiosis does not necessarily depend on nuclear localization, which is consistent with the finding that most if not all Xrn1p is cytoplasmic in yeast and mammalian cells (6, 28, 33). The influence on meiotic processes might be indirect, for example by transporting important molecules to their proper sites of action. A more direct role is envisioned if Xrn1p acts as a connection between microtubules and DNA, possibly telomeres because of its specificity for G4 substrates that may form at telomeric sequences (47, 48). Electron micrographs of meiotic prophase sections in lily show a physical connection of cytoplasmic microtubules to positions of the nuclear envelope to which chromatin (possibly telomeres) is attached (72; reviewed in reference 50). In summary, it appears that microtubule-mediated processes are important in meiotic prophase for chromosome synapsis with likely relevance to meiotic recombination (reviewed in references 49 and 90). We speculate that the meiosis-specific role of Xrn1 protein might be in this process, since the Xrn1-D208A mutant protein is largely proficient for meiosis and is still able to act as a microtubule assembly protein.
ACKNOWLEDGMENTS
We thank D. Botstein, N. Kleckner, and R. Kolodner for kindly supplying strains. The pRDK249 overexpression vector was kindly provided by A. Johnson and R. Kolodner. The plasmid containing the mouse β-actin gene for transcription was kindly supplied by T. Seebeck. We are grateful to S. Edelstein for support and helpful discussion and to I. Andrey Tornare for skillful technical assistance. We thank all members of the Heyer laboratory, especially V. Bashkirov, for help and useful discussions.
This work was supported by a career development award (START) to W.D.H., research grants of the Swiss National Science Foundation to W.D.H. and S. Edelstein, and funds from UC Davis to W.D.H.
REFERENCES
- 1.Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggest an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- 2.Amberg D C, Goldstein A L, Cole C N. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. doi: 10.1101/gad.6.7.1173. [DOI] [PubMed] [Google Scholar]
- 3.Bähler J, Hagens G, Holzinger G, Scherthan H, Heyer W D. Saccharomyces cerevisiae cells lacking the homologous pairing protein p175(SEP1) arrest at pachytene during meiotic prophase. Chromosoma. 1994;103:129–141. doi: 10.1007/BF00352322. [DOI] [PubMed] [Google Scholar]
- 4.Bang D D, Timmermans V, Verhage R, Zeeman A M, Vandeputte P, Brouwer J. Regulation of the Saccharomyces cerevisiae DNA repair gene RAD16. Nucleic Acids Res. 1995;23:1679–1685. doi: 10.1093/nar/23.10.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BascomSlack C A, Dawson D S. The yeast motor protein, Kar3p, is essential for meiosis I. J Cell Biol. 1997;139:459–467. doi: 10.1083/jcb.139.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashkirov V I, Scherthan H, Solinger J A, Buerstedde J M, Heyer W D. A mouse cytoplasmic exoribonuclease (mXRN1p) with preference for G4 tetraplex substrates. J Cell Biol. 1997;136:761–773. doi: 10.1083/jcb.136.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashkirov V I, Solinger J A, Heyer W D. Identification of functional domains in the Sep1 protein (=Kem1, Xrn1), which is required for transition through meiotic prophase in Saccharomyces cerevisiae. Chromosoma. 1995;104:215–222. doi: 10.1007/BF00352186. [DOI] [PubMed] [Google Scholar]
- 8.Beck P J, Gonzalez S, Ward C L, Molineux I J. Sequence of bacteriophage T3 DNA from gene 2.5 through gene 9. J Mol Biol. 1989;210:687–701. doi: 10.1016/0022-2836(89)90102-2. [DOI] [PubMed] [Google Scholar]
- 9.Bellocq C, Andreytornare I, Doret A M P, Maeder B, Paturle L, Job D, Haiech J, Edelstein S J. Purification of assembly-competent tubulin from Saccharomyces cerevisiae. Eur J Biochem. 1992;210:343–349. doi: 10.1111/j.1432-1033.1992.tb17427.x. [DOI] [PubMed] [Google Scholar]
- 10.Bishop D K, Park D, Xu L, Kleckner N. DMC1: A meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- 11.Borodovsky M, Rudd K E, Koonin E V. Intrinsic and extrinsic approaches for detecting genes in a bacterial genome. Nucleic Acids Res. 1994;22:4756–4767. doi: 10.1093/nar/22.22.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caponigro G, Parker R. Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol Rev. 1996;60:233. doi: 10.1128/mr.60.1.233-249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr A M, Sheldrick K S, Murray J M, al-Harithy R, Watts F Z, Lehmann A R. Evolutionary conservation of excision repair in Schizosaccharomyces pombe: evidence for a family of sequences related to the Saccharomyces cerevisiae RAD2 gene. Nucleic Acids Res. 1993;21:1345–1349. doi: 10.1093/nar/21.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceska T A, Sayers J R, Stier G, Suck D. A helical arch allowing single-stranded DNA to thread through T5 5′-exonuclease. Nature. 1996;382:90–93. doi: 10.1038/382090a0. [DOI] [PubMed] [Google Scholar]
- 15.Chen J H, Kanaar R, Cozzarelli N R. The Sep1 strand exchange protein from Saccharomyces cerevisiae promotes a paranemic joint between homologous DNA molecules. Genes Dev. 1994;8:1356–1366. doi: 10.1101/gad.8.11.1356. [DOI] [PubMed] [Google Scholar]
- 16.Chikashige Y, Ding D Q, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, Hiraoka Y. Telomere-led premeiotic chromosome movement in fission yeast. Science. 1994;264:270–273. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]
- 17.Decker C J, Parker R. A turnover pathway for both stable and unstable messenger RNAs in yeast—evidence for a requirement for deadenylation. Genes Dev. 1993;7:1632–1643. doi: 10.1101/gad.7.8.1632. [DOI] [PubMed] [Google Scholar]
- 18.Dykstra C C, Hamatake R K, Sugino A. DNA strand transferase protein β from yeast mitotic cells differs from strand transfer protein α from meiotic cells. J Biol Chem. 1990;265:10968–10973. [PubMed] [Google Scholar]
- 19.Dykstra C C, Kitata K, Clarke A B, Hamatake R K, Sugino A. Cloning and characterization of DST2, the gene for DNA strand transfer protein β from Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2583–2592. doi: 10.1128/mcb.11.5.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann R D, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 21.Fsihi H, Cole S T. The Mycobacterium leprae genome: systematic sequence analysis identifies key catabolic enzymes, ATP-dependent transport systems and a novel polA locus associated with genomic variability. Mol Microbiol. 1995;16:909–919. doi: 10.1111/j.1365-2958.1995.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 22.Gutman P D, Minton K W. Conserved sites in the 5′-3′ exonuclease domain of Escherichia coli DNA polymerase. Nucleic Acids Res. 1993;21:4406–4407. doi: 10.1093/nar/21.18.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington J J, Lieber M R. The characterization of a mammalian DNA structure-specific endonuclease. EMBO J. 1994;13:1235–1246. doi: 10.1002/j.1460-2075.1994.tb06373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington J J, Lieber M R. Functional domains within FEN-1 and RAD2 define a family of structure-specific endonucleases: implications for nucleotide excision repair. Genes Dev. 1994;8:1344–1355. doi: 10.1101/gad.8.11.1344. [DOI] [PubMed] [Google Scholar]
- 25.Henry Y, Wood H, Morrissey J P, Petfalski E, Kearsey S, Tollervey D. The 5′ end of yeast 5.8S rRNA is generated by exonucleases from an upstream cleavage site. EMBO J. 1994;13:2452–2463. doi: 10.1002/j.1460-2075.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heyer W D. The search for the right partner—homologous pairing and DNA strand exchange proteins in eukaryotes. Experientia. 1994;50:223–233. doi: 10.1007/BF01924005. [DOI] [PubMed] [Google Scholar]
- 27.Heyer W D, Evans D H, Kolodner R D. Renaturation of DNA by a Saccharomyces cerevisiae protein that catalyzes homologous pairing and strand exchange. J Biol Chem. 1988;263:15189–15195. [PubMed] [Google Scholar]
- 28.Heyer W D, Johnson A W, Reinhart U, Kolodner R D. Regulation and intracellular localization of Saccharomyces cerevisiae strand exchange protein 1 (Sep1/Xrn1/Kem1), a multifunctional exonuclease. Mol Cell Biol. 1995;15:2728–2736. doi: 10.1128/mcb.15.5.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holler A, Bashkirov V I, Solinger J A, Reinhart U, Heyer W D. Use of monoclonal antibodies in the functional characterization of the Saccharomyces cerevisiae Sep1 protein. Eur J Biochem. 1995;231:329–336. doi: 10.1111/j.1432-1033.1995.tb20704.x. [DOI] [PubMed] [Google Scholar]
- 30.Hollingsworth H C, Nossal N G. Bacteriophage T4 encodes an RNaseH which removes RNA primers made by the T4 DNA replication system in vitro. J Biol Chem. 1991;266:1888–1897. [PubMed] [Google Scholar]
- 31.Hsu C L, Stevens A. Yeast cells lacking 5′→3′ exoribonuclease 1 contain messenger RNA species that are Poly(A) deficient and partially lack the 5′ cap structure. Mol Cell Biol. 1993;13:4826–4835. doi: 10.1128/mcb.13.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Interthal H, Bellocq C, Bähler J, Bashkirov V I, Edelstein S, Heyer W D. A role of Sep1 (=Kem1, Xrn1) as a microtubule-associated protein in Saccharomyces cerevisiae. EMBO J. 1995;14:1057–1066. doi: 10.1002/j.1460-2075.1995.tb07088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson A W. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol Cell Biol. 1997;17:6122–6130. doi: 10.1128/mcb.17.10.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson A W, Kolodner R D. Strand exchange protein 1 from Saccharomyces cerevisiae. A novel multifunctional protein that contains DNA strand exchange and exonuclease activities. J Biol Chem. 1991;266:14046–14054. [PubMed] [Google Scholar]
- 35.Johnson A W, Kolodner R D. Synthetic lethality of sep1 (xrn1) ski2 and sep1 (xrn1) ski3 mutants of Saccharomyces cerevisiae is independent of killer virus and suggests a general role for these genes in translation control. Mol Cell Biol. 1995;15:2719–2727. doi: 10.1128/mcb.15.5.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kane S M, Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974;118:8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Käslin E, Heyer W-D. A multifunctional exonuclease from vegetative Schizosaccharomyces pombe cells exhibiting in vitro strand exchange activity. J Biol Chem. 1994;269:14094–14102. [PubMed] [Google Scholar]
- 38.Käslin E, Heyer W-D. Schizosaccharomyces pombe fatty acid synthetase mediates DNA strand exchange in vitro. J Biol Chem. 1994;269:14103–14110. [PubMed] [Google Scholar]
- 39.Kim J, Ljungdahl P O, Fink G R. kem mutations affect nuclear fusion in Saccharomyces cerevisiae. Genetics. 1990;126:799–812. doi: 10.1093/genetics/126.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y, Eom S H, Wang J, Lee D S, Suh S W, Steitz T A. Crystal structure of Thermus aquaticus DNA polymerase. Nature. 1995;376:612–616. doi: 10.1038/376612a0. [DOI] [PubMed] [Google Scholar]
- 41.Kipling D, Tambini C, Kearsey S E. rar mutations which increase artificial chromosome stability in Saccharomyces cerevisiae identify transcription and recombination proteins. Nucleic Acids Res. 1991;19:1385–1391. doi: 10.1093/nar/19.7.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohli J. Meiosis—telomeres lead chromosome movement. Curr Biol. 1994;4:724–727. doi: 10.1016/s0960-9822(00)00160-3. [DOI] [PubMed] [Google Scholar]
- 43.Kolodner R, Evans D H, Morrison P T. Purification and characterization of an activity from Saccharomyces cerevisiae that catalyzes homologous pairing and strand exchange. Proc Natl Acad Sci USA. 1987;84:5560–5564. doi: 10.1073/pnas.84.16.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolodner R, Hall S D, Luisideluca C. Homologous pairing proteins encoded by the Escherichia coli recE and recT genes. Mol Microbiol. 1994;11:23–30. doi: 10.1111/j.1365-2958.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 45.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 46.Larimer W F, Stevens A. Disruption of the gene XRN1, coding for a 5′-3′ exoribonuclease, restricts yeast cell growth. Gene. 1990;95:85–90. doi: 10.1016/0378-1119(90)90417-p. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z P, Gilbert W. The yeast KEM1 gene encodes a nuclease specific for G4 tetraplex DNA: implication of in vivo functions for this novel DNA structure. Cell. 1994;77:1083–1092. doi: 10.1016/0092-8674(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z P, Lee A, Gilbert W. Gene disruption of a G4-DNA-dependent nuclease in yeast leads to cellular senescence and telomere shortening. Proc Natl Acad Sci USA. 1995;92:6002–6006. doi: 10.1073/pnas.92.13.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loidl J. Cytological aspects of meiotic recombination. Experientia. 1994;50:285–294. doi: 10.1007/BF01924012. [DOI] [PubMed] [Google Scholar]
- 50.Loidl J. The initiation of meiotic chromosome pairing: the cytological view. Genome. 1990;33:759–778. doi: 10.1139/g90-115. [DOI] [PubMed] [Google Scholar]
- 51.Longhese M P, Plevani P, Lucchini G. Replication factor A is required in vivo for DNA replication, repair, and recombination. Mol Cell Biol. 1994;14:7884–7890. doi: 10.1128/mcb.14.12.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez P, Martinez S, Diaz A, Espinosa M, Lacks S A. Characterization of the polA gene of Streptococcus pneumoniae and comparison of the DNA polymerase I it encodes to homologous enzymes from Escherichia coli and phage T7. J Biol Chem. 1989;264:4255–4263. [PubMed] [Google Scholar]
- 53.Marini F, Pellicioli A, Paciotti V, Lucchini G, Plevani P, Stern D F, Foiani M. A role for DNA primase in coupling DNA replication to DNA damage response. EMBO J. 1997;16:639–650. doi: 10.1093/emboj/16.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McEwen B, Edelstein S J. Evidence for a mixed lattice in microtubules in vitro. J Mol Biol. 1980;139:123–145. doi: 10.1016/0022-2836(80)90300-9. [DOI] [PubMed] [Google Scholar]
- 55.Meluh P B, Rose M D. KAR3, a kinesin-related gene required for nuclear fusion. Cell. 1990;60:1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- 56.Mizrahi V, Huberts P. Deoxy- and dideoxynucleotide discrimination and identification of critical 5′ nuclease domain residues of the DNA polymerase I from Mycobacterium tuberculosis. Nucleic Acids Res. 1996;24:4845–4852. doi: 10.1093/nar/24.24.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mueser T C, Nossal N G, Hyde C C. Structure of bacteriophage T4 RNase H, a 5′ to 3′ RNA-DNA and DNA-DNA exonuclease with sequence similarity to the RAD2 family of eukaryotic proteins. Cell. 1996;85:1101–1112. doi: 10.1016/s0092-8674(00)81310-0. [DOI] [PubMed] [Google Scholar]
- 58.Muhlrad D, Decker C J, Parker R. Deadenylation of the unstable mRNA encoded by the yeast Mfa2 gene leads to decapping followed by 5′→3′ digestion of the transcript. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- 59.Muhlrad D, Decker C J, Parker R. Turnover mechanisms of the stable yeast PGK1 mRNA. Mol Cell Biol. 1995;15:2145–2156. doi: 10.1128/mcb.15.4.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obar R A, Collins C A, Hammarback J A, Shpetner H S, Vallee R B. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990;347:256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- 61.Odonovan A, Scherly D, Clarkson S G, Wood R D. Isolation of active recombinant XPG protein, a human DNA repair endonuclease. J Biol Chem. 1994;269:15965–15968. [PubMed] [Google Scholar]
- 62.Padmore R, Cao L, Kleckner N. Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell. 1991;66:1239–1256. doi: 10.1016/0092-8674(91)90046-2. [DOI] [PubMed] [Google Scholar]
- 63.Page A M, Davis K, Molineux C, Kolodner R D, Johnson A W. Mutational analysis of exoribonuclease I from Saccharomyces cerevisiae. Nucleic Acids Res. 1998;26:3707–3716. doi: 10.1093/nar/26.16.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Page B D, Satterwhite L L, Rose M D, Snyder M. Localization of the Kar3 kinesin heavy chain-related protein requires the Cik1 interacting protein. J Cell Biol. 1994;124:507–519. doi: 10.1083/jcb.124.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polaina J, Conde J. Genes involved in the control of nuclear fusion during the sexual cycle of Saccharomyces cerevisiae. Mol Gen Genet. 1982;186:253–258. doi: 10.1007/BF00331858. [DOI] [PubMed] [Google Scholar]
- 66.Robins P, Pappin D J C, Wood R D, Lindahl T. Structural and functional homology between mammalian DNase IV and the 5′-nuclease domain of Escherichia coli DNA polymerase I. J Biol Chem. 1994;269:28535–28538. [PubMed] [Google Scholar]
- 67.Rose M D. Nuclear fusion in the yeast Saccharomyces cerevisiae. Annu Rev Cell Dev Biol. 1996;12:663–695. doi: 10.1146/annurev.cellbio.12.1.663. [DOI] [PubMed] [Google Scholar]
- 68.Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santocanale C, Neecke H, Longhese M P, Lucchini G, Plevani P. Mutations in the gene encoding the 34 kDa subunit of yeast replication protein A cause defective S phase progression. J Mol Biol. 1995;254:595–607. doi: 10.1006/jmbi.1995.0641. [DOI] [PubMed] [Google Scholar]
- 70.Sayers J R. Computer aided identification of a potential 5′-3′ exonuclease gene encoded by Escherichia coli. J Theor Biol. 1994;170:415–421. doi: 10.1006/jtbi.1994.1202. [DOI] [PubMed] [Google Scholar]
- 71.Shelanski M L, Gaskin F, Cantor C R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci USA. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheldon J, Willson C, Dickinson H G. Interaction between the nucleus and cytoskeleton during pairing stages of male meiosis in flowering plants. In: Brandham O E, editor. Kew Chromosome Conference III. London, United Kingdom: HMSO; 1988. pp. 27–35. [Google Scholar]
- 73.Shen B H, Nolan J P, Sklar L A, Park M S. Essential amino acids for substrate binding and catalysis of human flap endonuclease 1. J Biol Chem. 1996;271:9173–9176. doi: 10.1074/jbc.271.16.9173. [DOI] [PubMed] [Google Scholar]
- 74.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 75.Shiomi T, Harada Y N, Saito T, Shiomi N, Okuno Y, Yamaizumi M. An Ercc5 gene with homology to yeast Rad2 is involved in group G xeroderma pigmentosum. Mutat Res. 1994;314:167–175. doi: 10.1016/0921-8777(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 76.Shobuike T, Sugano S, Yamashita T, Ikeda H. Characterization of cDNA encoding mouse homolog of fission yeast dhp1(+) gene: structural and functional conservation. Nucleic Acids Res. 1995;23:357–361. doi: 10.1093/nar/23.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sloboda R D, Rosenbaum J L. Purification and assay of microtubule-associated proteins (MAPs) Methods Enzymol. 1982;85:409–416. doi: 10.1016/0076-6879(82)85041-6. [DOI] [PubMed] [Google Scholar]
- 77a.Solinger, J. A., and W.-D. Heyer. Unpublished observations.
- 78.Stevens A. Eukaryotic nucleases and mRNA turnover. In: Belasco J G, Brawerman G, editors. Control of messenger RNA stability. San Diego, Calif: Academic Press, Inc.; 1993. pp. 449–471. [Google Scholar]
- 79.Stevens A. An exoribonuclease from Saccharomyces cerevisiae: effect of modifications of 5′ end groups on the hydrolysis of substrates to 5′ mononucleotides. Biochem Biophys Res Commun. 1978;81:656–661. doi: 10.1016/0006-291x(78)91586-3. [DOI] [PubMed] [Google Scholar]
- 80.Stevens A. Purification and characterization of a Saccharomyces cerevisiae exoribonuclease which yields 5′-mononucleotides by a 5′-3′ mode of hydrolysis. J Biol Chem. 1980;255:3080–3085. [PubMed] [Google Scholar]
- 81.Stevens A, Hsu C L, Isham K R, Larimer F W. Fragments of the internal transcribed spacer 1 of pre-rRNA accumulate in Saccharomyces cerevisiae lacking 5′-3′ exoribonuclease 1. J Bacteriol. 1991;173:7024–7028. doi: 10.1128/jb.173.21.7024-7028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sugano S, Shobuike T, Takeda T, Sugino A, Ikeda H. Molecular analysis of the Dhp1(+) gene of Schizosaccharomyces pombe—an essential gene that has homology to the Dst2 and Rat1 genes of Saccharomyces cerevisiae. Mol Gen Genet. 1994;243:1–8. doi: 10.1007/BF00283869. [DOI] [PubMed] [Google Scholar]
- 83.Svoboda A, Bahler J, Kohli J. Microtubule-driven nuclear movements and linear elements as meiosis-specific characteristics of the fission yeasts Schizosaccharomyces versatilis and Schizosaccharomyces pombe. Chromosoma. 1995;104:203–214. doi: 10.1007/BF00352185. [DOI] [PubMed] [Google Scholar]
- 84.Szankasi P, Smith G R. Requirement of S. pombe exonuclease II, a homologue of S. cerevisiae Sep1, for normal mitotic growth and viability. Curr Genet. 1996;30:284–293. doi: 10.1007/s002940050134. [DOI] [PubMed] [Google Scholar]
- 85.Tishkoff D X, Johnson A W, Kolodner R D. Molecular and genetic analysis of the gene encoding the Saccharomyces cerevisiae strand exchange protein Sep1. Mol Cell Biol. 1991;11:2593–2608. doi: 10.1128/mcb.11.5.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tishkoff D X, Rockmill B, Roeder G S, Kolodner R D. The sep1 mutant of Saccharomyces cerevisiae arrests in pachytene and is deficient in meiotic recombination. Genetics. 1995;139:495–509. doi: 10.1093/genetics/139.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waga S, Bauer G, Stillman B. Reconstitution of complete SV40 DNA replication with purified replication factors. J Biol Chem. 1994;269:10923–10934. [PubMed] [Google Scholar]
- 88.Yang X, Derbyshire V, Ng K, Sun X C, Grindley N D F, Joyce C M. Biochemical and mutational studies of the 5′-3′ exonuclease of DNA polymerase I of Escherichia coli. J Mol Biol. 1997;268:284–302. doi: 10.1006/jmbi.1997.0967. [DOI] [PubMed] [Google Scholar]
- 89.Yeh E, Driscoll R, Coltrera M, Olins A, Bloom K. A dynamin-like protein encoded by the yeast sporulation gene SPO15. Nature. 1991;349:713–714. doi: 10.1038/349713a0. [DOI] [PubMed] [Google Scholar]
- 90.Zickler D, Kleckner N. The leptotene-zygotene transition of meiosis. Annu Rev Genet. 1998;32:619–697. doi: 10.1146/annurev.genet.32.1.619. [DOI] [PubMed] [Google Scholar]