Abstract
Background:
An improved understanding of the neurodevelopmental differences between attention deficit hyperactivity disorder with and without prenatal alcohol exposure (ADHD + PAE and ADHD−PAE, respectively) is needed. Herein, we evaluated gyrification (cortical folding) in children with ADHD + PAE compared to that in children with familial ADHD−PAE and typically developing (TD) children.
Methods:
ADHD + PAE (n = 37), ADHD−PAE (n = 25), and TD children (n = 27), aged 8–13 years, were compared on facial morphological, neurobehavioral, and neuroimaging assessments. Local gyrification index (LGI) maps were compared between groups using general linear modelling. Relationships between LGI and clincobehavioral parameters in children with ADHD ± PAE were evaluated using multivariate partial least squares.
Results:
ADHD + PAE and ADHD−PAE groups showed significantly lower LGI (relative to TD) in numerous regions, overlapping in medial prefrontal, parietal, and temporo-occipital cortices (p < 0.001). However, LGI in left mid-dorsolateral prefrontal cortex was uniquely lower in the ADHD + PAE group (p < 0.001). Partial least squares analysis identified one significant latent variable (accounting for 59.3 % of the crossblock correlation, p < 0.001), reflecting a significant relationship between a profile of lower LGI in prefrontal (including left mid-dorsolateral), insular, cingulate, temporal, and parietal cortices and a clinicobehavioral profile of PAE, including a flat philtrum and upper vermillion border, lower IQ, poorer behavioral regulation scores, and greater hyperactivity/impulsivity.
Conclusions:
Children with ADHD + PAE uniquely demonstrate lower mid-dorsolateral LGI, with widespread lower LGI related to more severe facial dysmorphia and neurobehavioral impairments. These findings add insight into the brain bases of PAE symptoms, potentially informing more targeted ADHD treatments based on an objective differential diagnosis of ADHD + PAE vs. ADHD−PAE.
Keywords: Attention deficit hyperactivity disorder, Facial dysmorphology, Fetal alcohol spectrum disorders, Gyrification, Magnetic resonance imaging, Prenatal alcohol exposure
1. Introduction
Prenatal alcohol exposure (PAE) can cause abnormal brain development, facial dysmorphia, deficient growth, and behavioral and neurocognitive deficits (O’Connor, 2014), as well as increased risk for preadolescent alcohol experimentation (Lees et al., 2020) and alcohol use disorders in adulthood (Duko et al., 2020), resulting in a significant public health burden. While fetal alcohol spectrum disorders (FASD) affect up to 5% of children in the United States (May et al., 2018), it still remains greatly underdiagnosed in the general population. One issue is that children with PAE typically exhibit symptoms of attention deficit hyperactivity disorder (ADHD); thus, they are often diagnosed with ADHD and treated with stimulants despite limited evidence for their efficacy in children with ADHD + PAE (Doig et al., 2008; Frankel et al., 2006; Fryer et al., 2007; O’Connor, 2014; O’Malley and Nanson, 2002; Oesterheld et al., 1998; Peadon et al., 2009; Snyder et al., 1997). As ADHD + PAE is likely to be a distinct ADHD subtype, with an earlier onset, different clinical presentation, and different white-matter pathology than in non-PAE subtypes (ADHD−PAE) (Coles et al., 1997; Mattson et al., 2013; O’Neill et al., 2019), further research on the neurodevelopmental differences between ADHD + PAE and ADHD−PAE is imperative in order to refine future treatment approaches.
Neuroimaging studies have reported numerous PAE-related brain alterations, from infancy into adulthood (Wozniak et al., 2019). One important brain morphologic feature is gyrification (cortical folding), which enables more cortex to fit within the cranium and promotes the efficiency of neuronal connections (White et al., 2010). Greater gyrification, especially in frontal areas, is associated with better executive function performance and higher IQ (Gautam et al., 2015; Luders et al., 2008). The vast majority of the gyrification process occurs during gestation and, thus, may be especially susceptible to prenatal environmental stressors, such as PAE (Ishii and Hashimoto-Torii, 2015; Raznahan et al., 2011). After the first few months of life, gyrification generally shows a slight gradual decline lasting into early adulthood (Armstrong et al., 1995; Raznahan et al., 2011). Previous studies have shown widespread lower gyrification in children and adolescents with PAE compared to that in typically developing (TD) individuals (Hendrickson et al., 2018, 2017; Infante et al., 2015). However, potential differences between ADHD + PAE and ADHD−PAE in terms of gyrification remain unexplored.
Based on the above, we evaluated gyrification in children (aged 8–13 years) with ADHD + PAE compared to that in children with ADHD−PAE and TD children. To obtain a homogeneous ADHD−PAE comparison group with a well-defined ADHD etiology, this group only included children who met criteria for familial ADHD, verified by at least one first-degree relative with a similar diagnosis. To our knowledge, this is the first study to use this design to understand the differences between children with ADHD with and without PAE. We hypothesized that children with ADHD + PAE would have lower gyrification compared to both children with familial ADHD−PAE and TD children, especially in frontal regions, related to more impaired neurocognitive and behavioral functions.
2. Materials and methods
2.1. Participants
Three groups of children aged 8–13 years were included in the sample: 1) children with ADHD and PAE (ADHD + PAE; n = 37); 2) children with familial ADHD and without PAE (ADHD−PAE; n = 25); and 3) typically developing children with neither ADHD nor PAE (TD; n = 27). Children were recruited from community organizations, FASD parent organizations, national websites, other pediatric research studies, or physician referrals from local child psychiatry or pediatric clinics. This study was approved by the UCLA Institutional Review Board (IRB); written informed assent/consent was obtained from all participants.
Inclusion criteria for the ADHD + PAE group were as follows: ADHD by DSM-5 criteria for ADHD (any subtype) according to the clinician-administered computerized Schedule for Affective Disorders and Schizophrenia for School Aged Children, Parent Version (K-SADS) (Kaufman et al., 1997; Townsend et al., 2020) and the Conners-3 Parent Form (Conners, 2008); and diagnostic features of fetal alcohol syndrome (FAS), partial fetal alcohol syndrome (pFAS), or alcohol-related neurodevelopmental disorder (ARND) according to the Institute of Medicine (IOM) criteria proposed in updated guidelines (Hoyme et al., 2016; O’Connor et al., 2019). Four key diagnostic features of FAS were evaluated: 1) growth retardation; 2) the FAS facial phenotype, including short palpebral fissures, flat philtrum, and flat upper vermillion border (see details below); 3) neurodevelopmental dysfunction; and 4) gestational alcohol exposure. Alcohol exposure was assessed using the Health Interview for Women (HIW) or the Health Interview for Adoptive and Foster Parents (HIAFP) (Quattlebaum and O’Connor, 2013). The HIW assesses frequency and quantity of typical and binge drinking and use of other teratogens prior to and following recognition of pregnancy. For adopted/fostered participants, information on prenatal exposure to alcohol and other teratogens was obtained via birth, medical, or adoption records or reports by reliable informants. Because many individuals with FASD are adopted or fostered, it is often necessary to use such records, and this is a method accepted in the scientific community for establishing PAE (CDC, 2004). No child in the ADHD + PAE group was accepted without a clear history of PAE. Criteria for alcohol exposure included >6 drinks/wk for ≥2 wk and/or ≥3 drinks on ≥2 occasions including the time periods prior to and following pregnancy recognition. These criteria are based on findings that 1 drink/day (or >6 drinks/wk) is an adequate measure of exposure for FASD, and on epidemiologic studies demonstrating adverse fetal effects of episodic drinking of ≥3 drinks per occasion (Hoyme et al., 2016). Although alcohol use was measured retrospectively, studies show that recall of drinking during pregnancy predicts neurodevelopmental outcomes as far back as 14 years past delivery (Hannigan et al., 2010).
Inclusion criteria for the ADHD−PAE group were as follows: ADHD (defined as above); at least one first-degree family member (i.e., biological parent or sibling) diagnosed with ADHD; and PAE < 2 standard drinks (1.20 oz absolute alcohol) during gestation. Inclusion criteria for the TD group were as follows: no current or lifetime history of an Axis I mental disorder by K-SADS interview; and PAE < 2 standard drinks during gestation.
The exclusion criteria for all participants were as follows: estimated Full-scale IQ < 70 on the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) (Wechsler, 2011); known genetic syndrome associated with ADHD, including fragile X, tuberous sclerosis, and generalized resistance to thyroid hormone; pervasive developmental disorder; serious medical or neurologic illness likely to influence cognition or brain function or brain anatomy (e.g. a seizure disorder or history of closed-head trauma); gestation <34 weeks; history of claustrophobia; ferromagnetic metal implant, braces, or other contraindication to MRI; primary language at home was not English; unable to comply with study procedures; or poor MRI scan quality, resulting in inaccurate surface models despite manual editing.
2.2. Facial morphological assessments
The philtrum was rated from 1 to 5 based on the updated IOM guidelines for FAS, with a rating of 5 reflecting a flat philtrum and ratings of 4-5 meeting FAS criteria (Hoyme et al., 2016). The upper vermillion border was similarly rated from 1 to 5, with a rating of 5 reflecting a flat upper vermillion border and ratings of 4-5 meeting FAS criteria. In addition, the lengths of the right and left palpebral fissures were automatically calculated from digital photos of the participants’ faces (with the exception of 4 participants who only had manual assessments of palpebral fissure length). The average of the right and left palpebral fissures was used for analysis.
2.3. Neurobehavioral assessments
Participants completed the WASI-II (Wechsler, 2011) as a measure of IQ (Full-scale IQ); higher scores indicate higher IQ. Parents completed the Conners, Third Edition (Conners-3) (Conners, 2008) and the Behavioral Rating Inventory of Executive Functions (BRIEF2) (Gioia et al., 2015). The Conners-3 is used to assess the degree to which a child displays clinically significant symptoms of ADHD, with higher scores indicating greater symptom severity. In the present analysis, we utilized the scores of the Inattention and Hyperactivity/Impulsivity subscales. The BRIEF2 measures a child’s executive functioning skills in everyday settings as reported by the parent(s). The BRIEF2 comprises 9 scales that form 3 index scores (the Behavioral Regulation Index [BRI], comprising the Inhibit and Self Monitor scales; the Emotional Regulation Index [ERI], comprising the Shift and Emotional Control scales; and the Cognitive Regulation Index [CRI], comprising the Initiate, Working Memory, Plan/Organize, Task Monitor, and Organization of Materials scales). Higher scores indicate poorer executive function. All neurobehavioral parameters were converted into age-normed T-scores (mean = 50, standard deviation = 10).
2.4. Neuroimaging acquisition and pre-processing
Participants underwent extensive desensitization and training in keeping the head and other body parts still prior to MRI scanning. During scanning, participants watched a children’s movie of their choice and were monitored via video feed from within the MRI unit; participants were reminded to hold still when motion was detected. A high-resolution T1-weighted MRI was acquired as a sagittal whole-brain multi-echo magnetization-prepared rapid gradient-echo (MPRAGE) scan with real-time motion correction (TR/TEs 2500/1.81, 3.6, 5.39, 7.18 ms; TI 1000 ms; flip angle 8°; voxel dimensions 0.8 × 0.8 × 0.8 mm3) using a 3 T Siemens Prisma-Fit system (Siemens, Erlangen, Germany) with a 32-channel head coil. The four echoes were combined into one root mean squared image. Subsequently, a T2-weighted MRI was acquired (TR/TE 3200/564 ms). The T1- and T2-weighted pulse-sequences were from the Human Connectome Project (HCP; http://protocols.humanconnectome.org/HCP/3T/imaging-protocols.html).
Cortical reconstruction and volumetric segmentation were performed using updated HCP pipelines (Glasser et al., 2013), which utilize both the T1- and T2-weighted images to reconstruct the white and pial matter surfaces in FreeSurfer 6.0 (http://surfer.nmr.harvard.edu) (Dale et al., 1999). The resulting surfaces were visually inspected for accuracy and manually edited if necessary. Subsequently, local gyrification index (LGI) maps were calculated in FreeSurfer 6.0, using a 3-dimensional approach (Schaer et al., 2012, 2008). The LGI reflects the ratio of the amount of exposed cortical surface to the amount of cortical surface buried within the sulcul folds at each vertex. LGI values range from 1 to 5, with higher values representing greater gyrification and a value of 1 indicating a flat cortical surface. Individual LGI maps were normalized to a common template (fsaverage). As LGI maps are inherently smoothed, no additional smoothing was performed.
2.5. Statistical analysis
Group differences in demographic, morphological, and behavioral characteristics were evaluated in analyses of variance (ANOVAs) for continuous variables and in chi-square or Fisher’s exact tests for categorical variables using SPSS v26 (IBM Corp., Armonk, NY).
Group differences in LGI were evaluated in a general linear model (GLM) using mri_glmfit in FreeSurfer, controlling for sex and age. Although an estimate of the total intracranial volume or total brain volume is often, but not always, entered as a nuisance variable in studies of gyrification, a smaller cranial size is an intrinsic feature of PAE and cannot be separated from PAE; thus, controlling for this variable could obscure and produce anomalous results (Miller and Chapman, 2001). Contrasts comprised simple pairwise comparisons. In addition, we performed conjunction analyses to identify differences from TD common to both ADHD + PAE and ADHD−PAE (overlap of ADHD + PAE vs TD and ADHD−PAE vs TD) and unique to ADHD + PAE (overlap of ADHD + PAE vs ADHD−PAE and ADHD + PAE vs TD). Cluster-wise correction for multiple comparisons with adjustment for two hemispheres was performed using Monte Carlo simulation by mri_glmfit-sim in FreeSurfer (10,000 iterations).
Partial least squares (PLS) analysis was applied to evaluate the relationship between LGI and clinical/behavioral parameters among children with ADHD ± PAE, using freely available code (http://www.rotman-baycrest.on.ca/pls) (McIntosh et al., 1996). PLS is a multivariate statistical technique that identifies weighted patterns of variables in two blocks of variables (in this case, an LGI block and a clinicobehavioral block) that maximally covary with each other (Krishnan et al., 2011; McIntosh et al., 1996; McIntosh and Lobaugh, 2004). The clinicobehavioral block included the following measures: PAE; Full-scale IQ; the philtrum rating, upper vermillion border rating, and average length of the bilateral palpebral fissures, as facial parameters; BRI, ERI, and CRI scores, as neurobehavioral parameters; and Conners-3 Inattention and Hyperactivity/Impulsivity scores as ADHD severity parameters. In the present setting, PLS produced a set of latent variables (LVs), each comprising a spatial map (vertex loadings) paired with a clinicobehavioral profile (clinicobehavioral parameter loadings) and a singular value indicating the amount of the cross-block covariance accounted for by the LV. The statistical significance of the overall patterns identified by PLS was evaluated using permutation testing (1000 permutations); importance of each feature (LGI at each vertex, each clinicobehavioral measure) was assessed using bootstrap estimation (1000 samples); and stability of the patterns was assessed using split-half resampling (100 times).
3. Results
3.1. Participant characteristics
Demographic and clinicobehavioral data are summarized in Table 1. Groups did not differ on demographic characteristics. Significantly, the philtrum and upper vermillion border received higher ratings (indicating flatter philtrum and upper vermillion border) and the Full-scale IQ was significantly lower in the ADHD + PAE group than in the ADHD−PAE and the TD groups (p’s<0.01); and the BRI, ERI, and CRI on the BRIEF2, and Inattentive and Hyperactivity/Impulsivity scores on the Conners-3 were significantly higher in the ADHD + PAE and ADHD−PAE groups than in the TD group (p’s<0.001).
Table 1.
Demographic and clinical characteristics.
| ADHD + PAE |
ADHD− PAE |
TD | Overall p- value |
|
|---|---|---|---|---|
| N | 37 | 25 | 27 | |
| Male Sex, N (%) | 24(64.9) | 17(68.0) | 15(55.6) | .62 |
| Age, yrs | 9.7 ± 0.2 | 10.3 ± 0.3 | 10.4 ± 0.3 | .08 |
| Race/ethnicity, N (%) | .15‡ | |||
| White | 11(29.7) | 16(64.0) | 11(40.7) | |
| Black | 6(16.2) | 1(4.0) | 0(0) | |
| Latino | 7(18.9) | 1(4.0) | 8(29.6) | |
| Asian | 2(5.4) | 4(16) | 3(11.1) | |
| Other | 2(5.4) | 0(0) | 1(3.7) | |
| Mixed | 9(18.0) | 3(12.0) | 4(14.8) | |
| Mother’s education, yrs | 15.9 ± 0.3 | 17.0 ± 0.7 | 17.6 ± 1.0 | .15 |
| Philtrum rating | 3.6 ± 0.1† | 3.3 ± 0.1* | 2.8 ± 0.1 | <.001 |
| Upper lip rating | 3.6 ± 0.1† | 3.1 ± 0.1* | 2.7 ± 0.1 | <.001 |
| Palpebral fissures | 24.0 ± 0.3* | 24.5 ± 0.4* | 25.6 ±0.4 | .006 |
| Full-scale IQ | 96.7 ± 2.3† | 109.7 ± 2.7 | 113.4 ± 3.4 | <.001 |
| BRI | 71.5 ± 1.3* | 68.0 ± 2.0* | 43.3 ± 1.1 | <.001 |
| ERI | 68.0 ± 1.5* | 66.0 ± 1.8* | 44.9 ± 1.0 | <.001 |
| CRI | 70.1 ± 1.1* | 69.2 ± 1.6* | 45.1 ± 1.5 | <.001 |
| Inattention | 82.8 ± 1.4* | 81.8 ± 1.9* | 48.2 ± 1.9 | <.001 |
| Hyperactivity/impulsivity | 84.1 ± 1.4* | 80.3 ± 2.5* | 49.3 ± 2.2 | <.001 |
Mother’s education is a proxy for socioeconomic status.
ADHD, attention deficit hyperactivity disorder; BRI, Behavioral Regulation Index; CRI, Cognitive Regulation Index; ERI, Emotional Regulation Index; PAE, prenatal alcohol exposure; TD, typically developing.
p < 0.05 ADHD+/−PAE vs TD.
p < 0.05 ADHD + PAE vs ADHD−PAE.
White vs non-White.
3.2. Group differences in LGI
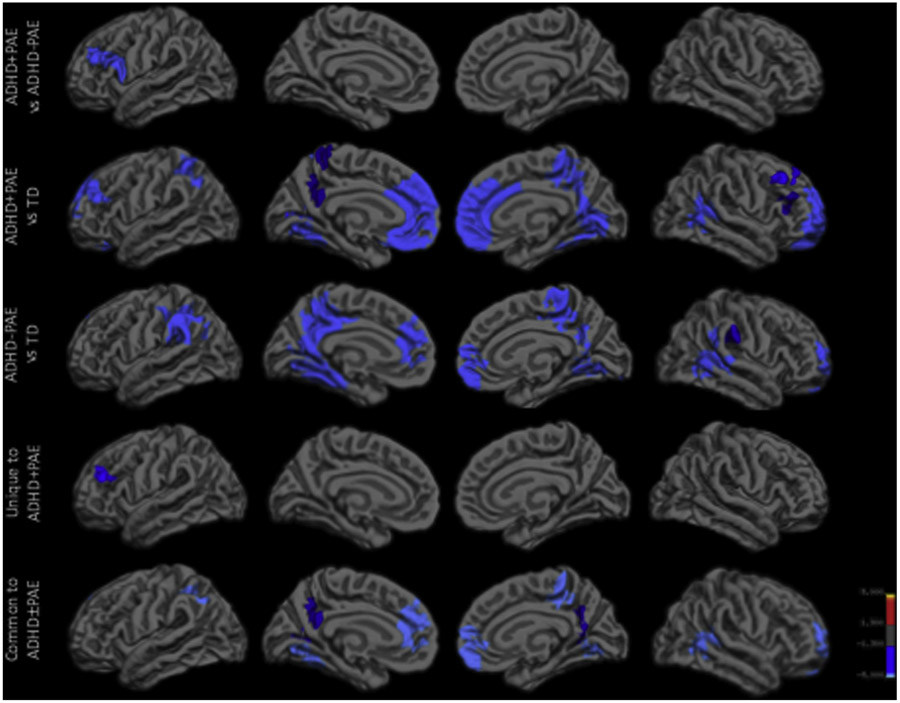
Controlling for sex and age, the ADHD + PAE group had significantly lower LGI compared to that in both the ADHD−PAE and TD groups in the left prefrontal cortex (Fig. 1; Table 2). Additionally, the ADHD + PAE group had significantly lower LGI compared to that in the TD group in cingulate and parietal cortices (Fig. 1; Table 2). There were no areas of higher LGI in the ADHD + PAE group compared to that in ADHD−PAE and TD groups. The conjunction analysis of significance on both ADHD + PAE vs TD and ADHD−PAE vs TD revealed overlapping areas of lower LGI in children with ADHD regardless of PAE, including medial prefrontal, parietal, and temporo-occipital cortices. The conjunction analysis of significance on both ADHD + PAE vs ADHD−PAE and ADHD + PAE vs TD revealed that ADHD + PAE group had uniquely lower LGI in the left mid-dorsolateral prefrontal cortex (Fig. 1; Table 2). A conjunction analysis of significance on both ADHD + PAE vs ADHD−PAE and ADHD−PAE vs TD failed to show any areas of uniquely lower LGI in children with ADHD−PAE.
Fig. 1.
Group differences in LGI, controlling for sex and age, are shown for each pairwise contrast and conjunction analysis. ADHD, attention deficit hyperactivity disorder; LGI, local gyrification index; PAE, prenatal alcohol exposure; TD, typically developing.
Table 2.
Group differences in LGI.
| Contrast | ||||
|---|---|---|---|---|
| Peak Region | MNI coordinates |
Peak Value |
Size (mm2) |
Corrected p- value |
| ADHD + PAE vs ADHD−PAE | ||||
| Left 46 | −28 32 30 | −4.4 | 2342 | <.001 |
| ADHD + PAE vs TD | ||||
| Left 9p | −17 48 35 | −4.8 | 5757 | <.001 |
| Left 7PC | −33 −53 62 | −3.8 | 2314 | <.001 |
| Left V3 | −18 −75 −10 | −2.7 | 1428 | <.001 |
| Left V1 | −14 −82 5 | −2.3 | 982 | <.001 |
| Left d23ab | −5 −48 26 | −2.4 | 552 | .03 |
| Left 5L | −16 −45 58 | −2.4 | 543 | .03 |
| Right a10p | 28 58 −10 | −4.5 | 6938 | <.001 |
| Right V2 | 15 −72 −5 | −3.8 | 6375 | <.001 |
| Right TPOJ2 | 58 −56 6 | −3.3 | 2321 | <.001 |
| Right 46 | 25 28 35 | −3.1 | 765 | .003 |
| Right IFSp | 41 25 21 | −3.1 | 587 | .02 |
| ADHD−PAE vs TD | ||||
| Left 31pd | −9 −54 38 | −3.5 | 7058 | <.001 |
| Left PF | −55 −45 30 | −4.3 | 3879 | <.001 |
| Left 9 m | −9 46 22 | −3.1 | 1371 | <.001 |
| Right TPOJ1 | 49 −35 5 | −3.8 | 3263 | <.001 |
| Right V2 | 14 −72 −4 | −2.7 | 2578 | <.001 |
| Right p32 | 12 48 0 | −2.6 | 2013 | <.001 |
| Right 5 m | 7 −35 54 | −2.8 | 1577 | <.001 |
| Right PF | 50 −32 31 | −3.8 | 651 | .009 |
| ADHD ± PAE vs TDa | ||||
| Left 9 m | −9 46 22 | −3.1 | 1370 | <.001 |
| Left IP2 | −32 −52 34 | −2.3 | 1240 | <.001 |
| Left V2 | −13 −64 −3 | −2.3 | 1028 | <.001 |
| Left V1 | −17 −75 10 | −1.9 | 702 | .001 |
| Left d23ab | −5 −48 26 | −2.4 | 544 | .02 |
| Right p32 | 12 48 −1 | −2.6 | 2001 | <.001 |
| Right TPOJ2 | 58 −56 6 | −3.1 | 1864 | <.001 |
| Right V2 | 14 −72 −4 | −2.9 | 1451 | <.001 |
| Right 5 m | 7 −35 54 | −2.7 | 1073 | <.001 |
| Right 7m | 9 −57 35 | −2.0 | 540 | .02 |
| ADHD + PAE vs ADHD−PAE/CONa | ||||
| Left 46 | −41 40 325 | −2.9 | 734 | <.001 |
ADHD, attention deficit hyperactivity disorder; LGI, local gyrification index; MNI, Montreal Neurological Institute; PAE, prenatal alcohol exposure; TD, typically developing; TPOJ, temporo-parieto-occipital junction.
Conjunction analysis performed to determine regions common to both pairwise contrasts (areas of overlap).
3.3. Relationships between LGI and clinicobehavioral parameters
The PLS analysis identified one significant LV (accounting for 59.3 % of the covariance between LGI and clinicobehavioral parameters; overall: p = 0.001, split-half: p_brain = 0.03, p_behav = 0.04), indicating that among children with ADHD, a profile of lower LGI in large portions of the prefrontal, insular, cingulate, temporal, and parietal cortices was associated with a clinicobehavioral profile of PAE, including higher philtrum and vermillion border ratings (greater dysmorphia); lower Full-scale IQ; higher BRI scores on the BRIEF2 (poorer behavioral regulation); and higher Hyperactivity/Impulsivity scores on the Conners-3 (greater ADHD severity) (Fig. 2; Table 3). The relationship between the LGI and clinicobehavioral profiles remained significant when controlling for sex and age. Palpebral fissure length, ERI and CRI on the BRIEF2, and Inattention scores on the Conners-3 did not reach statistical significance.
Fig. 2.
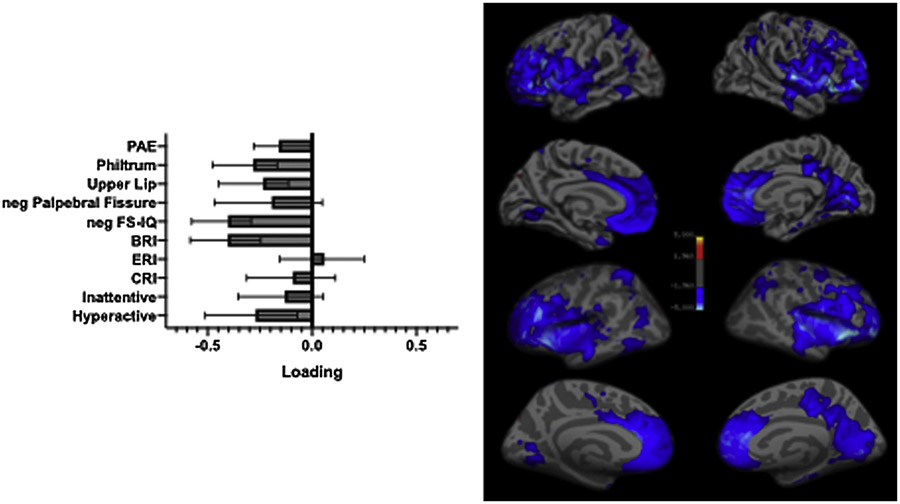
Significant relationship between LGI and clincobehavioral profiles as identified partial least squares analysis. The left panel indicates the importance of individual clinicobehavioral features to the overall pattern, with error bars indicating bootstrap-estimated confidence intervals. The negative of the palpebral fissure length and Full-scale IQ are plotted so that the worse/more severe endpoint of all clinicobehavioral features is toward the left. The right panel indicates the importance of individual vertices to the overall pattern according to the bootstrap ratio (approximately equivalent to a z-score). The results are shown on both pial (upper) and inflated surfaces (lower). ADHD, attention deficit hyperactivity disorder; BRI, behavioral regulation index; CRI, cognitive regulation index; ERI, emotional regulation index; FS-IQ, Full-scale IQ; LGI, local gyrification index.
Table 3.
Location of peak vertices from the partial least squares analyses, depicted in Fig. 2.
| Peak Region | MNI coordinates | Peak Value | Size (mm2) |
|---|---|---|---|
| Left anterior ventral insula | −28 27 −6 | 5.67 | 20547 |
| Left 7A (lateral parietal) | −20 −52 59 | 3.6 | 1298 |
| Left PH (temporo-occipital | −43 −53 −9 | 3.5 | 1043 |
| Left V2 | −10 −71 −2 | 3.2 | 760 |
| Left TPOJ2 | −46 −64 10 | 2.7 | 595 |
| Right anterior ventral insula | 31 29 −4 | 6.3 | 20468 |
| Right V1 | 5 −73 4 | 4.2 | 5642 |
| Right LIPd | 30 −59 46 | 3.5 | 1169 |
| Right frontal eye field | 35 −2 44 | 2.8 | 772 |
All p’s<0.001 by bootstrap resampling.
LIPd, dorsal lateral intraparietal; MNI, Montreal Neurological Institute; TPOJ, temporo-parieto-occipital junction.
4. Discussion
In the present study, we evaluated LGI in preadolescent children with ADHD + PAE compared to that in children with familial ADHD−PAE and TD children, using both a traditional univariate approach and a multivariate approach. The results supported our hypothesis that prefrontal LGI is particularly lower in children with ADHD + PAE, and that these lower values are related to facial dysmorphia and neurobehavioral impairments. Further, the results add to the accumulating evidence of differences between ADHD + PAE and ADHD−PAE.
Children with ADHD + PAE had lower LGI in the left mid-dorsolateral cortex than did children with ADHD−PAE, as well as lower LGI in frontal (including the left mid-dorsolateral cortex) and anterior cingulate cortices than did TD children. This latter result is consistent with previous studies that compared children with PAE to TD children, without regard to ADHD (Hendrickson et al., 2017; Infante et al., 2015). However, as we included a group of children with familial ADHD without PAE, the present study more clearly shows an association between lower LGI in the frontal cortex and PAE, localized to the left mid-dorsolateral cortex. The left mid-dorsolateral cluster comprised portions of areas 46, p9-46v, 9-46d, and 9p (Baker et al., 2018b). These areas play a role in the conscious, active control of planned behavior (Petrides, 2005). Furthermore, dissociable contributions of the left and right mid-dorsolateral prefrontal cortex to planning have been demonstrated. For example, activity in the left mid-dorsolateral prefrontal cortex seems to be more strongly impacted by goal hierarchy (clear or ambiguous sequence for attaining the overall goal) than by search depth (degree of interdependence between consecutive steps), and vise-versa for the activity in the right mid-dorsolateral prefrontal cortex (Kaller et al., 2011). However, controversies remain, as a neuroimaging meta-analysis published in 2017 failed to find strong evidence of lateralized involvement of the mid-dorsolateral prefrontal cortex in planning-related processes (Nitschke et al., 2017). In contrast, transcranial direct current stimulation studies show clear dissociations between the right and left prefrontal cortex in self-regulation, with differential effects of lateralized stimulation/inhibition protocols on a variety of self-regulation activities such as delay discounting, emotional regulation, and risk-taking (Kelley et al., 2018). Other stimulation studies support the greater involvement of the left prefrontal cortex in the initial planning and the right prefrontal cortex in monitoring/updating the planning process (Basso and Saracini, 2020). Thus, the lower LGI in the mid-dorsolateral cortex may underlie symptoms such as poor behavioral regulation (as supported by the PLS analysis discussed below) and sensitivity to initiating planning under ambiguous conditions as described in individuals with PAE (Schonfeld et al., 2009).
The PLS analysis showed that, among children with ADHD, a profile of lower LGI in prefrontal (including left dorsolateral cortex), insular, cingulate, and parietal cortices was significantly associated with a clinicobehavioral profile of features central to the diagnosis of ADHD + PAE, including PAE, facial dysmorphia in terms of philtrum and the upper vermillion border of the lip, lower Full-scale IQ, poorer behavioral regulation, and greater hyperactivity/impulsivity. Although smaller palpebral fissure length, another facial morphology feature central to PAE, was also associated with lower LGI, the reliability of its correlation failed to reach significance, as did that for the neurobehavioral parameters of ERI and CRI on the BRIEF2 and the Conners-3 inattention score. To our knowledge, this is the first analysis to relate facial dysmorphia to brain morphology in these well-defined etiologies, and suggests that some facial features may be more robustly associated with brain alterations impacting developmental dysfunction.
Two previous large-scale studies found no significant differences in gyrification between children (Shaw et al., 2012) and adolescents/young adults (Forde et al., 2017) with and without ADHD. However, two smaller studies found some significant differences, with lower gyrification in the left medial temporal lobe in adolescents with ADHD than in TD adolescents (Mous et al., 2014), and lower gyrification overall and in the right frontal lobe, as well as a tendency toward lower gyrification in the right parietal lobe, in preadolescents with ADHD than in TD preadolescents (Wolosin et al., 2009). The present study included a group of children with ADHD with a more homogenous etiology (familial and not due to PAE) than that in previous studies, and some significant differences between children with ADHD−PAE and TD children were observed. Compared to that in TD children, children with ADHD−PAE had lower LGI extending into the left medial temporal cortex, similar to the findings of Mous et al. (2014), as well as in additional regions, overlapping those reported in Wolosin et al. (2009). These additional regions comprised areas of somatosensory and visuomotor integration (5 m; mirror system, PF) and default mode network regions (lateral, TPOJ, IP2; posterior, 31pd; and anterior, 32, 9 m) (Baker et al., 2018a, c, d). Most of these regions also showed altered LGI in the ADHD + PAE group. The results of the present study, as well as our previous study, generally suggest that future ADHD-related neuroimaging studies would benefit from greater consideration of the diagnostic etiology (O’Neill et al., 2019), particularly PAE.
The present study has some limitations. First, the sample size was relatively small, but was comparable to some prior gyrification studies of ADHD (Mous et al., 2014; Palaniyappan et al., 2019) and PAE (De Guio et al., 2014; Infante et al., 2015) in children. Second, the Full-scale IQ was lower in the ADHD + PAE group; however, all participants had IQ > 70. This differs from some research samples of children meeting criteria for an intellectual disability, which often include results from extreme cases. Furthermore, lower IQ is a common symptom of PAE and it is not conventional to correct for it as there are valid arguments against such practice in groups with this inherent disability (Dennis et al., 2009). Third, although there is some evidence that the effects of PAE vary with the sex of the offspring (Fontaine et al., 2016; May et al., 2017; Weinberg et al., 2008), this is an underexplored area. A previous study showed no sex differences in the rate of decline in LGI during preadolescence in children with PAE (Hendrickson et al., 2018). Similarly, the current study failed to find notable sex differences; however, larger sample sizes may be necessary to determine an impact of sex. Fourth, only LGI was evaluated; the inclusion of cortical thickness and surface area could have added to the characterization of the cortical morphology and potentially compliment the LGI results. Finally, various epidemiological epiphenomena may co-occur alongside PAE (e.g. neglect, maternal stress, etc.) and contribute to the observed neurodevelopmental disruptions. Thus, the current study design should be reapplied to a larger number of children to enable the evaluation of interactions between PAE and these epiphenomena in terms of LGI in the context of ADHD.
4.1. Conclusions
The present study used a novel design, in which preadolescent children with ADHD and PAE were compared not only to TD children, but also to children with a well-defined ADHD etiology (familial) without PAE. The results demonstrated uniquely lower gyrification in the left mid-dorsolateral cortex, in children with ADHD due to PAE than in children with familial ADHD or TD children. These findings add insight into the brain bases of PAE symptoms. Moreover, our findings support the notion that ADHD in the presence of PAE represents a distinct subtype with specific neurodevelopmental abnormalities that may inform the development of more targeted ADHD treatments based on an objective differential diagnosis of ADHD + PAE vs. ADHD−PAE.
Acknowledgement
We wish to thank the patients and families who participated in this research.
Funding
This work was supported by the National Institutes of Health (grant number NIAAA R01AA025066).
Role of funding source
The funder was not involved in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K, 1995. The ontogeny of human gyrification. Cereb. Cortex 5 (1), 56–63. 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Baker CM, Burks JD, Briggs RG, Conner AK, Glenn CA, Manohar K, Milton CK, Sali G, McCoy TM, Battiste JD, O’Donoghue DL, Sughrue ME, 2018a. A connectomic atlas of the human cerebrum-chapter 8: the posterior cingulate cortex, medial parietal lobe, and parieto-occipital sulcus. Oper. Neurosurg. (Hagerstown) 15 (Suppl_1), S350–S371. 10.1093/ons/opy262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CM, Burks JD, Briggs RG, Conner AK, Glenn CA, Morgan JP, Stafford J, Sali G, McCoy TM, Battiste JD, O’Donoghue DL, Sughrue ME, 2018b. A connectomic atlas of the human cerebrum-chapter 2: the lateral frontal lobe. Oper. Neurosurg. (Hagerstown) 15 (Suppl_1), S10–S74. 10.1093/ons/opy254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CM, Burks JD, Briggs RG, Conner AK, Glenn CA, Taylor KN, Sali G, McCoy TM, Battiste JD, O’Donoghue DL, Sughrue ME, 2018c. A connectomic atlas of the human cerebrum-chapter 7: the lateral parietal lobe. Oper. Neurosurg. (Hagerstown) 15 (Suppl_1), S295–S349. 10.1093/ons/opy261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CM, Burks JD, Briggs RG, Sheets JR, Conner AK, Glenn CA, Sali G, McCoy TM, Battiste JD, O’Donoghue DL, Sughrue ME, 2018d. A connectomic atlas of the human cerebrum-chapter 3: the motor, premotor, and sensory cortices. Oper. Neurosurg. (Hagerstown) 15 (Suppl_1), S75–S121. 10.1093/ons/opy256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso D, Saracini C, 2020. Differential involvement of left and right frontoparietal areas in visuospatial planning: an rTMS study. Neuropsychologia 136, 107260. 10.1016/j.neuropsychologia.2019.107260. [DOI] [PubMed] [Google Scholar]
- CDC, 2004. Centers for Disease Control and Prevention. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. National Center on Birth Defects and Developmental Disabilities. Department of Health and Human Services. [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE, 1997. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol. Clin. Exp. Res 21 (1), 150–161. [PubMed] [Google Scholar]
- Conners KC, 2008. Conners, 3rd edition. Multi-Health Systems, Toronto. [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9 (2), 179–194. 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Guio F, Mangin JF, Riviere D, Perrot M, Molteno CD, Jacobson SW, Meintjes EM, Jacobson JL, 2014. A study of cortical morphology in children with fetal alcohol spectrum disorders. Hum. Brain Mapp 35 (5), 2285–2296. 10.1002/hbm.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM, 2009. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J. Int. Neuropsychol. Soc 15 (3), 331–343. 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig J, McLennan JD, Gibbard WB, 2008. Medication effects on symptoms of attention-deficit/hyperactivity disorder in children with fetal alcohol spectrum disorder. J. Child Adolesc. Psychopharmacol 18 (4), 365–371. 10.1089/cap.2007.0121. [DOI] [PubMed] [Google Scholar]
- Duko B, Pereira G, Betts K, Tait RJ, Newnham J, Alati R, 2020. Associations of prenatal alcohol exposure and offspring harmful alcohol use: findings from the Raine Study. Drug Alcohol Depend. 217, 108305. 10.1016/j.drugalcdep.2020.108305. [DOI] [PubMed] [Google Scholar]
- Fontaine CJ, Patten AR, Sickmann HM, Helfer JL, Christie BR, 2016. Effects of pre-natal alcohol exposure on hippocampal synaptic plasticity: sex, age and methodological considerations. Neurosci. Biobehav. Rev 64, 12–34. 10.1016/j.neubiorev.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Forde NJ, Ronan L, Zwiers MP, Alexander-Bloch AF, Faraone SV, Oosterlaan J, Heslenfeld DJ, Hartman CA, Buitelaar JK, Hoekstra PJ, 2017. No association between cortical gyrification or intrinsic curvature and attention-deficit/hyperactivity disorder in adolescents and young adults. Front. Neurosci 11, 218. 10.3389/fnins.2017.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F, Paley B, Marquardt R, O’Connor M, 2006. Stimulants, neuroleptics, and children’s friendship training for children with fetal alcohol spectrum disorders. J. Child Adolesc. Psychopharmacol 16 (6), 777–789. 10.1089/cap.2006.16.777. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN, 2007. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics 119 (3), e733–741. 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Gautam P, Anstey KJ, Wen W, Sachdev PS, Cherbuin N, 2015. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav. Brain Res 287, 331–339. 10.1016/j.bbr.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Gioia G, Isquith PK, Guy SC, Kenworthy L, 2015. Behavior Rating Inventory of Executive Function. Second Psychological Assessment Resources. PAR Inc., Lutz, FL. [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, Consortium WU-MH, 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwald MK, Delaney-Black V, 2010. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol 44 (7-8), 583–594. 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson TJ, Mueller BA, Sowell ER, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lim KO, Riley EP, Wozniak JR, 2017. Cortical gyrification is abnormal in children with prenatal alcohol exposure. Neuroimage Clin. 15, 391–400. 10.1016/j.nicl.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson TJ, Mueller BA, Sowell ER, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lee S, Lim KO, Riley EP, Wozniak JR, 2018. Two-year cortical trajectories are abnormal in children and adolescents with prenatal alcohol exposure. Dev. Cogn. Neurosci 30, 123–133. 10.1016/j.dcn.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA, 2016. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 138 (2). 10.1542/peds.2015-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante MA, Moore EM, Bischoff-Grethe A, Migliorini R, Mattson SN, Riley EP, 2015. Atypical cortical gyrification in adolescents with histories of heavy prenatal alcohol exposure. Brain Res. 1624, 446–454. 10.1016/j.brainres.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Hashimoto-Torii K, 2015. Impact of prenatal environmental stress on cortical development. Front. Cell. Neurosci 9, 207. 10.3389/fncel.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaller CP, Rahm B, Spreer J, Weiller C, Unterrainer JM, 2011. Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cereb. Cortex 21 (2), 307–317. 10.1093/cercor/bhq096. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36 (7), 980–988. 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelley NJ, Gallucci A, Riva P, Romero Lauro LJ, Schmeichel BJ, 2018. Stimulating self-regulation: a review of non-invasive brain stimulation studies of goal-directed behavior. Front. Behav. Neurosci 12, 337. 10.3389/fnbeh.2018.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H, 2011. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage 56 (2), 455–475. 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Lees B, Mewton L, Stapinski LA, Teesson M, Squeglia LM, 2020. Association of prenatal alcohol exposure with preadolescent alcohol sipping in the ABCD study(R). Drug Alcohol Depend. 214, 108187. 10.1016/j.drugalcdep.2020.108187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Szeszko PR, Gurbani MN, Hamilton L, Toga AW, Gaser C, 2008. Mapping the relationship between cortical convolution and intelligence: effects of gender. Cereb. Cortex 18 (9), 2019–2026. 10.1093/cercor/bhm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP, Cifasd, 2013. Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res 37 (3), 517–528. 10.1111/j.1530-0277.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick B, Hasken JM, Marais AS, de Vries MM, Barnard R, Joubert B, Cloete M, Botha I, Kalberg WO, Buckley D, Burroughs ZR, Bezuidenhout H, Robinson LK, Manning MA, Adnams CM, Seedat S, Parry CDH, Hoyme HE, 2017. Who is most affected by prenatal alcohol exposure: boys or girls? Drug Alcohol Depend. 177, 258–267. 10.1016/j.drugalcdep.2017.04.010. [DOI] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE, 2018. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA 319 (5), 474–482. 10.1001/jama.2017.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ, 2004. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage 23 (Suppl. 1), S250–263. 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL, 1996. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3 (3 Pt 1), 143–157. 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP, 2001. Misunderstanding analysis of covariance. J. Abnorm. Psychol 110 (1), 40–48. 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mous SE, Karatekin C, Kao CY, Gottesman II, Posthuma D, White T, 2014. Gyrification differences in children and adolescents with velocardiofacial syndrome and attention-deficit/hyperactivity disorder: a pilot study. Psychiatry Res. 221 (2), 169–171. 10.1016/j.pscychresns.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Nitschke K, Kostering L, Finkel L, Weiller C, Kaller CP, 2017. A meta-analysis on the neural basis of planning: activation likelihood estimation of functional brain imaging results in the Tower of London task. Hum. Brain Mapp 38 (1), 396–413. 10.1002/hbm.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, 2014. Mental health outcomes associated with prenatal alcohol exposure: genetic and environmental factors. Curr. Dev. Disord. Rep 1, 181–188. 10.1007/s40474-014-0021-0027. [DOI] [Google Scholar]
- O’Connor MJ, Portnoff LC, Lebsack-Coleman M, Dipple KM, 2019. Suicide risk in adolescents with fetal alcohol spectrum disorders. Birth Defects Res. 111 (12), 822–828. 10.1002/bdr2.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley KD, Nanson J, 2002. Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. Can. J. Psychiatry 47 (4), 349–354. 10.1177/070674370204700405. [DOI] [PubMed] [Google Scholar]
- O’Neill J, O’Connor MJ, Yee V, Ly R, Narr K, Alger JR, Levitt JG, 2019. Differential neuroimaging indices in prefrontal white matter in prenatal alcohol-associated ADHD versus idiopathic ADHD. Birth Defects Res. 111 (12), 797–811. 10.1002/bdr2.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterheld JR, Kofoed L, Tervo R, Fogas B, Wilson A, Fiechtner H, 1998. Effectiveness of methylphenidate in Native American children with fetal alcohol syndrome and attention deficit/hyperactivity disorder: a controlled pilot study. J. Child Adolesc. Psychopharmacol 8 (1), 39–48. 10.1089/cap.1998.8.39. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Batty MJ, Liddle PF, Liddle EB, Groom MJ, Hollis C, Scerif G, 2019. Reduced prefrontal gyrification in carriers of the dopamine D4 receptor 7-repeat allele with attention deficit/hyperactivity disorder: a preliminary report. Front. Psychiatry 10, 235. 10.3389/fpsyt.2019.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peadon E, Rhys-Jones B, Bower C, Elliott EJ, 2009. Systematic review of interventions for children with Fetal Alcohol Spectrum Disorders. BMC Pediatr. 9, 35. 10.1186/1471-2431-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, 2005. Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. Lond. B Biol. Sci 360 (1456), 781–795. 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattlebaum JL, O’Connor MJ, 2013. Higher functioning children with prenatal alcohol exposure: is there a specific neurocognitive profile? Child Neuropsychol. 19 (6), 561–578. 10.1080/09297049.2012.713466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN, 2011. How does your cortex grow? J. Neurosci 31 (19), 7174–7177. 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP, 2008. A surface-based approach to quantify local cortical gyrification. IEEE Trans. Med. Imaging 27 (2), 161–170. 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran JP, Eliez S, 2012. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J. Vis. Exp (59), e3417 10.3791/3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ, 2009. Behavioral regulation as a predictor of response to Children’s Friendship Training in children with fetal alcohol spectrum disorders. Clin. Neuropsychol 23 (3), 428–445. 10.1080/13854040802389177. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D, 2012. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol. Psychiatry 72 (3), 191–197. 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J, Nanson J, Snyder R, Block G, 1997. A study of stimulant medication in children with FAS. In: Streissguth A, Kanter J (Eds.), Overcoming and Preventing Secondary Disabilities in Fetal Alcohol Syndrome and Fetal Alcohol Effects. University of Washington Press, Seattle, WA, pp. 64–77. [Google Scholar]
- Townsend L, Kobak K, Kearney C, Milham M, Andreotti C, Escalera J, Alexander L, Gill MK, Birmaher B, Sylvester R, Rice D, Deep A, Kaufman J, 2020. Development of three web-based computerized versions of the kiddie schedule for affective disorders and schizophrenia child psychiatric diagnostic interview: preliminary validity data. J. Am. Acad. Child Adolesc. Psychiatry 59 (2), 309–325. 10.1016/j.jaac.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 2011. Wechsler Abbreviated Scale of Intelligence, second edition. NCS Pearson, San Antonio (WASI-II). [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG, 2008. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J. Neuroendocrinol 20 (4), 470–488. 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G, 2010. The development of gyrification in childhood and adolescence. Brain Cogn. 72 (1), 36–45. 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH, 2009. Abnormal cerebral cortex structure in children with ADHD. Hum. Brain Mapp 30 (1), 175–184. 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Riley EP, Charness ME, 2019. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 18 (8), 760–770. 10.1016/S1474-4422(19)30150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]