Abstract
Objective:
To determine the prevalence of clinically important masses among incidental hyper-enhancing liver observations on portal venous phase CT in patients without known malignancy or liver disease.
Methods:
Retrospective search of portal venous phase CTs to identify hyper-enhancing liver observations in patients without cancer or liver disease. Observations were assigned a morphology of homogeneous, hemangioma, or heterogeneous. The reference standard was pathology (n=2), liver protocol CT/MRI (n=40), follow-up portal venous phase CT for ≥ 2 years (n=81), or clinical follow-up for ≥ 5 years (n=107).
Results:
There were no clinically important masses among 83 observations with homogeneous morphology or 110 with hemangioma morphology. There were 2 clinically important masses (1 HCC and 1 hepatic adenoma) among 37 (5.4%) heterogeneous morphology observations.
Conclusions:
Incidental hyper-enhancing liver observations on portal venous phase CT with homogeneous or typical hemangioma morphology in patients without known cancer or liver disease are highly likely benign.
Introduction:
Incidental liver lesions are encountered in up to 30% of patients over 40 years old who undergo computed tomography (CT) of the abdomen (1–4). The majority of such lesions are benign in patients without risk factors such as known primary malignancy or chronic liver disease, and require no further follow-up (3). In particular, hyper-enhancing liver observations are a common source of the incidental liver lesion. Hemangiomas are the most common liver tumor with a prevalence up to 20% and the second most common incidental liver lesion behind hepatic cysts (5, 6). Small hemangiomas typically demonstrate homogeneous hyper-enhancement, the so-called flash-filling appearance, that parallels blood pool enhancement over time. Larger hemangiomas demonstrate peripheral, nodular, and discontinuous hyper-enhancement that progressively fills in over time (7). Other common hyper-enhancing liver observations include focal nodular hyperplasia (FNH), the second most common liver tumor, and transient hepatic attenuation differences (THADs) (8–10).
It is usually possible to differentiate the above benign liver lesions from malignancy on multi-phasic CT or MRI given their characteristic appearances on different phases. However, routine abdominal CT is most commonly performed during a single portal venous phase, and it can be challenging to differentiate benign hyper-enhancing lesions from hyper-enhancing metastases, primary liver malignancies (including hepatocellular carcinoma [HCC] and cholangiocarcinoma), and potentially malignant masses such as hepatic adenomas. Despite the high prevalence of hyper-enhancing liver observations, there is little data regarding the risk of malignancy when they are incidentally detected on portal venous phase CT. Therefore, the purpose of this study is to determine the prevalence of clinically important masses among incidental hyper-enhancing liver observations seen on portal venous phase CT in patients without known malignancy or liver disease.
Materials and Methods:
Patient Population
This was a retrospective study and was approved by the institutional review board. It was Health Insurance Portability and Accountability Act compliant. We performed a search of the radiology database for subjects 18 years and older who underwent portal venous phase CT of the abdomen from January 1, 2011 to March 1, 2015. We performed a keyword search of the radiology reports to identify cases where the following terms occurred within 10 words of either “liver” or “hepatic”: “enhancing,” “hyper-enhancing,” “hypervascular,” “flash,” “hemangioma,” “thad,” and “perfusional.” The electronic medical record was reviewed to exclude subjects with a history of cancer (other than non-melanoma skin cancers) (n=192) or chronic liver disease (n=110). Cases where the CT exam was specifically performed for evaluation of liver lesions were not considered incidental and were excluded (n=86). All reports were manually reviewed by a radiology resident (second year of radiology residency) to confirm a description of a potentially hyper-enhancing liver lesion, and those with no relevant description were excluded. The images of the remaining subjects were then reviewed by the same radiology resident who was blinded to any further clinical, pathological, or follow-up imaging data. Cases with no relevant liver observations upon report or image review (n=880) and those with no reference standard (n=425) were then excluded, leaving a final study population of 230 subjects (111 males and 119 females, mean age 52.5 years ± 13.7 years) (Figure 1).
Figure 1.

Study flow diagram.
Image Analysis
The liver was initially reviewed by the same radiology resident to identify focal observations with enhancement of any part of the observation greater than surrounding liver parenchyma. The single largest diameter in the axial plane was measured on the index and any follow up examinations. In cases with multiple observations, the largest was recorded to avoid clustering bias. Clustering bias occurs when multiple observations are obtained from the same patient and are therefore no longer independent measurements (11). As hemangiomas and THADs can commonly be numerous in a given patient, including multiple observations per patient would introduce clustering bias and could have led to a misleading elevated sample size. Two abdominal radiologists (with 9 and 2 years of post-fellowship experience) blinded to clinical, pathological, and follow-up imaging data then independently reviewed the images to characterize the observations as homogeneous (uniform attenuation throughout the observation), definite hemangioma (all of the following: peripheral, nodular, and discontinuous enhancement), or heterogeneous (neither homogeneous nor definite hemangioma). In cases where the 2 reviewers were discordant (n=60), a third abdominal radiologist independently reviewed the observations and assigned a morphology according to the same definitions above. The morphology assigned by 2 of the 3 reviewers was designated the final morphology for each observation. No case received three different morphologies from all three reviewers.
Reference Standard
The observations were determined to be benign/clinically unimportant or clinically important based on pathology, diagnostic imaging, follow-up imaging, or clinical follow-up. The electronic medical record was searched in all subjects with liver observations for pathologic correlation. If there was no relevant pathology, the picture archiving and communication system was searched for liver protocol MRI or CT to serve as diagnostic imaging. Observations were considered benign if they had the typical appearance of a hemangioma (hypo-attenuating on unenhanced CT or markedly hyper-intense on T2-weighted imaging, and either homogeneous hyper-enhancement paralleling blood pool on all post contrast phases or peripheral, nodular, and discontinuous enhancement with progressive fill in on delayed post contrast phases), or FNH (iso-intensity on T1 and T2-weighted images or mildly hyperintense on T2-weighted images, and homogeneous hyper-enhancement in the late arterial phase with iso-enhancement on portal venous and delayed phases, and iso- or hyper-enhancement on the hepatobiliary phase with a hepatobiliary agent). In the case of FNH, we did not assess for the presence of a central scar as this is not a highly specific or sensitive finding, particularly in small FNHs. CT or MRI with an extracellular contrast agent was not used as diagnostic imaging for FNH. Follow-up imaging was used as a reference standard if there was no pathology or diagnostic imaging. Benignity was defined as resolution of the observation on any subsequent portal venous phase CT or stability or decreased size on subsequent or prior portal venous phase CT ≥ 2 years from the index CT. Growth of the observation at a rate of ≤ 2 mm/year was considered benign (12). Clinical follow-up was used if no other reference standard was available (13–16)Lack of clinically important mass was defined as no clinical evidence of liver malignancy (primary or secondary) and lack of liver tumor related complication (i.e. bleeding) ≥ 5 years from the index CT. Clinically important masses were defined as any malignant mass or potentially malignant mass such as hepatic adenoma. The distribution of reference standard types is shown in Table 1.
Table 1.
Reference standard types for hyper-enhancing liver observations. Numbers in parentheses are ranges.
| Characterization method | Number of observations | Mean time from index CT to reference standard |
|---|---|---|
| Pathology* | 2 | 1.1 years (0–2.2) |
| Diagnostic Imaging** | 40 | 0.4 years (0–4.2) |
| Follow-up Imaging | 81 | 4.7 years (2.0–8.0) |
| Clinical follow up | 107 | 6.5 years (5.0–12.0) |
| Total | 230 |
One HCC and 1 hemangioma
MRI with and without contrast in 34 cases and liver protocol CT in 6 cases.
Imaging Technique
Index CT examinations were performed on a variety of CT equipment. CT scanners included GE 16 & 64 detector row scanners (General Electric Medical Systems, Milwaukee, WI) and Siemens 64 and 128 detector row scanners (Siemens Medical System, Forchheim, Germany). All scans were obtained using a fixed kV of 120. A variable mAs was used for all scans using automated dose modulation. The pitch varied across the scanners. The slice thickness was 5mm and interval was 5.0 mm for all CT examinations. In addition, all CT examinations had 1.25 mm axial reconstructions which were available for review if deemed necessary by the reviewers to better characterize an observation. All examinations were obtained in the portal venous phase (80 seconds after the initiation of the contrast injection) following intravenous administration of 100–125 mL of Omnipaque-350 (GE Healthcare, Cork, Ireland) at a rate of 2 mL/second. For the liver protocol CT examinations, imaging was acquired in the unenhanced, late arterial, portal venous and 3 minute delayed phases after injection of 125 mL of contrast at a rate of 4 cc/second. Otherwise, the CT parameters were as above.
All MRI examinations were performed on a 1.5-T system (Signa, GE Healthcare) with a phased-array torso coil. All patients fasted for at least 4 hours before the examination. All examinations included transverse T2-single shot fast spin echo (SSFSE) (field of view (FOV), 32 cm; slice thickness, 5mm; spacing, 6 mm; repetition time (TR), 2400 msec; echo time (TE), 90 msec; flip angle (FA), 90; matrix, 288 × 192), coronal T2-SSFSE (FOV, 42 cm; slice thickness, 5mm; spacing, 6 mm; TR, 2400 msec; TE, 90 msec; FA, 90; matrix, 288 × 192), transverse T2-fast spin echo (FOV, 32 cm; slice thickness, 5mm; spacing, 6 mm; TR, 1000 msec; TE, 82 msec; FA, 90; matrix, 256 × 192, and axial 2D in/out of phase T1-weighted imaging (FOV, 40 cm; slice thickness, 5 mm; spacing 6 mm; matrix 288 × 160; TR, 150 msec; TE, 2.2/4.4 msec; FA, 90). Transverse pre- and post-contrast T1-weighted 3D spoiled gradient echo pulse (LAVA) sequences (FOV, 36 cm; slice thickness, 5.0 mm; spacing, 2.5 mm; TR, 3.2 msec; TE, 1.4 msec; FA, 12; matrix, 288 × 192) were used. Post-contrast imaging was acquired during the late hepatic arterial, portal venous and 3 minute delayed phases after intravenous administration of 0.1 mmol/kg of gadodiamide (Omniscan, GE Healthcare) at 2 mL/s. For MRI exams with gadoxetic acid, additional 18 minute coronal and 20 minute transverse delayed LAVA sequences were obtained, and 0.025 mmol/kg of contrast was injected intravenously at 2 mL/s.
Statistical Analysis
We estimated Cohen’s kappa with 95% confidence interval to evaluate agreement between readers using the FREQ procedure in SAS® software for Windows® version 9.4. One-sided and two-sided 95% confidence intervals (CI) were calculated using the exact method for binomial proportions. Unpaired t-test was used to compare observation diameter means.
Results:
There were 230 hyper-enhancing liver observations in 230 patients with a mean observation diameter of 2.1 cm ± 14.9 cm (range 0.6–13.8 cm). 83 observations were homogeneous (Figure 2) (mean diameter 1.4 cm ± 7.2 cm) and none were clinically important (0%, 95% CI 0.0–3.5%). Fifteen were characterized by diagnostic imaging and there were 12 hemangiomas and 3 FNHs. Thirty were characterized by follow-up imaging, of which 15 had no growth, 12 resolved, and 3 decreased in size. The remainder had clinical follow-up (n=38).
Figure 2.




61 year-old woman with hemangioma. (a) Axial contrast-enhanced CT image shows a 0.9 cm homogenously hyper-enhancing liver observation in the peripheral right hepatic lobe (black arrow). (B) Axial T2-weighted MR image shows homogeneous marked hyper-intensity (white arrow). Axial T1-weighted images in the arterial (c) and delayed (d) phases shows homogenous arterial phase hyper-enhancement (black arrow) with persistent hyper-enhancement following blood pool on delayed images (black arrow) consistent with a flash-filling hemangioma. The mass was determined to be homogeneous by both reviewers.
There were 110 observations with hemangioma morphology (mean diameter 2.4 cm ± 16.5 cm) and none were clinically significant (0%, 95% CI 0.0–2.7%) (Figure 3). Ten were characterized by diagnostic imaging and all were hemangiomas. Thirty-five were characterized by follow-up imaging and 32 had no growth, 1 resolved, 1 decreased, and 1 grew. The hemangioma that grew was deemed benign and not clinically important owing to its slow growth of minimally above 2 mm/yr [2.0 to 3.5 cm over 5.7 years (2.6 mm/yr)], homogeneous marked hyperintensity on T2-weighted images from an unenhanced MRI, typical hemangioma appearance on multiple subsequent ultrasounds (homogeneous hyperechoic without flow on color Doppler), and typical peripheral, nodular, discontinuous enhancement pattern on multiple single phase CT examinations. One hemangioma had pathologic proof and 64 had clinical follow-up.
Figure 3.

90 year-old woman with hemangioma. Axial contrast-enhanced CT shows a 2.9 cm left liver lesion (arrow) with peripheral, nodular, discontinuous enhancement. Follow-up CT 4.9 years later (not shown) showed no change in size. The mass was determined to be a hemangioma by both reviewers.
There were 37 heterogeneous observations (mean diameter 2.6 cm ± 17.9 cm) and 2 were clinically important (5.4%, 95% CI 0.7–18.2%). One observation was a 3.4 cm hepatic adenoma in a 42 year-old woman (Figure 4). The diagnosis was made by MRI with a hepatobiliary contrast agent. The other was a pathologically proven 10.0 cm HCC in a 26 year-old man with no risk factors for HCC (Figure 5). Fifteen heterogeneous observations were characterized with diagnostic imaging, of which 13 were hemangiomas, 1 FNH, and 1 hepatic adenoma. Sixteen were characterized with follow-up imaging, of which 7 had no growth, 6 decreased and 3 resolved. The remainder had clinical follow-up (n=4).
Figure 4.

42 year-old woman with hepatic adenoma. Axial contrast-enhanced CT shows a 3.4 cm heterogeneously hyper-enhancing liver mass (arrow) in the left hepatic lobe. The mass was determined to be heterogeneous by both reviewers.
Figure 5.
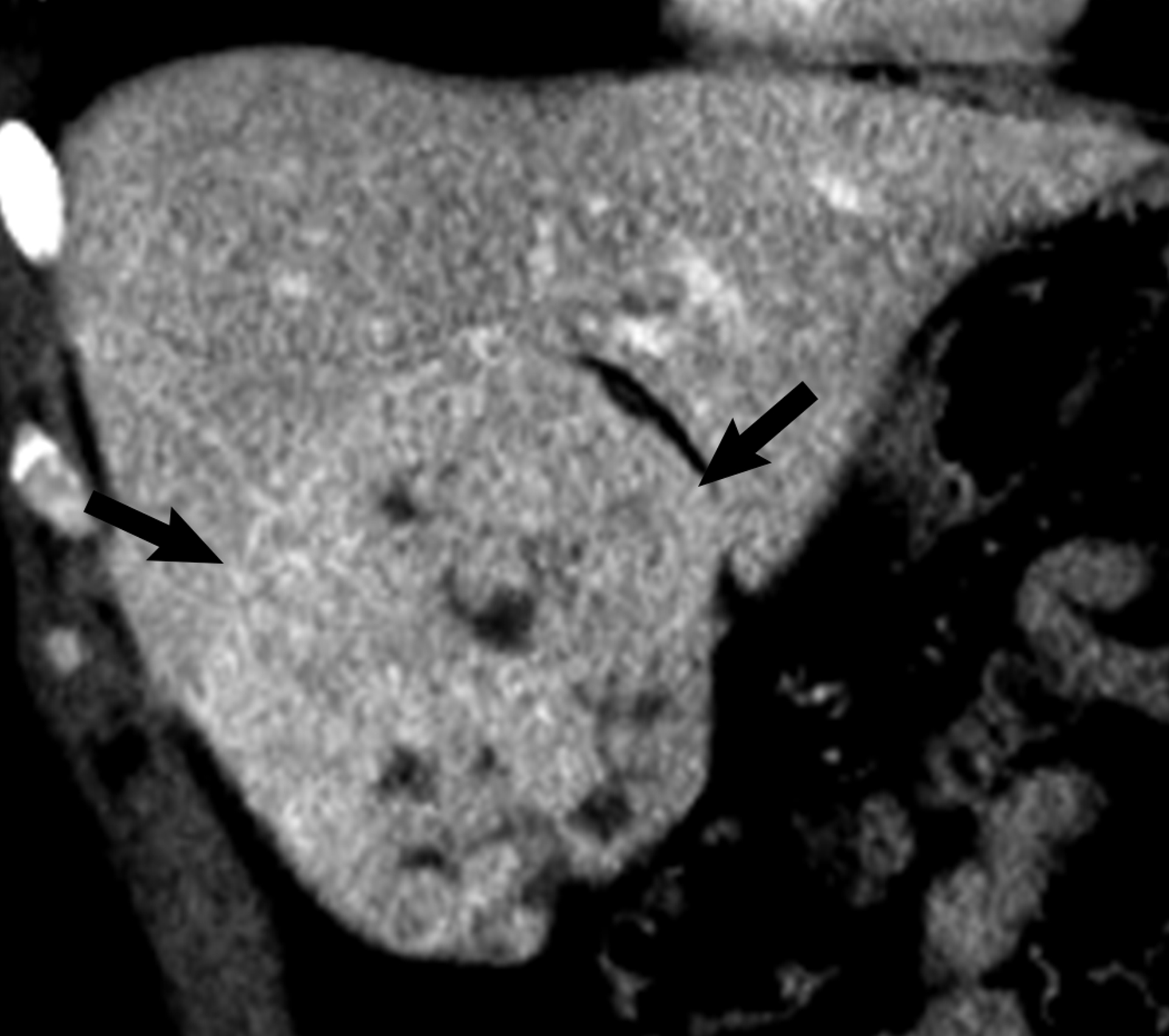
26 year-old man with hepatocellular carcinoma. Coronal contrast-enhanced CT shows a 10.0 cm heterogeneously mildly hyper-enhancing liver mass (arrows). The mass was determined to be heterogeneous by both reviewers.
The mean diameter of the homogeneous observations was significantly less than that of the hemangioma or heterogeneous observations (p<.0001).
Agreement between the two readers for observation morphology was substantial with a weighted kappa of 0.73 (p<.001, 95% CI 0.66–0.79). Both the HCC and hepatic adenoma were deemed heterogeneous by both reviewers.
Discussion:
We found that incidental hyper-enhancing liver observations seen on portal venous phase CT in patients without known cancer or chronic liver disease are highly likely benign if they have homogeneous or typical hemangioma morphology. A small but important proportion (5.4%) of observations with heterogeneous morphology are clinically important.
The typical enhancement pattern of hemangiomas is well described and accepted (7, 17). However, a full assessment requires multiple post-contrast phases to visualize the progressive centripetal enhancement pattern or persistent hyper-enhancement following blood pool in the case of flash-filling hemangiomas. Our study suggests that hemangiomas can be diagnosed with confidence on a single phased portal venous CT when they demonstrate all of the typical enhancement features (peripheral, nodular, and discontinuous enhancement), as all 110 hemangioma observations were benign. Our study also confirms that incidental small homogeneous hyper-enhancing liver observations are highly likely benign. Most of these likely represent small flash filling hemangiomas and THADs with a smaller proportion of FNHs. This is supported by the fact that 12/15 homogeneous observations with diagnostic imaging were hemangiomas and 3/15 were FNH. 15/30 homogeneous observations with follow-up imaging resolved or decreased on subsequent studies and thus were likely THADs. It is important to note the smaller size of the homogeneous observations (mean 1.4 cm). This is likely because small hemangiomas are more likely to be flash-filling than larger ones (18).
We found 2 clinically important lesions among 37 incidental heterogeneous hyper-enhancing liver observations (1 HCC and 1 adenoma). Both observations were large (3.4 and 10.0 cm) and thus size is likely an important factor in determining clinical importance. Most of the heterogeneous observations with diagnostic imaging (13/15) proved to be hemangiomas. These were likely hemangiomas that demonstrated some but not all of the required features of a typical hemangioma during the portal venous phase and therefore could not be diagnosed with confidence.
Our study assessed the clinical importance of incidental hyper-enhancing liver observations stratified by morphology. We are aware of only one other publication addressing the prevalence of malignancy in hypervascular liver lesions in the normal liver. This study by Amico et al. found malignancy in 12.5% of 88 patients with hypervascular liver lesions (19). The study population is different from that in our study as typical hemangiomas were excluded, and one-third of lesions were biopsied, which could result in verification bias towards more suspicious lesions. Furthermore, patients with personal history of cancer and abnormal liver function tests were included and thus these did not represent truly incidental lesions.
Our findings support the 2017 ACR white paper on the management of incidental liver lesions on CT (3). In that paper, flash-filling lesions ≤ 1.5 cm are considered benign in the low risk patient whereas those > 1.5 cm should receive further imaging. Our results support the lack of need for follow-up of incidental small homogeneously hyper-enhancing liver observations in patients without risk factors for hepatic malignancy. Of note, the ACR white paper defines flash-filling as uniform hyper-enhancement relative to hepatic parenchyma on arterial and early portal venous phase images. The CT examinations in our study were performed in the portal venous phase and so our data support extrapolating the ACR guidelines to the portal venous phase, the most common CT phase for routine abdominal imaging. Lesions with “suspicious” features in the ACR white paper correspond with heterogeneous category in our study. Further imaging is recommended for such observations regardless of size. Although many heterogeneous hyper-enhancing observations may prove to be hemangiomas, it is appropriate to maintain a high level of sensitivity for clinically important masses in order not to miss masses such as HCC or hepatic adenoma. Although less common, HCC can occur in patients without underlying liver disease as in one case in our study (20, 21). Although we found no cases in our study, intrahepatic cholangiocarcinoma is a potential mimic of hemangioma as it can demonstrate peripheral hyper-enhancement (22). The enhancement is typically irregular and continuous as opposed to nodular and discontinuous (23). However, on a single phase CT, a hemangioma may be at a point of progressive enhancement that can appear less nodular and be continuous.
Our study has limitations. We performed a key-word search of radiology reports and thus could not assess the prevalence of hyper-enhancing liver observations and may not have captured all relevant lesions. However, we performed a wide keyword search and manually reviewed a large number of reports and images to minimize the chance of missing relevant observations. There were a large number of observations without a reference standard and their outcomes are unknown. We also relied on clinical follow-up as a reference standard when follow-up imaging was not available. Although the exact nature of the observations is unknown in these cases, the minimum time to clinical follow-up of five years ensures that no aggressive malignancies were missed. The determination of observation morphology was subjective. However, our inter-reader agreement was substantial and importantly, both clinically significant lesions were deemed heterogeneous by both reviewers. Although we included 230 observations in total, the sample size for each individual morphology was relatively low. The CT examinations were performed on a variety of equipment, however this may be considered a strength as it may allow for widespread applicability of our findings.
In conclusion, incidental hyper-enhancing liver observations seen on portal venous phase CT in patients without known cancer or liver disease are highly likely benign if they demonstrate homogeneous or typical hemangioma morphology, and no follow-up imaging is necessary. A small but important number of heterogeneous hyper-enhancing liver observations are clinically important, and further imaging should be performed. Our findings support the 2017 ACR white paper management guidelines.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Morgan AE, Berland LL, Ananyev SS, Lockhart ME, Kolettis PN. Extraurinary Incidental Findings on CT for Hematuria: The Radiologist’s Role and Downstream Cost Analysis. AJR American journal of roentgenology. 2015;204(6):1160–7. doi: 10.2214/AJR.14.12483. [DOI] [PubMed] [Google Scholar]
- 2.Kaltenbach TE, Engler P, Kratzer W, Oeztuerk S, Seufferlein T, Haenle MM, et al. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdominal radiology. 2016;41(1):25–32. doi: 10.1007/s00261-015-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gore RM, Pickhardt PJ, Mortele KJ, Fishman EK, Horowitz JM, Fimmel CJ, et al. Management of Incidental Liver Lesions on CT: A White Paper of the ACR Incidental Findings Committee. Journal of the American College of Radiology : JACR. 2017;14(11):1429–37. doi: 10.1016/j.jacr.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Green DE, Woodward PJ. The management of indeterminate incidental findings detected at abdominal CT. Seminars in ultrasound, CT, and MR. 2005;26(1):2–13. doi: 10.1053/j.sult.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Horta G, Lopez M, Dotte A, Cordero J, Chesta C, Castro A, et al. [Benign focal liver lesions detected by computed tomography: Review of 1,184 examinations]. Revista medica de Chile. 2015;143(2):197–202. doi: 10.4067/S0034-98872015000200007. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the L. EASL Clinical Practice Guidelines on the management of benign liver tumours. Journal of hepatology. 2016;65(2):386–98. doi: 10.1016/j.jhep.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Lamba R, Fananapazir G, Corwin MT, Khatri VP. Diagnostic imaging of hepatic lesions in adults. Surgical oncology clinics of North America. 2014;23(4):789–820. doi: 10.1016/j.soc.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Murakami T, Tsurusaki M. Hypervascular benign and malignant liver tumors that require differentiation from hepatocellular carcinoma: key points of imaging diagnosis. Liver cancer. 2014;3(2):85–96. doi: 10.1159/000343864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desser TS. Understanding transient hepatic attenuation differences. Seminars in ultrasound, CT, and MR. 2009;30(5):408–17. doi: 10.1053/j.sult.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Marrero JA, Ahn J, Rajender Reddy K, Americal College of G. ACG clinical guideline: the diagnosis and management of focal liver lesions. The American journal of gastroenterology. 2014;109(9):1328–47; quiz 48. doi: 10.1038/ajg.2014.213. [DOI] [PubMed] [Google Scholar]
- 11.Sica GT. Bias in research studies. Radiology. 2006;238(3):780–9. Epub 2006/03/01. doi: 10.1148/radiol.2383041109. [DOI] [PubMed] [Google Scholar]
- 12.Hasan HY, Hinshaw JL, Borman EJ, Gegios A, Leverson G, Winslow ER. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA surgery. 2014;149(12):1266–71. doi: 10.1001/jamasurg.2014.477. [DOI] [PubMed] [Google Scholar]
- 13.Ip PP, Irving JA, McCluggage WG, Clement PB, Young RH. Papillary proliferation of the endometrium: a clinicopathologic study of 59 cases of simple and complex papillae without cytologic atypia. Am J Surg Pathol. 2013;37(2):167–77. Epub 2012/12/06. doi: 10.1097/PAS.0b013e318272d428. [DOI] [PubMed] [Google Scholar]
- 14.Corwin MT, Altinmakas E, Asch D, Bishop KA, Boge M, Curci NE, et al. Clinical importance of incidental homogeneous renal masses 10–40 mm and 21–39 Hounsfield Units at portal venous-phase CT: A 12-institution retrospective cohort study. AJR American journal of roentgenology. 2020. Epub 2020/08/28. doi: 10.2214/AJR.20.24245. [DOI] [PubMed] [Google Scholar]
- 15.Corwin MT, Siewert B, Sheiman RG, Kane RA. Incidentally detected gallbladder polyps: is follow-up necessary?--Long-term clinical and US analysis of 346 patients. Radiology. 2011;258(1):277–82. Epub 2010/08/11. doi: 10.1148/radiol.10100273. [DOI] [PubMed] [Google Scholar]
- 16.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental indeterminate adrenal mass on CT (> 10 H) in patients without cancer: is further imaging necessary? Follow-up of 321 consecutive indeterminate adrenal masses. AJR American journal of roentgenology. 2007;189(5):1119–23. Epub 2007/10/24. doi: 10.2214/AJR.07.2167. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita Y, Ogata I, Urata J, Takahashi M. Cavernous hemangioma of the liver: pathologic correlation with dynamic CT findings. Radiology. 1997;203(1):121–5. doi: 10.1148/radiology.203.1.9122378. [DOI] [PubMed] [Google Scholar]
- 18.Vilgrain V, Boulos L, Vullierme MP, Denys A, Terris B, Menu Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc 2000;20(2):379–97. doi: 10.1148/radiographics.20.2.g00mc01379. [DOI] [PubMed] [Google Scholar]
- 19.Amico EC, Alves JR, Souza DLB, Salviano FAM, Joao SA, Liguori AAL. Hypervascular Liver Lesions in Radiologically Normal Liver. Arquivos brasileiros de cirurgia digestiva : ABCD = Brazilian archives of digestive surgery. 2017;30(1):21–6. doi: 10.1590/0102-6720201700010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaddikeri S, McNeeley MF, Wang CL, Bhargava P, Dighe MK, Yeh MM, et al. Hepatocellular carcinoma in the noncirrhotic liver. AJR American journal of roentgenology. 2014;203(1):W34–47. doi: 10.2214/AJR.13.11511. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wang C, Xu H, Xiao P, Gao Y. Hepatocellular carcinoma in the noncirrhotic liver: a literature review. European journal of gastroenterology & hepatology. 2019;31(7):743–8. doi: 10.1097/MEG.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 22.Seo N, Kim DY, Choi JY. Cross-Sectional Imaging of Intrahepatic Cholangiocarcinoma: Development, Growth, Spread, and Prognosis. AJR American journal of roentgenology. 2017;209(2):W64–W75. doi: 10.2214/AJR.16.16923. [DOI] [PubMed] [Google Scholar]
- 23.Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2009;29(3):683–700. doi: 10.1148/rg.293085729. [DOI] [PubMed] [Google Scholar]


