Abstract
Aims
In the randomized, placebo-controlled Colchicine Cardiovascular Outcomes Trial (COLCOT) of 4745 patients enrolled within 30 days after myocardial infarction (MI), low-dose colchicine (0.5 mg once daily) reduced the incidence of the primary composite endpoint of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina leading to coronary revascularization. To assess the in-trial period and lifetime cost-effectiveness of low-dose colchicine therapy compared to placebo in post-MI patients on standard-of-care therapy.
Methods and results
A multistate Markov model was developed incorporating the primary efficacy and safety results from COLCOT, as well as healthcare costs and utilities from the Canadian healthcare system perspective. All components of the primary outcome, non-cardiovascular deaths, and pneumonia were included as health states in the model as both primary and recurrent events. In the main analysis, a deterministic approach was used to estimate the incremental cost-effectiveness ratio (ICER) for the trial period (24 months) and lifetime (20 years). Over the in-trial period, the addition of colchicine to post-MI standard-of-care treatment decreased the mean overall per-patient costs by 47%, from $502 to $265 Canadian dollar (CAD), and increased the quality-adjusted life years (QALYs) from 1.30 to 1.34. The lifetime per-patient costs were further reduced (69%) and QALYs increased with colchicine therapy (from 8.82 to 11.68). As a result, both in-trial and lifetime ICERs indicated colchicine therapy was a dominant strategy.
Conclusion
Cost-effectiveness analyses indicate that the addition of colchicine to standard-of-care therapy after MI is economically dominant and therefore generates cost savings.
Keywords: Myocardial infarction, Cost effectiveness, Colchicine
Introduction
Approximately 870 000 North Americans suffer from a myocardial infarction (MI) each year and it is estimated that 18.7 million North Americans currently live with the associated risks and consequences of a prior MI event.1,2 Despite advancements in pharmacologic therapy, post-MI patients maintain a substantial residual risk for additional MIs, strokes, cardiac arrests, and all-cause mortality.2,3 In addition, these subsequent debilitating events in post-MI patients lead to a large burden on healthcare systems and reductions in quality of life.4–6
Colchicine, an anti-inflammatory medication commonly prescribed to treat gout,7,8 has been shown to be a viable therapeutic option for secondary prevention in post-MI patients.9,10 Results from the Colchicine Cardiovascular Outcomes Trial (COLCOT) of 4745 patients enrolled within 30 days after MI showed that the addition of low-dose colchicine (0.5 mg once daily) to standard-of-care medical therapy decreased the incidence of the primary composite endpoint of cardiovascular death, resuscitated cardiac arrest, MI, stroke, and urgent hospitalization for angina leading to coronary revascularization.9
Colchicine is a well-established medication, but whether the risk reductions reported in COLCOT translate into a change in the cost-effectiveness of post-MI treatment has yet to be evaluated. As a new indication and applied population for therapy, a quantitative assessment of the economic value of concomitant colchicine therapy post-MI would further aid clinicians and health policy decision-makers about long-term management of post-MI patients. Therefore, the objective of the present study was to assess the in-trial period and lifetime cost-effectiveness of low-dose colchicine therapy compared to placebo in post-MI patients on standard-of-care therapy.
Methods
Clinical trial
Detailed trial design characteristics and results of COLCOT were previously published9 and relevant results are summarized in Tables 1 and2. COLCOT was a randomized, double-blind placebo-controlled trial in which patients with a prior MI treated with standard medical therapy were randomized (1:1) to low-dose colchicine (0.5 mg per day) or placebo for a median follow-up duration of approximately 2 years.9 The primary efficacy endpoint was a composite of death from cardiovascular causes, resuscitated cardiac arrest, MI, stroke, and urgent hospitalization for angina leading to coronary revascularization.9
Table 1.
Baseline characteristics
| Characteristics | Colchicine (N = 2366) | Placebo (N = 2379) |
|---|---|---|
| Age, mean ± SD | 60.6 ± 10.7 | 60.5 ± 10.6 |
| Female sex | 472 (19.9) | 437 (18.4) |
| Hypertension | 1185 (50.1) | 1236 (52.0) |
| Diabetes | 462 (19.5) | 497 (20.9) |
| Priormyocardial infarction | 370 (15.6) | 397 (16.7) |
| Priorpercutaneous coronary intervention | 392 (16.6) | 406 (17.1) |
| Heart failure | 48 (2.0) | 42 (1.8) |
| Prior stroke or transient ischaemic attack | 55 (2.3) | 67 (2.8) |
| Medication use | ||
| Aspirin | 2334 (98.6) | 2352 (98.9) |
| Otherantiplatelet agent | 2310 (97.6) | 2337 (98.2) |
| Statin | 2339 (98.9) | 2357 (99.1) |
| Beta-blocker | 2116 (89.4) | 2101 (88.3) |
Values are represented as N (%).
Table 2.
Clinical and safety endpoints included in cost-effectiveness analyses
| Endpoints | Colchicine (N = 2366) | Placebo (N = 2379) | Hazards ratio (95% CI) |
|---|---|---|---|
| First event | |||
| Composite primary outcome | 131 (5.5) | 170 (7.1) | 0.77 (0.61–0.96) |
| Death from cardiovascular causes | 20 (0.8) | 24 (1.0) | 0.84 (0.46–1.52) |
| Resuscitated cardiac arrest | 5 (0.2) | 6 (0.3) | 0.83 (0.25–2.73) |
| Myocardial infarction | 89 (3.8) | 98 (4.1) | 0.91 (0.68–1.21) |
| Stroke | 5 (0.2) | 19 (0.8) | 0.26 (0.10–0.70) |
| Urgent hospitalization for angina leading to revascularization | 25 (1.1) | 50 (2.1) | 0.50 (0.31–0.81) |
| Other clinical and safety endpoints | |||
| Death from non-cardiovascular causes | 23 (1.0) | 20 (0.8) | — |
| All coronary revascularizationsa | 132 (5.6) | 164 (6.9) | — |
| Pneumonia | 21 (0.9) | 9 (0.4) | — |
| Number of events per patient | |||
| Resuscitated cardiac arrest | — | ||
| 1 | 4 | 5 | |
| 2 | 1 | 1 | |
| Myocardial infarction | — | ||
| 1 | 80 | 84 | |
| 2 | 9 | 9 | |
| 3 | — | 5 | |
| Stroke | — | ||
| 1 | 5 | 18 | |
| 2 | — | 1 | |
| Urgent hospitalization for angina leading to revascularization | — | ||
| 1 | 25 | 46 | |
| 2 | — | 3 | |
| 3 | — | 1 | |
| All coronary revascularizations | — | ||
| 1 | 124 | 143 | |
| 2 | 6 | 18 | |
| 3 | 2 | 1 | |
| 4 | — | 1 | |
| 5 | — | 1 | |
Includes urgent and elective coronary revascularizations.
Healthcare costs
All healthcare costs were estimated from the Canadian healthcare perspective using the Ontario Case Costing Initiative (OCCI) for costs associated with acute events11 and Régie de l’Assurance Maladie du Québec (RAMQ) for the price of colchicine and medication dispensing fees.12 Per-patient chronic care and treatment costs associated with each event were obtained from published literature on the Canadian population enrolled in a single-payer healthcare system.5,13–15 Chronic care costs were based on the average health care utilization for each cardiovascular event and were obtained from population-level studies using administrative databases. Costs of physician visits, hospitalizations, emergency room visits, medications, rehabilitation, and healthcare home visits were included in chronic care costs. All costs were based on an average value, inflated to 2019 rates, and chronic care costs were applied to the 2 and 20-year time horizons. Cost inputs incorporated in cost-effectiveness models are reported in Table 3.
Table 3.
Cost inputs
| Event/medication | Base valuea | Low valuea | High valuea | Distribution |
|---|---|---|---|---|
| Colchicine (per pill)12 | $0.26 | — | — | — |
| Acute costs11 | ||||
| Resuscitated cardiac arrest | $9673 | $7255 | $12 090 | Gamma |
| Myocardial infarction | $7769 | $5827 | $9711 | Gamma |
| Stroke | $10 224 | $7668 | $$12 780 | Gamma |
| Coronary revascularization | Gamma | |||
| Coronary artery bypass graft surgery | $24 283 | $18 213 | $30 354 | |
| Percutaneous coronary intervention | $8894 | $6670 | $11 117 | |
| Pneumonia | $8206 | $6154 | $10 257 | Gamma |
| Long-term costsb | ||||
| Resuscitated cardiac arrest13 | $458 | $343 | $572 | Gamma |
| Myocardial infarction5 | $766 | $575 | $958 | Gamma |
| Stroke14 | $1557 | $1168 | $1947 | Gamma |
| Coronary artery bypass graft surgery13 | $1276 | $957 | $1595 | Gamma |
| Percutaneous coronary intervention13 | $766 | $575 | $958 | Gamma |
All costs are reported in Canadian dollars (CAD $).
Long-term follow-up costs are presented yearly.
Utility measures
Utility weights were used to calculate quality-adjusted life years (QALYs). As COLCOT did not collect data on quality of life measures, all utilities were estimated from published literature on similar patient populations16–19 and presented in Table 4. Utility weights range from 0 to 1 per year, with a utility of one denoting perfect health.
Table 4.
Utility inputs
| Utilities/disutilities | Base value | Low value | High value | Distribution |
|---|---|---|---|---|
| Baseline utilitya18 | 0.682 | 0.512 | 0.853 | Beta |
| Disutilities | ||||
| Resuscitated cardiac arrest19 | 0.101 | 0.076 | 0.126 | Beta |
| Myocardial infarction18 | 0.147 | 0.110 | 0.184 | Beta |
| Stroke18 | 0.178 | 0.134 | 0.223 | Beta |
| Coronary revascularization17 | Beta | |||
| Coronary artery bypass graft surgery | 0.090 | 0.068 | 0.113 | |
| Percutaneous coronary intervention | 0.060 | 0.045 | 0.075 | |
| Pneumonia16 | 0.020 | 0.015 | 0.025 | Beta |
Utility is presented yearly.
The utility for the baseline health state for all patients was 0.682. COLCOT was a secondary prevention trial and therefore at the time of enrolment, patients were in a diminished health state. At an average age of 60 years for the trial population, the initial utility value was set at 0.829.18 To qualify for trial inclusion, all patients had a prior MI, which further reduced the baseline utility to 0.682 (disutility for MI of 0.147).18
Base case cost-effectiveness models
Multistate Markov models were developed incorporating the primary efficacy endpoint components, non-cardiovascular death, and pneumonia as health states. Pneumonia was the only serious adverse event that was statistically significantly different (P < 0.05) between groups and, hence, was included in the Markov models. All event rates were derived from the intent-to-treat results of the trial and included the first and second events.9
A deterministic approach was used to calculate the incremental cost-effectiveness ratio (ICER) for the primary in-trial and lifetime cost-effectiveness analyses. The discount rate was set at 1.5% and the cycle length was 3 months. The time horizon for the in-trial analysis was 2 years and increased to 20 years for the lifetime analysis. For the in-trial and lifetime perspectives, it was assumed that patients took the medication (colchicine or placebo) throughout and that the hazards for each event were constant over the 2 and 20-year time horizons. A negative ICER value implied dominance, in which treatment decreased costs and increased effectiveness.
Sensitivity analyses
Multiple sensitivity analyses were performed. These included modelling any coronary revascularization as an endpoint, incorporating all recurrent events, as well as accounting for variations in costs and utilities with a one-way sensitivity analysis and using the probabilistic approach. Deterministic in-trial and lifetime ICERs were calculated to include all recurrent events captured in the trial (maximum six events) and all coronary revascularizations. For the one-way sensitivity analysis, costs, utilities, and disutilities were varied individually by ±25% of the base case values, while other inputs were held constant (model inputs presented in Tables 3 and4). A tornado diagram was created to display the sensitivity of the Markov model to specific model inputs. In the probabilistic approach, all model inputs were simultaneously varied (stochastic) based on specific variable distributions (Tables 3 and4) using Monte Carlo simulations (n = 1000 bootstrap resamples). Incremental cost-effectiveness scatterplots and acceptability curves were generated to present results for the probabilistic approach. All sensitivity analyses were conducted for the in-trial and lifetime time horizons.
Cost-effectiveness analyses were conducted using the TreeAge Pro 2019, R2 (TreeAge Software, Williamstown, MA, USA; software available at http://www.treeage.com). Clinical efficacy and descriptive data analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). The trial protocol was approved by the institutional review board of all participating centres.
Results
Trial population and clinical outcomes
The intent-to-treat population included a total of 4745 patients, which consisted of 2366 patients in the colchicine arm and 2379 in the placebo arm. Baseline characteristics were balanced between treatment arms and are presented in Table 1.
Over the median 23 months of follow-up, 5.5% of patients in the colchicine arm and 7.1% of patients in the placebo arm had at least one event included in the primary efficacy endpoint [hazards ratio (HR) 0.77, 95% confidence interval (CI) 0.61–0.96); Table 2]. Of the specific events included in the primary composite endpoint, colchicine had a statistically significant protective effect against stroke (HR 0.26, 95% CI 0.10–0.70) and urgent rehospitalization for angina leading to revascularization (HR 0.50, 95% CI 0.31–0.81) (Table 2). In addition, colchicine was shown to reduce the incidence of the primary endpoint with the inclusion of all recurrent events (rate ratio 0.66, 95% CI 0.51–0.86).
Base case analyses
Over the 24-month period of the trial, the addition of colchicine to post-MI standard-of-care treatment decreased the mean overall per-patient costs by 47%, from $502 to $265 Canadian dollar (CAD), and increased the QALYs from 1.30 to 1.34. Per-patient costs were further reduced (69%) with colchicine ($2590 CAD) compared to placebo ($8239 CAD) for the lifetime perspective. The difference in QALYs also increased with colchicine therapy over the lifetime (11.68 vs. 8.82 QALYs, colchicine vs. placebo, respectively). As a result, both in-trial and lifetime ICERs were negative thereby indicating that colchicine therapy was a dominant strategy (Table 5).
Table 5.
In-trial and lifetime incremental cost-effectiveness ratios (ICERs)
| Analysis | Average cost, CAD $ |
Average QALYs gained |
ICERb | ||||
|---|---|---|---|---|---|---|---|
| Colchicine | Placebo | Differencea | Colchicine | Placebo | Differencea | ||
| In-trial | |||||||
| Base case | $265 | $502 | −$237 | 1.34 | 1.30 | −0.04 | Dominant |
| Primary endpoints, non-cardiovascular deaths, pneumonia | |||||||
| 1st and 2nd (recurrent) events | |||||||
| Sensitivity analyses | |||||||
| Base case and inclusion of all recurrent events | $265 | $494 | −$222 | 1.34 | 1.30 | −0.04 | Dominant |
| Base case and inclusion of tertiary endpoint: elective coronary revascularization | $745 | $855 | −$111 | 1.30 | 1.29 | −0.01 | Dominant |
| Base case and inclusion of: elective coronary revascularization and all recurrent events | $749 | $858 | −$98 | 1.30 | 1.29 | −0.01 | Dominant |
| Lifetime | |||||||
| Base case | $2590 | $8239 | −$5647 | 11.68 | 8.82 | −2.86 | Dominant |
| Primary endpoints, non-cardiovascular deaths, pneumonia | |||||||
| 1st and 2nd (recurrent) events | |||||||
| Sensitivity analyses | |||||||
| Base case and inclusion of all recurrent events | $2597 | $8172 | −$5539 | 11.69 | 8.73 | −2.96 | Dominant |
| Base case and inclusion of tertiary endpoint: elective coronary revascularization | $13 737 | $14 175 | −$438 | 8.51 | 7.98 | −0.53 | Dominant |
| Base case and inclusion of: elective coronary revascularization and all recurrent events | $13 825 | $14 284 | −$400 | 8.51 | 7.98 | −0.53 | Dominant |
Differences compare average costs and QALYs of colchicine to placebo.
Dominant ICERs are not presented and results from lower costs and higher QALYs for colchicine.
All sensitivity analyses using the deterministic approach produced similar results to the main analyses, suggesting a dominant strategy (Table 5).
One-way sensitivity analyses
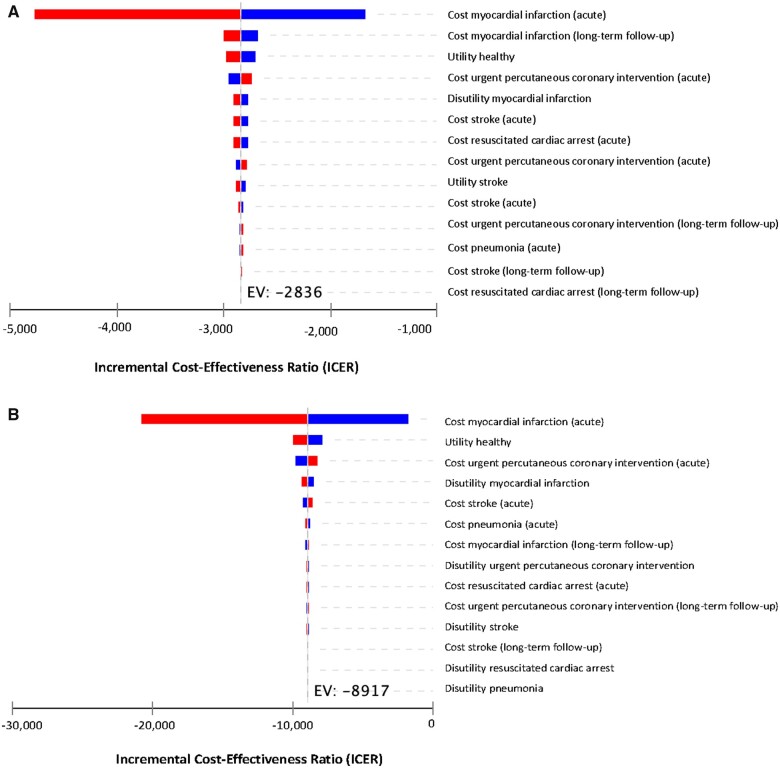
The parameter with the largest impact on ICER for both the in-trial and lifetime perspectives was the acute cost of an MI. Nevertheless, the ICER remained dominant for the range of costs (Figure1A andB). For the in-trial perspective, after the acute cost of MI, the model was the most sensitive to the cost of long-term follow-up for MI followed by the baseline utility (Figure 1A). For the lifetime perspective, variations in the baseline utility followed by the cost of urgent percutaneous coronary intervention effected the ICER the most after the acute cost of an MI (Figure 1B). Regardless, the ICER was dominant for all variations of costs, utilities, and disutilities for both the in-trial and lifetime perspectives (Figure1A andB).
Figure 1.
(A) Tornado diagram (in-trial). (B) Tornado diagram (lifetime).
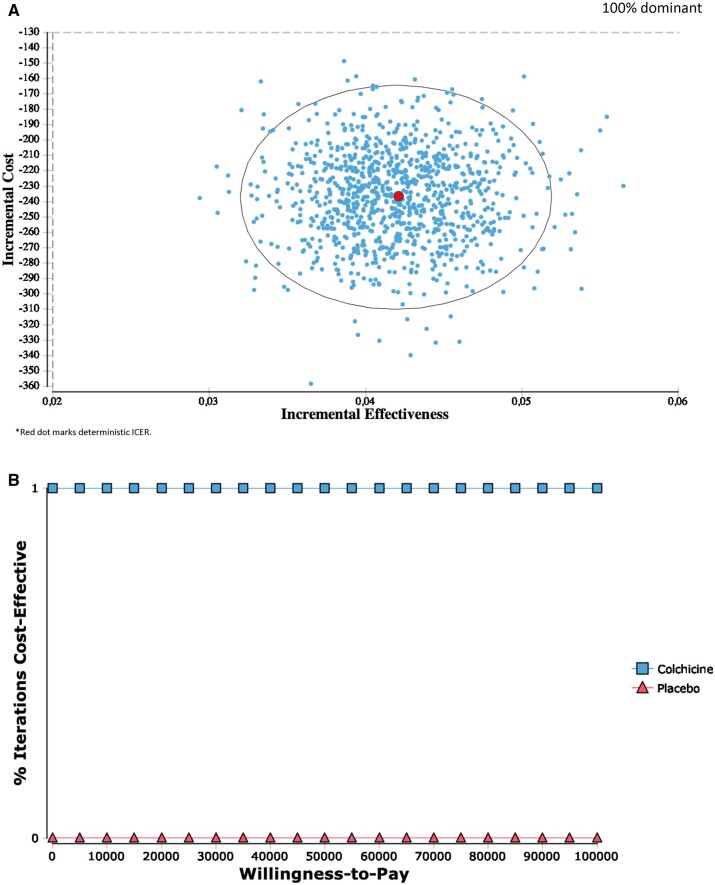
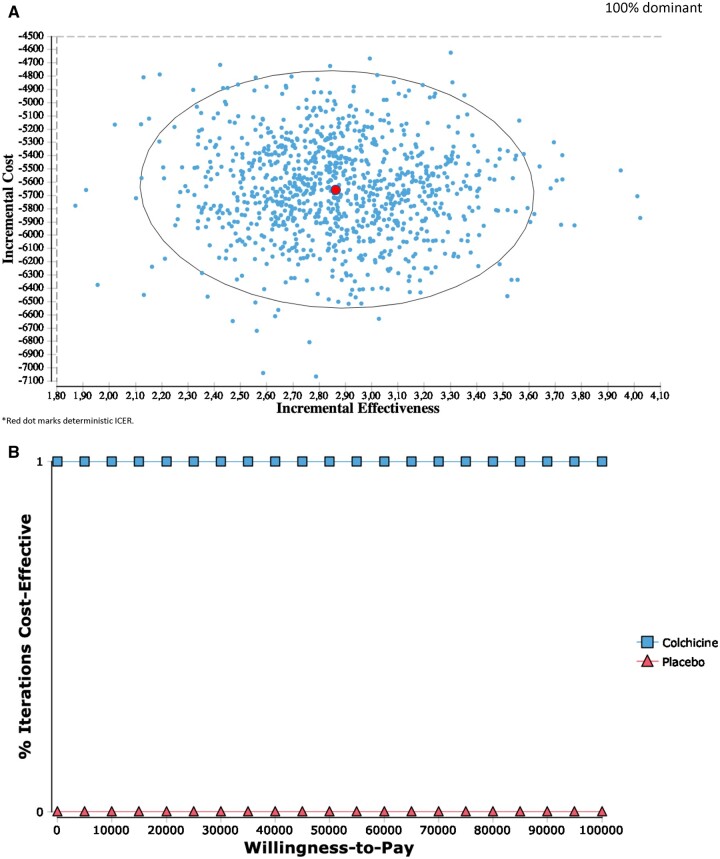
Probabilistic sensitivity analyses
Results for the in-trial and lifetime probabilistic sensitivity analyses were consistent with the deterministic ICERs, indicating a 100% dominant strategy after 1000 bootstrapped estimates (Figures2A and3A). Furthermore, at a willingness-to-pay of $0 per QALY, colchicine was 100% cost-effective for both the in-trial and lifetime perspectives (Figures2B and3B).
Figure 2.
(A) Incremental cost-effectiveness (in-trial). (B) Cost-effectiveness acceptability curve (in-trial).
Figure 3.
(A) Incremental cost-effectiveness (lifetime). (B) Cost-effectiveness acceptability curve (lifetime).
Discussion
COLCOT demonstrated that the addition of low-dose colchicine to standard medical therapy for post-MI patients decreases cardiovascular events, primarily stroke, and urgent hospitalization for angina requiring coronary revascularization.9 The present cost-effectiveness assessment indicates that the reduction in events reported in COLCOT translated into lower overall per-patient healthcare costs and increased utilities for both the in-trial and lifetime perspectives. Specifically, colchicine reduced in-trial and lifetime healthcare costs by 47% and 69%, respectively, and corresponding increases in QALY were 0.04 and 2.87. Therefore, colchicine was an economically dominant strategy for the primary analyses and these results were robust in all sensitivity analyses, which included all recurrent events, all coronary revascularizations, and variations in costs and utilities.
Colchicine therapy as a dominant strategy
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines state that a willingness-to-pay of <$50 000/QALY gained is considered high value and cost effective.20 The present study demonstrated that colchicine was 100% cost-effective at a willingness-to-pay of $0/QALY gained due to the dominant ICER. The economic dominance in addition to the clinical efficacy of colchicine further supports its use in post-MI patients.
The economically dominant strategy of colchicine is attributable to both a reduction in costly clinical events and the low price of this medication. Colchicine was isolated in the early 1800s and has been used as a treatment for gout and Familial Mediterranean Fever.7,8,21 It is currently available as a generic medication in most healthcare systems and in Canada, the cost of colchicine is $0.26 per pill.12
The components of the primary endpoint with the largest magnitude of reduction in events were stroke (HR 0.26, 95% CI 0.10–0.70) and urgent hospitalization for angina requiring coronary revascularization (HR 0.50, 95% CI 0.31–0.81). Of all primary endpoint components, the two events with the largest reduction were also the most expensive in the acute and long-term phases. Although the difference in QALYs was small between colchicine and placebo during the in-trial period, stroke has the highest disutility value (0.147) and the HR of 0.26 likely contributed to the increased effectiveness of colchicine, especially in the long term.
Although substantial reductions in the incidence of stroke and urgent hospitalization for angina requiring revascularization were pivotal for a dominant ICER, the model was most sensitive to the acute cost of MI for the in-trial and lifetime perspectives. This was due to the higher incidence of MIs compared to the other components of the primary endpoint.
Comparison of cost-effectiveness to other contemporary post-myocardial infarction medications
In recent years, several therapeutic options have been tested for secondary prevention in MI patients. The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) demonstrated a 15% reduction in cardiovascular endpoints; however, the medication was not cost-effective at a lifetime ICER of $6.4 million per QALY gained.22 Similarly, the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background Aspirin-Thrombolysis In Myocardial Infarction 54 (PEGASUS-TIMI 54) trial showed that treatment with ticagrelor resulted in an ICER of $94 917 per QALY gained,23 which suggests an intermediate value for cost-effectiveness according to the ACC/AHA guidelines.20 Few trials have demonstrated cost-effectiveness with ICERs below $50 000 per QALY gained, such as the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38)24 and a subgroup analysis of Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management, and Avoidance (CHARISMA) Trial.25 Even an established therapy such as high-dose statin did not demonstrate dominance when compared with low-dose statins in patients with acute coronary syndrome (cost-effective ICER of $44 000 per QALY gained).26 The only medication that also represents a dominant strategy, such as colchicine, is aspirin use in post-MI patients.27
Limitations
Although all results were consistent to show that colchicine was a dominant strategy despite variations in costs and utilities, important assumptions and limitations remained. First, quality of life measures that would have enabled utility values to be calculated directly from the subjects enrolled in COLCOT were not collected. Therefore, model inputs for utilities were obtained from previously published literature on populations that closely resembled the COLCOT study population, however, differences between the populations exist. Furthermore, few published studies measure the utilities of recurrent events, especially for three or more events. It is uncertain that the disutilities associated with a third event would be the same or augmented compared to the first or second event. For the present study, it was assumed that the magnitude of disutility was the same regardless of the number of prior events. In addition, mean costs of each event were incorporated into the Markov model instead of individual patient costs. Although some patients may have utilized differing magnitudes of healthcare resources due to different event severities, the use of an average cost ensures greater generalizability of results. Also, the present study used effect estimates from COLCOT (2-year follow-up) and assumed hazards of each event were constant over the 20-year lifetime perspective. Finally, although COLCOT was an international study, the cost-effectiveness estimates were based on the Canadian single-payer healthcare system. Therefore, future studies are warranted to investigate geographic variations in the cost-effectiveness of low-dose colchicine therapy in post-MI patients.
Conclusion
Cost-effectiveness analyses indicate that the addition of colchicine to standard-of-care therapy after MI is economically dominant and therefore generates costs savings and increased effectiveness.
What’s new?
For patients who suffered a recent myocardial infarction (MI), the addition of low-dose colchicine to standard medical therapy was highly cost-effective, with a decrease in overall per-patient costs and an increase in effectiveness.
Reductions in the incidence of cardiovascular events and an economically dominant cost-effectiveness strategy demonstrated in the Colchicine Cardiovascular Outcomes Trial (COLCOT) support the use of colchicine among post-MI patients.
Funding
This trial was supported by the Government of Quebec, the Canadian Institutes of Health Research and philanthropic funds, with the funds administered by the Montreal Heart Institute.
Data availability statement: No new data were generated or analysed in support of this research.
Conflict of interest: J.-C.T. reports receiving grant support from Amarin, Esperion, and Ionis Pharmaceuticals, receiving grant support and honoraria from AstraZeneca, Pfizer, Sanofi, and Servier, receiving grant support and honoraria from and having equity in DalCor Pharmaceuticals, holding a pending patent (US20170233812A1) on genetic markers for predicting responsiveness to therapy with a high-density lipoprotein (HDL) raising or HDL mimicking agent, and holding pending patents (62/935,751 and 62/935,865) on methods for using low-dose colchicine after myocardial infarction, licensed to Montreal Heart Institute, for which royalties are received. S.K. receiving advisory board fees and lecture fees and owning shares in Medtronic, Amgen, Bausch Health, and Bristol-Myers Squibb, receiving grant support, advisory board fees, and lecture fees and owning shares in Sanofi, AstraZeneca, and Novartis, receiving grant support and owning shares in GlaxoSmithKline, receiving grant support, advisory board fees, and lecture fees from Merck, Pfizer, and Boehringer Ingelheim, receiving advisory board fees and lecture fees from Eli Lilly, Bayer, and Servier, receiving grant support from Esperion, DalCor Pharmaceuticals, Eisai, Amarin, and Theracos, and owning shares in Johnson & Johnson, Celgene, Biogen, Gilead Sciences, Roche, Boston Scientific, TG Therapeutics, Becton Dickinson, and Spectrum Therapeutics. D.D.W. receiving fees for serving on a steering committee from DalCor Pharmaceuticals and Resverlogix, fees for serving on a data monitoring committee from the Medicines Company, and consulting fees from Pharmascience. R.D. receiving grant support, advisory board fees, and lecture fees from Sanofi and grant support from Amgen, Lepetit Pharma, and DalCor Pharmaceuticals, Astra Zeneca, Sanofi, Eli Lilly, Amgen, Population Heart Research Institute, Duke Clinical Research Institute, Montreal Health Research Coordinating Center, Cirius Therapeutics, Heart Initiative. A.P.M. receiving fees for participating on study committees from Bayer, Fresenius, and Novartis. F.J.P. receiving grant support, consulting fees, lecture fees, and advisory board fees from Bayer, AstraZeneca, Daiichi Sankyo, Biotronik, Servier, Philips, and Menarini, consulting fees, lecture fees, and advisory board fees from Boehringer Ingelheim, and grant support and consulting fees from Medtronic and Abbott. G.S.K. receiving lecture fees from Bayer, Servier, Novartis, AstraZeneca, Bristol-Myers Squibb, and Roche and Pfizer. C.B. receiving grant support and consulting fees, paid to the University of Glasgow, from Abbott Vascular, AstraZeneca, Coroventis, GlaxoSmithKline, HeartFlow, Novartis, and Siemens. J.L.-S. receiving grant support from Merck, Novartis, Sanofi, McMaster University, DalCor Pharmaceuticals, Boehringer Ingleheim, New York University School of Medicine and Pfizer and lecture fees from Menarini. W.K. receiving consulting fees and lecture fees from AstraZeneca, consulting fees from Novartis, Pfizer, the Medicines Company, DalCor Pharmaceuticals., Kowa Pharmaceuticals Ltd, Amgen, and Corvidia Therapeutics, lecture fees from Sanofi and Berlin-Chemie, and grant support and donated supplies from Roche Diagnostics, Beckmann Coulter, Singulex, and Abbott. J.C.G. receiving lecture fees and advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Sanofi, and Servier and advisory board fees from HLS Therapeutics. M.-P.D. receiving honoraria and fees for serving on an executive committee from and holding equity in DalCor Pharmaceuticals, holding a pending patent (US20190070178A1) on methods for treating or preventing cardiovascular disorders and lowering risk of cardiovascular events, licensed to DalCor Pharmaceuticals, holding a pending patent (US20170233812A1) on genetic markers for predicting responsiveness to therapy with an HDL-raising or HDL mimicking agent, licensed to DalCor Pharmaceuticals, and holding a pending patent (62/935,751) on methods for using low-dose colchicine after myocardial infarction, licensed to Montreal Heart Institute, for which royalties are received. N.B. was a consultant for AstraZeneca (2019). F.R. receiving consulting fees, lecture fees, and advisory board fees from Amgen, Abbott, and Sanofi, grant support, consulting fees, lecture fees, and advisory board fees from Novartis and cochicine, consulting fees and lecture fees from Servier, Medtronic, and Vifor Pharma, and grant support, consulting fees, and lecture fees from AstraZeneca. No other potential conflict of interest relevant to this article was reported.
Contributor Information
Michelle Samuel, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada.
Jean-Claude Tardif, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada.
Paul Khairy, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada.
François Roubille, Université de Montpellier, INSERM, CNRS, CHU de Montpellier, Cardiology Department, CHU Arnaud de Villeneuve, 371, avenue du Doyen Gaston-Giraud, 34090 Montpellier, France.
David D Waters, San Francisco General Hospital, Zuckerberg San Francisco General Hospital, 1001 Potrero Avenue, San Francisco, CA 94110, USA.
Jean C Grégoire, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada.
Fausto J Pinto, Santa Maria University Hospital (Centro Hospitalar Universitário Lisboa Norte), Centro Académico de Medicina de Lisboa, Centro Cardiovascular da Universidade de Lisboa, Faculdade de Medicina da Universidade de Lisboa, Lisbon, Portugal.
Aldo P Maggioni, ANMCO Research Center, Via La Marmora 34, 50121 Firenze, Italy.
Rafael Diaz, Estudios Clinicos Latinoamerica, Paraguay 160, 2000, Rosario, Argentina.
Colin Berry, University of Glasgow and NHS Glasgow Clinical Research Facility, 126 University Pl, University of Glasgow, Glasgow, G12 8TA, Scotland, UK.
Wolfgang Koenig, Deutsches Herzzentrum München, Technische Universität München, Munich, Institute of Epidemiology and Medical Biometry, University of Ulm, Ulm, Lazarettstr. 36, D-80636 Munchen, Germany.
Petr Ostadal, Cardiovascular Center, Na Homolce Hospital, Roentgenova 2, 150 00 Prague, Czech Republic.
Jose Lopez-Sendon, H La Paz, IdiPaz, UAM, Ciber-CV Madrid, La Paz University Hospital, Paseo de la Castellana, 261, 28046 Madrid, Spain.
Habib Gamra, Fattouma Bourguiba University Hospital, 5000 Monastir, Tunisia.
Ghassan S Kiwan, Bellevue Medical Center, Qanater Zubayda- Mansouriyeh, Mansourieh, Metn District, Beirut, Lebanon.
Marie-Pierre Dubé, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada.
Mylène Provencher, The Montreal Health Innovations Coordinating Center, 4100 Molson St. Suite 400 Montreal, Quebec H1Y 3N1, Canada.
Andreas Orfanos, The Montreal Health Innovations Coordinating Center, 4100 Molson St. Suite 400 Montreal, Quebec H1Y 3N1, Canada.
Lucie Blondeau, The Montreal Health Innovations Coordinating Center, 4100 Molson St. Suite 400 Montreal, Quebec H1Y 3N1, Canada.
Simon Kouz, Centre Hospitalier Régional de Lanaudière, 1000 Sainte-Anne Blvd Saint-Charles-Borromée, Quebec J6E 6J2, Canada.
Philippe L L’Allier, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada.
Reda Ibrahim, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada.
Nadia Bouabdallaoui, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada.
Dominic Mitchell, Logimetrix Inc., 3600 Rhodes Drive Windsor, Ontario N8W 5A4, Canada.
Marie-Claude Guertin, The Montreal Health Innovations Coordinating Center, 4100 Molson St. Suite 400 Montreal, Quebec H1Y 3N1, Canada.
Jacques Lelorier, Montreal Heart Institute, Université de Montréal, 5000 Belanger Street, Montréal, Québec H1T 1C8, Canada; Centre de recherche du Centre hospitalier de l’Université de Montréal, 900 St Denis St Montreal, Quebec H2X 0A9, Canada.
References
- 1.Heart Disease in Canada: Highlights From the Canadian Chronic Disease Surveillance System. Public Health Agency of Canada, Ottawa, Canada; 2017. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/heart-disease-canada-fact-sheet.html (20 January 2020).
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP. et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 update: a report from the American Heart Association . Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 3.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M.. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 4.Munyombwe T, Hall M, Dondo TB, Alabas OA, Gerard O, West RM. et al. Quality of life trajectories in survivors of acute myocardial infarction: a national longitudinal study. Heart 2020;106:33–39. [DOI] [PubMed] [Google Scholar]
- 5.Tran DT, Welsh RC, Ohinmaa A, Thanh NX, Kaul P.. Resource use and burden of hospitalization, outpatient, physician, and drug costs in short- and long-term care after acute myocardial infarction. Can J Cardiol 2018;34:1298–1306. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney EM, Wang K, Cohen DJ, Hirsch AT, Alberts MJ, Eagle K. et al. One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes 2008;1:38–45. [DOI] [PubMed] [Google Scholar]
- 7.Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F. et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005;112:2012–2016. [DOI] [PubMed] [Google Scholar]
- 8.Cerquaglia C, Diaco M, Nucera G, Regina M, Montalto M, Manna R.. Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: an update. Curr Drug Targets Inflamm Allergy 2005;4:117–124. [DOI] [PubMed] [Google Scholar]
- 9.Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 10.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL.. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–410. [DOI] [PubMed] [Google Scholar]
- 11.Ontario Case Costing Initiative. OCC Costing Analysis Tool 2018. https://stage/hsim/health.gov.on.ca/hdbportal (10 December 2019).
- 12.Régie de l’assurance maladie du Québec 2019. http://www.ramq.gouv.qc.ca (10 December 2019).
- 13.Fernando SM, McIsaac DI, Rochwerg B, Cook DJ, Bagshaw SM, Muscedere J. et al. Frailty and associated outcomes and resource utilization following in-hospital cardiac arrest. Resuscitation 2020;146:138–144. [DOI] [PubMed] [Google Scholar]
- 14.Griffith LE, Gruneir A, Fisher K, Panjwani D, Gafni A, Patterson C. et al. Insights on multimorbidity and associated health service use and costs from three population-based studies of older adults in Ontario with diabetes, dementia and stroke. BMC Health Serv Res 2019;19:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schedule of Benefits: Physician Services Under the Health Insurance Act. Ontario, ON: Ministry of Health and Long Term Care; 2020. http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20200429.pdf (20 January 2020).
- 16.Atwood M, Beausoleil L, Breton MC, Laferriere C, Sato R, Weycker D.. Cost-effectiveness of alternative strategies for use of 13-valent pneumococcal conjugate vaccine (PCV13) in Canadian adults. Can J Public Health 2018;109:756–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ariyaratne TV, Yap CH, Ademi Z, Rosenfeldt F, Duffy SJ, Billah B. et al. A systematic review of cost-effectiveness of percutaneous coronary intervention vs. surgery for the treatment of multivessel coronary artery disease in the drug-eluting stent era. Eur Heart J Qual Care Clin Outcomes 2016;2:261–270. [DOI] [PubMed] [Google Scholar]
- 18.Nyman JA, Barleen NA, Dowd BE, Russell DW, Coons SJ, Sullivan PW.. Quality-of-life weights for the US population: self-reported health status and priority health conditions, by demographic characteristics. Med Care 2007;45:618–628. [DOI] [PubMed] [Google Scholar]
- 19.Marti J, Hulme C, Ferreira Z, Nikolova S, Lall R, Kaye C. et al. The cost-effectiveness of a mechanical compression device in out-of-hospital cardiac arrest. Resuscitation 2017;117:1–7. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ. et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2304–2322. [DOI] [PubMed] [Google Scholar]
- 21.Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S.. Colchicine: old and new. Am J Med 2015;128:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehested TSG, Bjerre J, Ku S, Chang A, Jahansouz A, Owens DK. et al. Cost-effectiveness of canakinumab for prevention of recurrent cardiovascular events. JAMA Cardiol 2019;4:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnuson EA, Li H, Wang K, Vilain K, Shafiq A, Bonaca MP. et al. Cost-effectiveness of long-term ticagrelor in patients with prior myocardial infarction: results from the PEGASUS-TIMI 54 trial. J Am Coll Cardiol 2017;70:527–538. [DOI] [PubMed] [Google Scholar]
- 24.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Bhatt DL, Dunn ES, Shi C, Caro JJ, Mahoney EM. et al. Cost-effectiveness of clopidogrel plus aspirin versus aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA trial. Value Health 2009;12:872–879. [DOI] [PubMed] [Google Scholar]
- 26.Chan PS, Nallamothu BK, Gurm HS, Hayward RA, Vijan S.. Incremental benefit and cost-effectiveness of high-dose statin therapy in high-risk patients with coronary artery disease. Circulation 2007;115:2398–2409. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Moran AE, Liu J, Coxson PG, Heidenreich PA, Gu D. et al. Cost-effectiveness of optimal use of acute myocardial infarction treatments and impact on coronary heart disease mortality in China. Circ Cardiovasc Qual Outcomes 2014;7:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]