Abstract
As the HIV-infected population ages and the burden of chronic comorbidities increases, adherence to medications for HIV and diabetes and hypertension is crucial to improve outcomes. We pilot-tested a pictorial aid intervention to improve medication adherence for both HIV and common chronic conditions. Adult patients with HIV and diabetes (DM) and/or hypertension (HTN) attending a clinic for underserved patients and at risk for poor health outcomes were enrolled. Patients were randomized to receive either a pictorial aid intervention (a photographic representation of their medications, the indications, and the dosing schedule) or a standard clinic visit discharge medication list. Adherence to antiretroviral therapy (ART) for HIV and therapy for DM or HTN was compared. Predictors of ART adherence at baseline were determined using logistic regression. Medication adherence was assessed using medication possession ratio (MPR) for the 6-month interval before and after the intervention. Change in adherence by treatment group was compared by ANOVA. Among the 46 participants, there was a trend towards higher adherence to medications for HIV compared with medications for hypertension/diabetes (baseline median MPR for ART 0.92; baseline median MPR for the medication for the comorbid condition 0.79, p=0.07). The intervention was feasible to implement and satisfaction with the intervention was high. With a small sample size, the intervention did not demonstrate significant improvement in adherence to medications for HIV or comorbid conditions. Patients with HIV are often medically complex and may have multiple barriers to medication adherence. Medication adherence is a multifaceted process and adherence promotion interventions require an approach that targets patient-specific barriers.
Keywords: medication adherence, HIV treatment, pictorial aid, diabetes, hypertension
Introduction
As the HIV-infected population ages, people living with HIV (PLWH) may suffer morbidity and mortality from multiple chronic conditions including diabetes and hypertension (Barbaro, 2006). With an increased risk of heart disease among PLWH (Freiberg et al., 2013), uncontrolled hypertension and diabetes may be more dangerous. HIV, diabetes (DM), and hypertension (HTN) all require high levels of medication adherence to achieve treatment goals and optimize clinical outcomes, and in the HIV-infected population, disease control is suboptimal (Adeyemi, Vibhakar, & Max, 2009). This is further complicated by the adverse impact of polypharmacy on adherence (Kripalani, Yao, & Haynes, 2007). A prior study of older PLWH showed that those with more comorbidities were less likely to demonstrate optimal ART adherence (Abara, Adekeye, Xu, Heiman, & Rust, 2016).
Pictorial aids have been tested to improve medication adherence. These aids ideally include a photograph of the actual pill, the dosage, the number of pills per dose, and a schedule (Katz, Kripalani, & Weiss, 2006). The pictorial aid aims to increase a patient’s understanding of the medication regimen to facilitate adherence. These may be particularly helpful interventions for individuals with complex regimens and low health literacy, both factors which have been associated with poor medication adherence (Gellad, Grenard, & Marcum, 2011; Kalichman, Ramachandran, & Catz, 1999; Van Servellen, Brown, Lombardi, & Herrera, 2003). Pictorial aid interventions have been tested in many patient populations and care venues to increase medication adherence (Dowse, Barford, & Browne, 2014; Gazmararian, Jacobson, Pan, Schmotzer, & Kripalani, 2010; S. C. Kalichman et al., 2013; Kripalani, Schmotzer, & Jacobson, 2012; Martin, Kripalani, & Durapau, 2012; Mohan, Riley, Schmotzer, Boyington, & Kripalani, 2014). The results of these interventions have revealed increases in knowledge (Dowse et al., 2014; Mohan et al., 2014) and self-efficacy (Dowse et al., 2014; Martin et al., 2012). A separate study showed improvement in adherence among individuals with polypharmacy or low self-efficacy (Kripalani et al., 2012). One study did not show an increase in ART adherence associated with a pictorial aid among marginal or low literacy individuals ( Kalichman et al., 2013), and another study did not show improvements adherence among patients with low health literacy (Gazmararian et al., 2010). In studies that did not show improvements, the increase in medication understanding and/or self-efficacy from the pictorial aid intervention may not have been sufficient to overcome other barriers to adherence.
Data on adherence to medications for diabetes and hypertension among PLWH is limited. Among PLWH and diabetes, those not achieving HIV control are less likely to achieve diabetes control ( Monroe, Chander, & Moore, 2011). Furthermore, diabetes and hypertension are a source of concern and frustration to PLWH, sometimes even eclipsing concern regarding HIV. Limited understanding of these health conditions and treatments hinders adherence. Some PLWH note placing higher importance on adherence to their HIV medications, reporting higher adherence to their HIV medications compared to other medications (Monroe, Rowe, Moore, & Chander, 2013). Although it is clear that HIV control is paramount, as individuals live longer with HIV there is increasing need to concurrently manage comorbidities.
Among an urban HIV clinic population managing multiple comorbidities, adherence support may be strengthened by the inclusion of a pictorial-based adherence aid. We pilot-tested a pictorial aid intervention among PLWH and diabetes and/or hypertension in a small randomized controlled trial (RCT). Our objectives were to explore acceptability of a pictorial aid intervention, to quantify and compare adherence to medications for HIV and another medical condition (either diabetes and/or hypertension) and to measure medication adherence before and after the intervention.
Methods
Participants
Eligibility criteria included English-speaking adults (≥ 18 years old) from the Johns Hopkins HIV Clinic with a diagnosis of HIV infection with either diabetes and/or hypertension and with either a detectable HIV RNA, a hemoglobin A1c (HbA1c) > 7%, or a blood pressure measurement above 140/90 mmHg at their most recent clinic visit. Additional eligibility criteria included using the HIV Clinic pharmacy for all prescriptions, being prescribed medications for HIV and diabetes and/or hypertension for ≥ 6 months and being prescribed ≥ 5 different medications per day total (for any condition). Insulin was counted as one medication although the complexity of adhering to insulin administration may be higher than the complexity of adhering to an oral medication. The study was approved by the Johns Hopkins Institutional Review Board and all participants provided informed consent.
Procedures
Baseline assessment
A baseline assessment included demographics, HIV duration, diagnosis of diabetes and/or hypertension, and the duration of relationship with current medical provider. We collected measures of self-reported medication adherence (Adherence to Refills and Medications Scale (ARMS)) (Kripalani, Risser, Gatti, & Jacobson, 2009), health literacy (Rapid Estimate of Adult Literacy in Medicine (REALM-R)) (Bass, Wilson, & Griffith, 2003), social support (Duke-UNC Functional Support Questionnaire) (Broadhead, Gehlbach, De Gruy, & Kaplan, 1988), self-efficacy for appropriate medication use (Self-efficacy for Appropriate Medication Use Scale (SEAMS)) (Risser, Jacobson, & Kripalani, 2007) depression (Patient Health Questionnaire (PHQ-9) (Spitzer et al, 1999), and medication understanding questionnaire (MUQ) (Marvanova et al., 2011). Baseline HIV RNA level, CD4 count, number of medications, and number of medical comorbidities were collected from medical records.
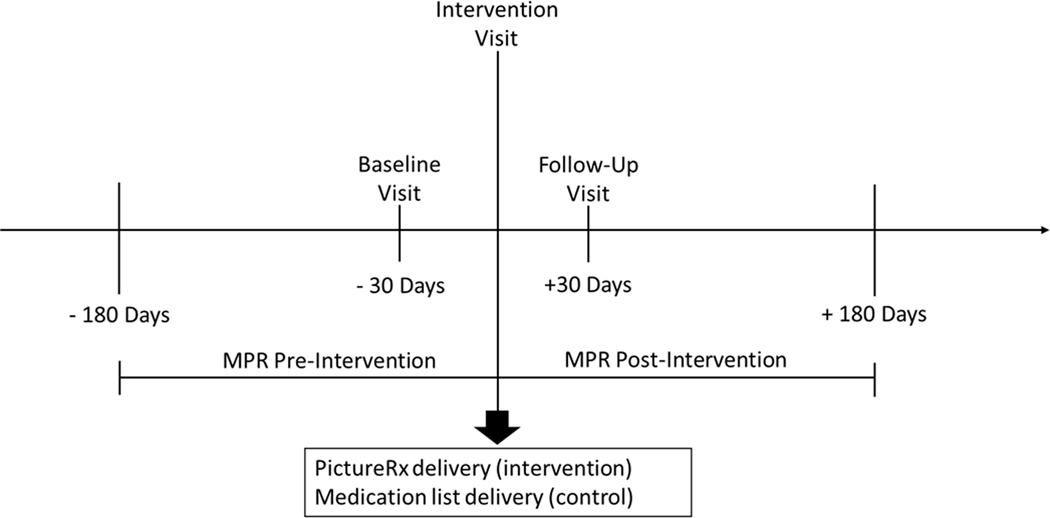
Study participants were randomized to the control or intervention groups using sealed opaque envelopes containing randomly generated treatment allocations. After opening the envelope, participants were informed of their randomization assignment and instructed to return for the intervention visit four weeks later. All study visits were scheduled separately from regular clinic visits (Figure 1).
Figure 1.
Study timeline
Intervention Visit
The intervention group received a pictorial representation of the appearance, indication, and daily dosing schedule for each of their medications. The pictorial aid was a PictureRx card generated by a study physician (AM) using mypicturerx.com (Mohan, Riley, Boyington, & Kripalani, 2012). The information that populated the card was obtained from the medication list in the patient’s medical chart and verified with the patient. The card was provided to the patient along with their regular clinic visit discharge medication list. The control group received only the regular clinic visit discharge medication list. Standardized counseling regarding the instructions on the pictorial aid and/or the medication list was provided by a study physician (AM).
Outcome assessment
The primary outcome measure for medication adherence was a calculated medication possession ratio (MPR) (Blandford, 1999; Okano, Rascati, Wilson, & Remund, 1999; Sclar et al., 1994), defined as the days covered by medication in a 180 day interval – 180 days before and after the intervention visit for each patient. MPRs were calculated using pharmacy fill data from the HIV Clinic pharmacy. An MPR was calculated for one drug in the patient’s HIV regimen (either a protease inhibitor, integrase inhibitor, non-nucleoside reverse transcriptase inhibitor, or nucleoside reverse transcriptase inhibitor). If the patient was on a single tablet HIV regimen, the MPR for that medication was calculated. For the diabetes or hypertension medication regimen, an agent from that regimen was selected using a standard scheme and an MPR was calculated for that agent. For individuals with DM and HTN, the HTN agent was selected. All medications were reviewed, and if changes were made to an individual’s HIV, diabetes, or hypertension regimen, the MPR was calculated to account for the new medication. This process was completed blinded to study assignment.
A satisfaction questionnaire was administered to all participants receiving the PictureRx card who presented for the follow-up assessment (Mohan et al., 2014). The questionnaire evaluated how helpful the PictureRx card was for remembering the appearance, name, dosage, use, and the time of day to take each medication, ease of use and clarity.
Statistical Analysis
Baseline characteristics were summarized for both the control and intervention groups. For continuous variables, we calculated means and standard deviations and used t-tests to assess differences between the intervention and control groups. For categorical variables, counts and proportions of key variables of interest were reported for the intervention and control groups, with differences between groups assessed by chi-square tests or Fisher’s exact test. We used the Wilcoxon signed-rank test because the data was not normally distributed to compare the MPR for ART with the MPR for the patient’s medication for diabetes or hypertension at both the baseline and follow up visits. We performed univariate logistic regression to determine factors associated with adequate adherence (MPR > 0.8) at baseline. Recent work in older adults showed that ART adherence levels > 80% produced favorable clinical outcomes (Abara, Adekeye, Xu, & Rust, 2017). Eighty percent has been used in the general medicine literature as a threshold for adequate adherence and empiric data supports this threshold (Karve et al., 2009).
A repeated measures analysis of variance test (ANOVA) was used to determine intervention effect on adherence in the pre- and post-intervention periods. Summary statistics were generated for participant satisfaction data. A formal sample size calculation was not performed due to the pilot nature of the study.
Results
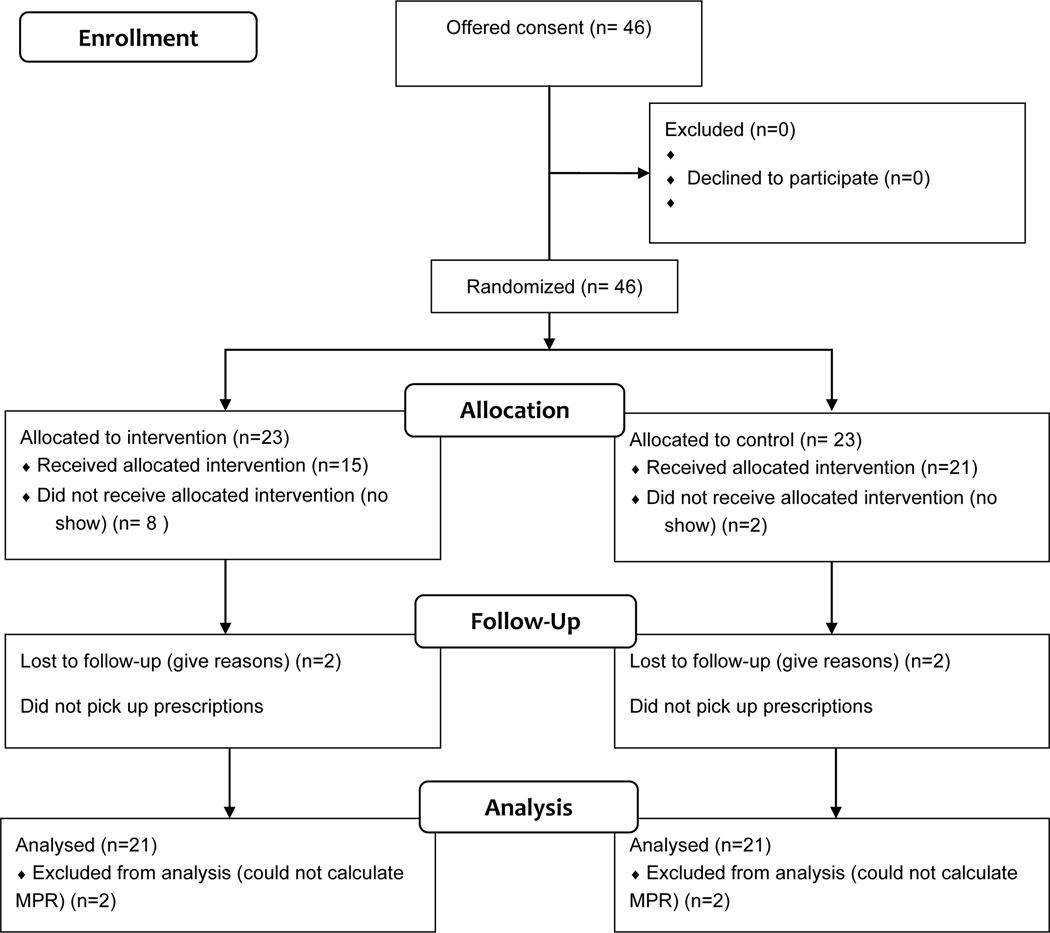
Forty-six participants enrolled in the study, with 23 participants per group. Thirty-two participants completed all three study visits (See Figure 2, Study Flow diagram). As shown in Table 1, the mean age was 52. The sample was predominantly African-American. The majority of the study sample had HTN only (57%). Over one-third (37%) had hypertension and diabetes. The remainder (6%) had DM only. The distribution of condition by randomization assignment was not statistically significant by group. Participants had a long duration of HIV infection (mean duration >15 years) and their other comorbidities (mean duration of hypertension>10 years, mean duration of diabetes >5 years). The type of insurance used by participants at baseline was significantly different with more individuals in the intervention group having public insurance (p = 0.02). Many of the participants reported moderate to severe depressive symptoms and were assessed as at risk for poor health literacy. Medication understanding values ranged from 0 to 1, with an average of 0.78 overall. The majority of participants (63.0%) had a medication understanding questionnaire score of 0.80 or higher.
Figure 2.
Study Flow Diagram
Table 1.
Demographic and Clinical Characteristics of Study Group
| Characteristic | Control Group (N=23) | Intervention Group (N=23) | P-value |
|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | ||
| Age | 52.6 (.90) | 51.96 (5.2) | 0.65 |
| Gender | 0.77 | ||
| Male | 57 | 61 | |
| Female | 43 | 39 | |
| Race | 0.35 | ||
| Black | 96 | 87 | |
| White | 4 | 4 | |
| Other | 0 | 9 | |
| Years since HIV Dx | 15 (1.62) | 16.17 (1.57) | 0.61 |
| Years since BP Dx | 10.14 (1.95) | 12.3 (2.37) | 0.48 |
| Years since DM Dx | 6.5 (1.58) | 5.5 (1.41) | 0.66 |
| CD4 cell count (cells/mm 3 ) | |||
| ≤200 | 13 | 13 | 0.90 |
| 201–499 | 48 | 57 | |
| ≥500 | 30 | 26 | |
| Missing | 9 | 4 | |
| HIV1 RNALevel (copies/ml) | |||
| <200 | 52 | 61 | 0.76 |
| ≥200 | 39 | 35 | |
| Missing | 9 | 4 | |
| At Risk for Poor Health Literacy (REALM-R) | 0.30 | ||
| No | 39 | 43 | 0.77 |
| Yes | 61 | 57 | |
| Current Smoking | |||
| Yes | 65 | 61 | 0.76 |
| No | 35 | 39 | |
| Insurance | |||
| Private | 9 | 13 | 0.02 |
| Public | 87 | 52 | |
| Uninsured | 4 | 35 | |
| Uses pillbox | |||
| Yes | 47 | 61 | 0.38 |
| No | 52 | 39 | |
| Education | |||
| Less than college | 74 | 87 | 0.27 |
| Any college | 26 | 13 | |
| Depression screening (PHQ 9) | |||
| None/Mild symptoms | 35 | 39 | 0.90 |
| Moderate symptoms | 48 | 48 | |
| Severe symptoms | 17 | 13 | |
| Social Support (FSSQ) * | 3.73 (0.22) | 3.55 (0.28) | 0.61 |
| Self Efficacy (SEAMS) ** | 32 (6) | 31 (6) | 0.55 |
| Medication understanding (MUQ) ‡ | 0.78 (0.12) | 0.79 (0.10) | 0.74 |
| Baseline number of medications | 8.4 (2.8) | 8.0 (2.2) | 0.60 |
| Baseline number of comorbidities | 3.1 (1.9) | 3.7 (1.9) | 0.30 |
FSSQ score Range: 1 to 5 (low to high social support)
SEAMS score range: 13 to 39 (low to high self efficacy)
MUQ score range: 0 to 1 (low to high understanding)
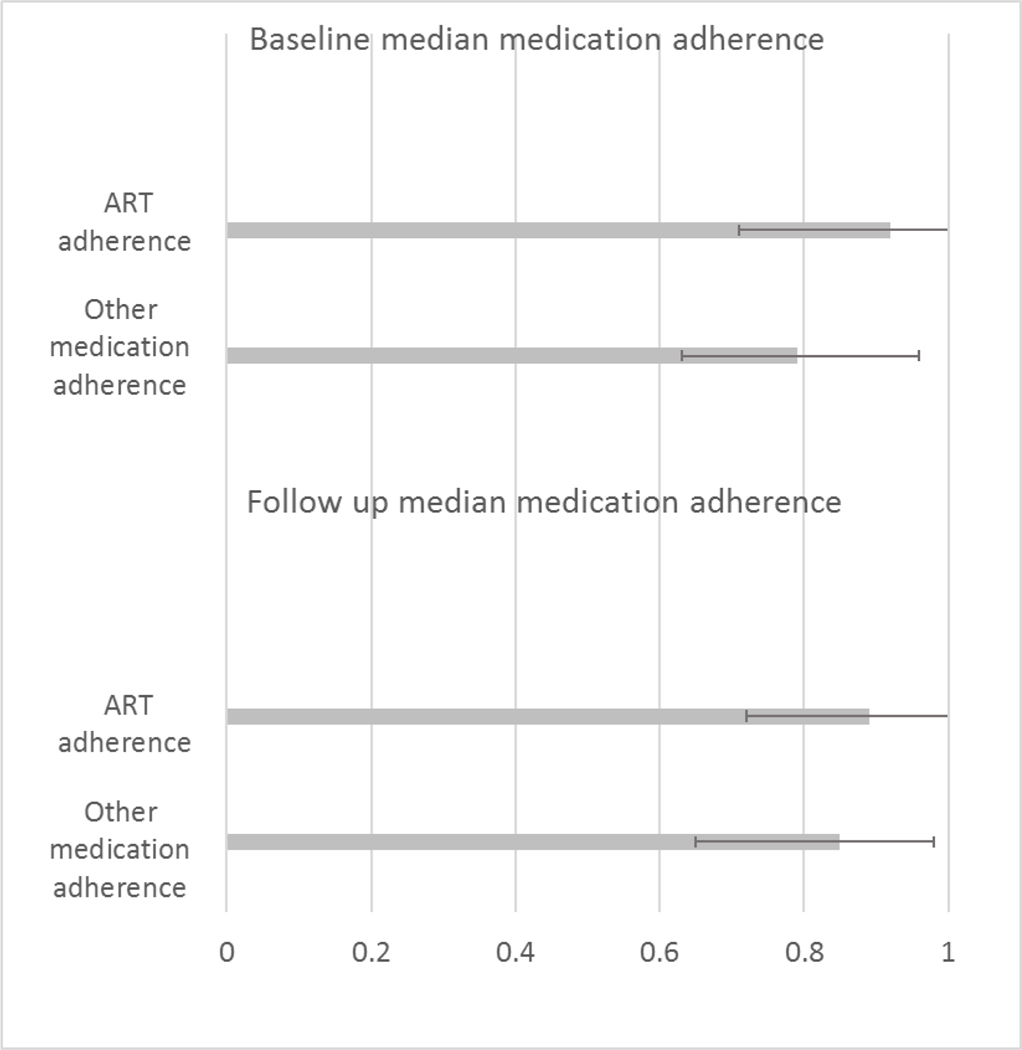
The median MPR for ART at baseline was 92% (IQR 71%–100%). This was higher than the MPR for the diabetes or hypertension medication, with a median MPR of 79% (IQR 63%–96%) (p-value for comparison of median MPR for ART vs MPR for other medication at baseline = 0.07). The median MPR for ART at follow-up was 89% (IQR 72%–100%). The median MPR for the other medication at follow up was 85% (IQR 65%–98%) (Table 2A) (p=0.06) (Figure 3).
Table 2A.
Median MPR for ART and Other Medications
| Baseline | Follow-Up | |
|---|---|---|
| Median MPR ART | 92% (IQR 71–100%) | 89% (IQR 72–100%) |
| Median MPR Other Meds | 79% (IQR 63–96%) | 85% (IQR 65–98%) |
Figure 3.
Adherence to ART vs Other Medication at Baseline and Follow up
The mean change in MPR for ART in the intervention group was 0.02 (SD = 0.25) while the mean change in the control group was 0.02 (SD = 0.33). Change in MPR for ART was not higher in the intervention group when compared to the control group (p = 0.96). The mean change in MPR for diabetes or hypertension medications in the intervention group was −0.02 (SD = 0.31) while the mean change in the control group was 0.08 (SD = 0.45). Change in MPR for diabetes or hypertension medication was not statistically significantly higher in intervention group (p=0.32), which was also true when restricted to people with baseline MPR for their diabetes or hypertension medication of <0.9 or <0.8 or those with baseline low health literacy (Table 2B). Factors associated with ART adherence (MPR > 0.8) at baseline are shown in Table 2.
Table 2B.
Mean change in MPR for ART and Other Medications by study group
| Intervention | Control | P-value | |
|---|---|---|---|
| Mean Change MPR ART | 0.02 (25) | 0.02 (0.33) | 0.96 |
| Mean Change MPR Other Meds | −0.02 (0.31) | 0.08 (0.45) | 0.32 |
Satisfaction with the intervention was high, with mean (SD) satisfaction score of 24.8 (4.0) on a 28-point scale. Participants found the intervention most helpful in helping them remember what medications they are supposed to take and the name of their medications (mean (SD) score of 2.9 (0.3) on a 3-point scale for both questions). Participants gave the aid high rankings for easy to understand instructions and for clear pictures depicting the medication’s purpose (mean (SD) 3.7 (0.7) and mean (SD) 3.7 (0.5) on a 4-point scale, respectively).
Discussion
Among PLWH and diabetes and/or hypertension, adherence to ART was higher compared to adherence to medication for the other comorbidities. Overall, participants reported that the pictorial aid helped them remember which medications to take and was easy to understand. In a study limited by a small sample size, the pictorial aid did not demonstrate an effect on adherence.
The patients enrolled in this study were predominantly low income and of low educational attainment with a significant proportion at risk for low health literacy. Pictorial aids are posited to have the largest effect on increasing medication understanding, and subsequently medication adherence, for individuals who do not understand their regimen (Kripalani et al., 2012). However, most participants had a long duration of HIV and engagement in care. Possibly because of this, they had a relatively high baseline medication understanding, making a pictorial aid less effective than it might have otherwise been. Patients newly engaged in care, who do not have a long history of medication taking which contributes to increased medication understanding, may be better candidates for a pictorial aid for adherence.
There is limited data on the effect of pictorial aid interventions among PLWH. Of those studies, two did not directly assess adherence (Dowse et al., 2014; Wilby et al., 2011). Improvements in ART knowledge (Dowse et al., 2014; Wilby et al., 2011) and self-efficacy (Wilby et al., 2011) were reported. A large RCT evaluated pictograph-guided adherence counseling for PLWH and limited health literacy. Both adherence (unannounced pill count) and viral suppression were measured. The intervention did not show any additional benefit of pictogram enhanced counseling versus standard counseling on the outcomes in individuals with marginal health literacy and did not show any benefit of HIV-specific counseling (with or without pictograph enhancement) versus general counseling among individuals with low health literacy (Kalichman et al., 2013).
The results of these studies, examined in conjunction with our findings, suggest that pictograms may not be effective in isolation in improving medication adherence. Our results suggest that the use of pictograms must be carefully targeted to those who could benefit the most from them, and future work in larger populations with a more narrowly defined population might demonstrate efficacy. Our results showed high satisfaction with the intervention; therefore, if a target population for whom the intervention is effective were determined, pictograms would likely be well received in future trials or clinical venues.
Among factors we collected that were potentially associated with ART adherence, self-efficacy, health literacy, and medication understanding were not associated with ART adherence at baseline. Prior work has shown that self-efficacy is associated with ART adherence (Langebeek et al., 2014). Additionally, low health literacy has been shown to be associated with low adherence (Osborn, Paasche-Orlow, Davis, & Wolf, 2007). Interestingly, self-efficacy can mediate the relationship between low health literacy and adherence (Wolf et al., 2007). We posit that we did not see expected associations between these factors and adherence because we had a treatment experienced sample with a high baseline level of adherence, and the influence of the duration of time taking medications outweighed other factors.
The adherence estimates in our work are similar to those presented in recent studies. A recent study which assessed ART adherence by three self-report measures among approximately one thousand individuals engaged in HIV care demonstrated high levels of ART adherence. Seventy-one percent of that sample reported >95% ART adherence, with an additional 22% reporting between 75 and 94% adherence.(Nance et al., 2017). A study looking at MPR for ART among individuals with commercial insurance and not accounting for all other medications showed that the mean (standard deviation) MPR was 0.92 (0.09) among patients receiving a single pill per day, 0.90 (0.10) among patients receiving two pills per day, and 0.90 (0.09) among patients receiving three or more pills per day (P<0.01 for single pill vs. two pills and for single pill vs. three or more pill (Sax, Meyers, Mugavero, & Davis, 2012). Although MPR is a useful measure of adherence, it is limited in its sensitivity to detect change. The pathway from patient understanding to persistent adherence (reflected by refills) includes multiple steps, including understanding the medication regimen, leading to improved self-efficacy, the intention to adhere, and to adherence and finally persistent adherence. It is difficult to effect change in a distal outcome, particularly with a short term intervention such as this one.
MPR was selected as the adherence outcome in this study. Good adherence based on MPR has been associated with important HIV-related clinical outcomes (Wood et al., 2004). The underlying assumption of MPR, and a limitation of the measure, is that patients take the medications that they pick up. Other modalities to assess adherence have various strengths and limitations. Self-report is a fast and inexpensive way to collect adherence data, and self-reported ART adherence is a valid predictor of virologic suppression (Simoni et al., 2006). The main difficulty with self-report is the tendency to overestimate adherence, although reports of non-adherence are quite accurate (Stirratt et al., 2015). Self-report measures are not standardized in terms of recall period, asking about medications individually compared with in combination, and whether they use visual analog scales or Liekert scale-based responses (Berg & Arnsten, 2006). Electronic drug monitoring (monitoring device in cap of drug bottle, e.g.) provides a detailed assessment of adherence, however is limited by expense, burden to user and staff, and potential for over-or under estimates of adherence if bottle is opened and no pills are removed/ingested or if multiple pills are removed at once to be taken over several days. Pill count is inexpensive, however, if it is planned and patients know how many pills should be in the bottle, it is possible to “game” the system.
Another limitation of the analysis is that an effect was difficult to detect due to the small sample size. Furthermore, analyses were univariate only. Our randomization was not balanced with regards to insurance status, with more individuals with public insurance in the intervention group. Individuals with public insurance would possibly have different barriers to obtaining medication, and therefore to medication adherence, limiting the ability to discern an intervention effect. However, the effect on our results was likely minimal.
PLWH and another comorbidity demonstrated higher ART adherence than adherence for their comorbid condition, suggesting that patients place higher value on their HIV medications than other medications. Limited by sample size and an outcome measure that was not very sensitive for detecting short-term adherence change, a pictorial aid intervention did not demonstrate an effect on medication adherence. Our results support that there is no “quick fix” with regards to medication adherence; interventions that address medication reconciliation issues and/or attempt to increase medication understanding in isolation will not likely improve adherence. In an era of HIV care where adherence to both ART and medications for comorbid conditions are crucial, different interventions to improve adherence are needed.
Table 3.
Factors associated with ART adherence at baseline (univariate analysis)
| Odds ratio (95% Confidence Interval) | |
|---|---|
| Race | |
| White (Ref: African American) | 1.16 (0.07, 19.8) |
| Education | |
| Some college or above (Ref: Less than college) | 0.89 (0.21, 3.88) |
| Insurance | |
| Private | 1 (Ref) |
| Public | 0.46 (0.07, 3.12) |
| Uninsured | 1.11 (0.11, 10.99) |
| CD4 cell count (cells/mm 3 ) | |
| ≤200 | 1 (Ref) |
| 201–499 | 6.5 (0.65, 64.82) |
| ≥500 | 4.29 (0.39, 47.62) |
| Depression screening (PHQ 9) | |
| None/Mild symptoms | 1 (Ref) |
| Moderate symptoms | 0.89 (0.24, 3.28) |
| Severe symptoms | 3.21 (0.47, 21.8) |
| Social Support (each 1 point increase in FSSQ) | 1.87 (0.75, 6.11) |
| High self-efficacy (Ref: Low self-efficacy) | 0.30 (0.09, 1.02) |
| Health Literacy (REALM) | |
| At risk for low health literacy (Ref: not at risk) | 0.55 (0.16, 1.84) |
| High medication understanding (Ref: low understanding) | 0.93 (0.29, 3.01) |
| Above median number of medications (Ref: below median) | 0.98 (0.78, 1.35) |
| Above median number of comorbidities (Ref: below median) | 1.00 (0.74, 1.35) |
Acknowledgments
Conflicts of Interest and Sources of Funding:
Dr. Monroe is supported by K23MH105-284-03. This project was funded by the Lawrence Linn Award, Society for General Internal Medicine. Dr. Moore is supported by U01 DA036935 and P30 AI094189.
AKM, JSP, RDM, KAR, and GC have no conflicts of interest to report. SK has served as a consultant to SAI Interactive and has equity in Bioscape Digital, LLC. MNE received an honorarium from Praxis Pharamaceuticals.
References
- Abara WE, Adekeye OA, Xu J, Heiman HJ, & Rust G. (2016). Correlates of Combination Antiretroviral Adherence Among Recently Diagnosed Older HIV-Infected Adults Between 50 and 64 years. AIDS and Behavior, 1–8. [DOI] [PMC free article] [PubMed]
- Abara WE, Adekeye OA, Xu J, & Rust G. (2017). Adherence to combination antiretroviral treatment and clinical outcomes in a Medicaid sample of older HIV-infected adults. AIDS Care, 29(4), 441–448. doi: 10.1080/09540121.2016.1257774 [DOI] [PubMed] [Google Scholar]
- Adeyemi O, Vibhakar S, & Max B. (2009). Are We Meeting the American Diabetes Association Goals for HIV-Infected Patients with Diabetes Mellitus? Clinical Infectious Diseases, 49(Journal Article), 799–802. [DOI] [PubMed] [Google Scholar]
- Barbaro G. (2006). Highly Active Antiretroviral Therapy-Associated Metabolic Syndrome: Pathogenesis and Cardiovascular Risk. American journal of therapeutics, 13(3), 248–260. [DOI] [PubMed] [Google Scholar]
- Bass PF, Wilson JF, & Griffith CH (2003). A shortened instrument for literacy screening. Journal of General Internal Medicine, 18(12), 1036–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, & Arnsten JH (2006). Practical and conceptual challenges in measuring antiretroviral adherence. Journal of acquired immune deficiency syndromes (1999), 43(Suppl 1), S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandford L. (1999). Analyzing variations in medication compliance related to individual drug, drug class, and prescribing physician. Journal of Managed Care Pharmacy, 5(1), 47–51. [Google Scholar]
- Broadhead W, Gehlbach SH, De Gruy FV, & Kaplan BH (1988). The Duke-UNC Functional Social Support Questionnaire: Measurement of social support in family medicine patients. Medical Care, 709–723. [DOI] [PubMed]
- Dowse R, Barford K, & Browne SH (2014). Simple, illustrated medicines information improves ARV knowledge and patient self-efficacy in limited literacy South African HIV patients. AIDS Care, 26(11), 1400–1406. doi: 10.1080/09540121.2014.931559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg MS, Chang C-CH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, … Oursler KA (2013). HIV infection and the risk of acute myocardial infarction. JAMA Internal Medicine, 173(8), 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazmararian J, Jacobson K, Pan Y, Schmotzer B, & Kripalani S. (2010). Effect of a Pharmacy-Based Health Literacy Intervention and Patient Characteristics on Medication Refill Adherence in an Urban Health System. The Annals of pharmacotherapy, 44(1), 80. [DOI] [PubMed] [Google Scholar]
- Gellad WF, Grenard JL, & Marcum ZA (2011). A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother, 9(1), 11–23. doi: 10.1016/j.amjopharm.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S, Ramachandran B, & Catz S. (1999). Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of General Internal Medicine, 14(5), 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Cherry C, Kalichman MO, Amaral C, White D, Grebler T, … Schinazi RF (2013). Randomized clinical trial of HIV treatment adherence counseling interventions for people living with HIV and limited health literacy. J Acquir Immune Defic Syndr, 63(1), 42–50. doi: 10.1097/QAI.0b013e318286ce49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve S, Cleves MA, Helm M, Hudson TJ, West DS, & Martin BC (2009). Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin, 25(9), 2303–2310. doi: 10.1185/03007990903126833 [DOI] [PubMed] [Google Scholar]
- Katz M, Kripalani S, & Weiss B. (2006). Use of pictorial aids in medication instructions: a review of the literature. American Journal of Health-System Pharmacy, 63(23), 2391–2398. [DOI] [PubMed] [Google Scholar]
- Kripalani S, Risser J, Gatti ME, & Jacobson TA (2009). Development and Evaluation of the Adherence to Refills and Medications Scale (ARMS) among Low-Literacy Patients with Chronic Disease. Value in Health, 12(1), 118–123. [DOI] [PubMed] [Google Scholar]
- Kripalani S, Schmotzer B, & Jacobson TA (2012). Improving medication adherence through graphically enhanced interventions in coronary heart disease (IMAGE-CHD): a randomized controlled trial. Journal of General Internal Medicine, 27(12), 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripalani S, Yao X, & Haynes R. (2007). Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Archives of Internal Medicine, 167(6), 540. [DOI] [PubMed] [Google Scholar]
- Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdóttir TB, Richter C, … Nieuwkerk PT (2014). Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC medicine, 12(1), 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Kripalani S, & Durapau VJ Jr. (2012). Improving medication management among at-risk older adults. J Gerontol Nurs, 38(6), 24–34; quiz 36–27. doi: 10.3928/00989134-20120509-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvanova M, Roumie CL, Eden SK, Cawthon C, Schnipper JL, & Kripalani S. (2011). Health literacy and medication understanding among hospitalized adults. Journal of hospital medicine, 6(9), 488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Riley B, Schmotzer B, Boyington DR, & Kripalani S. (2014). Improving medication understanding among Latinos through illustrated medication lists. Am J Manag Care, 20(12), e547–555. [PubMed] [Google Scholar]
- Mohan A, Riley MB, Boyington D, & Kripalani S. (2012). PictureRx: Illustrated medication instructions for patients with limited health literacy. J Am Pharm Assoc (2003), 52(5), e122–129. doi: 10.1331/JAPhA.2012.11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe AK, Chander G, & Moore RD (2011). Control of medical comorbidities in individuals with HIV. Journal of Acquired Immune Deficiency Syndromes, 58(5), 458–462. doi: 10.1097/QAI.0b013e31823801c4 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe AK, Rowe TL, Moore RD, & Chander G. (2013). Medication adherence in HIV-positive patients with diabetes or hypertension: a focus group study. BMC health services research, 13(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance RM, Delaney JC, Golin CE, Wechsberg WM, Cunningham C, Altice F, … Gordon MS (2017). Co-calibration of two self-reported measures of adherence to antiretroviral therapy. AIDS Care, 29(4), 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano GJ, Rascati KL, Wilson JP, & Remund DD (1999). A comparison of antihypertensive medication utilization before and after guideline changes using the Department of Defense Prescription Database. Ann Pharmacother, 33(5), 548–553. [DOI] [PubMed] [Google Scholar]
- Osborn CY, Paasche-Orlow MK, Davis TC, & Wolf MS (2007). Health literacy: an overlooked factor in understanding HIV health disparities. American Journal of Preventive Medicine, 33(5), 374–378. [DOI] [PubMed] [Google Scholar]
- Risser J, Jacobson T, & Kripalani S. (2007). Development and psychometric evaluation of the self-efficacy for appropriate medication use scale (SEAMS) in low-literacy patients with chronic disease. Journal of Nursing Measurement, 15(3), 203–219. [DOI] [PubMed] [Google Scholar]
- Sax PE, Meyers JL, Mugavero M, & Davis KL (2012). Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS ONE, 7(2), e31591. doi: 10.1371/journal.pone.0031591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclar DA, Robison LM, Skaer TL, Legg RF, Nemec NL, Galin RS, … Buesching DP (1994). Antidepressant pharmacotherapy: economic outcomes in a health maintenance organization. Clin Ther, 16(4), 715–730; discussion 774. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, & Frick PA (2006). Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS and Behavior, 10(3), 227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Group PHQPCS (1999). Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA, 282(18), 1737–1744. [DOI] [PubMed] [Google Scholar]
- Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, … Huntley K. (2015). Self-report measures of medication adherence behavior: recommendations on optimal use. Translational behavioral medicine, 5(4), 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Servellen G, Brown JS, Lombardi E, & Herrera G. (2003). Health literacy in low-income Latino men and women receiving antiretroviral therapy in community-based treatment centers. AIDS Patient Care STDS, 17(6), 283–298. doi: 10.1089/108729103322108166 [DOI] [PubMed] [Google Scholar]
- Wilby K, Marra CA, da Silva JH, Grubisic M, Harvard S, & Lynd LD (2011). Randomized controlled trial evaluating pictogram augmentation of HIV medication information. Ann Pharmacother, 45(11), 1378–1383. doi: 10.1345/aph.1Q091 [DOI] [PubMed] [Google Scholar]
- Wolf MS, Davis TC, Osborn CY, Skripkauskas S, Bennett CL, & Makoul G. (2007). Literacy, self-efficacy, and HIV medication adherence. Patient Education and Counseling, 65(2), 253–260. [DOI] [PubMed] [Google Scholar]
- Wood E, Hogg RS, Yip B, Harrigan PR, O’shaughnessy MV, & Montaner JS (2004). The impact of adherence on CD4 cell count responses among HIV-infected patients. JAIDS Journal of Acquired Immune Deficiency Syndromes, 35(3), 261–268. [DOI] [PubMed] [Google Scholar]