Abstract
Background:
Tailored neo-adjuvant treatment of locally advanced rectal cancer (LARC) may improve outcomes. The aim of this study was to determine early MRI prognostic parameters to stratify neoadjuvant treatment in patient with LARC.
Methods:
All patients from a prospective phase II, multicenter, randomized study (GRECCAR4-NCT01333709) were included in this study and underwent rectal MRIs before treatment, 4 weeks after induction chemotherapy and after completion of chemoradiation (CRT). Tumor volumetry, MRI Tumor Regression Grade (mrTRG), T staging, N staging, Circumferential resection margin (CRM) status, and MRI extramural vascular invasion (EMVI) presence were evaluated.
Results:
133 randomized patients were analyzed. Median follow-up was 41.4 months [95% CI: 36.6–45.2]. 31 patients (23%) recurred. At baseline, in univariate analysis, mrEMVI was the only prognostic factor associated with poorer outcome (p=0.0152). After induction chemotherapy, a higher tumor volume on MRI (p=0.019), a tumor volume regression ≤60% (p=0.002), involvement of the CRM (p=0.037), mrEMVI (p=0.026) and a poor mrTRG (p=0.023) were associated with poor outcome. After completion of CRT, the absence of complete response on MRI (p=0.004), mrEMVI (p=0.04) and a poor mrTRG (p=0.005) were associated with shorter DFS. A final multivariate model including all significant variables (baseline, post-induction, post-CRT) found ECOG status (p=0.011), sphincter involvement (p=0.009), EMVI presence at baseline (p=0.002) and early tumor volume regression ≤ 60% after induction (p=0.007) were associated with relapse.
Conclusion:
Baseline and early post-treatment MRI parameters are associated with prognosis in LARC. Future preoperative treatment should stratify treatment according to baseline EMVI status and early tumor volume regression.
Keywords: Rectal Cancer, Magnetic Resonance Imaging, disease-free survival
Introduction
The standard treatment for of locally-advanced rectal cancer (LARC) is chemoradiotherapy (CRT) followed by surgical resection, irrespective of tumor response1,2. However, personalized cancer therapy potentially changes this management. Patients’ can now be stratified by their response to treatment to minimally-invasive treatments for potentially good responders and more “aggressive” approach for patients likely to show a poor response3–5. The CRT regimens used are also evolving. Several studies have demonstrated the feasibility of adding oxaliplatin to standard preoperative CRT with improved efficacy and acceptable toxicity and compliance6–8. Others have evaluated different radiation regimens9–11. In this context, finding reliable prognostic indicators for early monitoring of treatment response is critical in order to switch to alternative strategies if required12.
Several approaches to assess tumor response using MRI have been described. These include morphological changes with MRI tumor regression (mrTRG)13 and volumetry12,14–19, or functional changes using diffusion-weighted imaging (DWI)14,15,20–24 and dynamic contrast-enhanced MRI (DCE-MRI)25–29. Tumor volumetry is a strong predictor of tumor response and outcome, especially when combined with DWI12,15–18,30–36. The advantage of T2W-DWI volumetry is that it capitalizes on tumor biology rather than relying only on morphologic appearance. Thus better differentiating postradiation fibrosis from residual tumor14,16,17,37,38. Other studies have evaluated the mrTRG derived from the pathologic tumor regression grade (pTRG)13. In the MERCURY study, a mrTRG of 1 to 3 (good response)7 was associated with higher disease-free (DFS) and overall survival compared with a mrTRG of 4 or 5 (poor response) (p<0.001)33.
To validate the concept of a more individualized treatment strategy of LARC, a multicenter randomized phase II study was conducted from May 2011 to October 2014 (GRECCAR 4-NCT01333709). The trial objective was to determine whether induction chemotherapy with oxaliplatin could be used to identify subgroups of patients in whom treatments, especially the radiation doses and regimens, could be de-escalated or intensified. Primary results were reported in 20177. The objective of the present study was to determine early MRI prognostic indicators that may help personalizing the treatment in this population.
Patients and Methods
Study design and patients
All patients initially enrolled in the original prospective phase II multicenter randomized study (GRECCAR4-NCT01333709) were eligible for the present analysis (n=206). The inclusion criteria were as follows: primary tumor evaluated using MRI (initial stage: mriT3 ≥ c or mriT4; or the predictive CRM was ≤ 1 mm), age ≥ 18 years, ECOG performance status ≤ 2 and adequate hematological, hepatic and renal functions. The exclusion criteria were indications for immediate surgery, no MRI evaluation of the primary tumor available, and previous pelvic radiotherapy or contraindication to radio- and/or chemotherapy.
GRECCAR 4 stratified patients according to the MRI tumor volume reduction after 4 weeks of induction chemotherapy. Patients were then divided into two strata based on the tumor response. A favorable response (stratum I) was defined as shrinkage ≥ 75% from the initial tumor volume with a predictive CRM > 1mm. These patients were randomly assigned (1:1 ratio) to receive immediate surgery (experimental arm, arm A) or classical CRT (Cap 50) followed by surgery (standard arm, arm B). An unfavorable response (stratum II) was defined as shrinkage < 75% from the initial tumor volume or a predictive CRM ≤ 1mm. Patients were randomized to receive Cap 50 (standard arm, arm C) or intensified CRT (Cap 60; experimental arm, arm D). Randomization was centralized and stratified by center and initial tumor stage. The study was approved by the institutional review board and informed consent was obtained from all patients.
The GRECCAR 4 trial initially included 206 patients, among whom 194 underwent induction chemotherapy and initial MRI assessment. Finally, 133 patients were randomized. After the inclusion of the 51 planned patients in arm D (Cap60), randomization in stratum II was stopped and all patients with an unfavourable response were included in arm C (Cap50). Randomization kept going in stratum I to try to reach the number of patients required in arm A. Finally, after inclusion of 61 more patients in arm C, the study independent committee recommended stopping all inclusions because of the difficulty to recruit in stratum I. Analyses were performed on all patients included in stratum I and all randomized patients in stratum II to have a homogenous population with clean data7.
Treatments
Neoadjuvant Treatment
All patients received FOLFIRINOX as induction chemotherapy: four cycles of 180mg/m2 irinotecan, 85mg/m2 oxaliplatin, 200mg/m2 elvorin and 5-FU (400mg/m2 bolus followed by 2400mg/m2 continuous infusion for 46 hours) delivered over 8 weeks.
Chemoradiotherapy
The standard Cap50 CRT regimen comprised a 50 Gy radiation dose administered as conventional 3D conformal or intensity-modulated radiation therapy (IMRT) (2Gy/fraction, 5 fractions/week for 5 weeks: 44Gy as mini-pelvic radiotherapy with a 6-Gy boost to a reduced target peritumoral volume) with concomitant oral capecitabine (1600mg/m2 daily in two identical doses taken on the radiotherapy days). The experimental Cap60 regimen consisted of 60 Gy irradiation (2Gy/fraction, 5 fractions/week for six weeks: 44Gy as mini-pelvic radiotherapy with a 16-Gy boost to a reduced target peritumoral volume) with the same concomitant oral capecitabine intake as in the Cap50 regimen.
Surgery
Surgery was performed in each center by an expert colorectal surgeon. Total Mesorectal Excision (TME) was performed by laparoscopic, robotic surgery, or open surgery. Surgical quality was evaluated using the Quirke mesorectum grading system.
Magnetic Resonance Imaging (MRI)
Imaging
Rectal MRIs were performed in all patients prior to treatment and after 4 weeks of induction chemotherapy. A third MRI after completion of CRT prior to surgery was performed at the discretion of each center (n=94).
Imaging was performed using a phased array body coil at 1.5 or 3T (Supplementary Table 1). Briefly, the MRI protocol consisted of standard T2-weighted fast spin echo sequences in multiple acquisition planes. All exams included dedicated transverse planes angled along the long axis of the tumor, using the sagittal planes as reference39. An additional diffusion-weighted echo planar imaging sequence was acquired with b0 as the lowest and b1500 s/mm2 as the highest b-factor. The MRI parameters reported in the 16 participating centers are presented in Supplementary Table 1. All MRIs were performed in accordance with the European Society of Gastrointestinal and Abdominal Radiology guidelines40,41.
Interpretation
All images were independently analyzed by a single highly experienced radiologist (centralized reading), blinded to all clinical information, other imaging results, and histopathology.
Tumor evaluation before treatment, after 4 weeks of induction chemotherapy and after completion of CRT was evaluated according to “DISTANCE” parameters42: Dis: Distance from the inferior part of the tumor to the transitional skin, T:T staging, A: Anal complex, N: Nodal staging, C: Circumferential resection margin, and E: Extramural vascular invasion. Presence of mucin was also collected.
For each patient, tumor volumetry was performed manually, tracing the tumor boundaries on the axial oblique images on T2W-MR images in conjunction with the highest b value image as to exclude areas of T2 fibrosis and include only the residual tumor. The volume of the whole tumor was then calculated multiplying each cross-sectional area by section thickness (including the slice gap if present). For each patient, the reader determined (1) the “T2+DWI” pretreatment tumor volume, (2) the “T2+DWI” post induction treatment tumor volume, and (3) the “T2+DWI” post CRT tumor volume. The tumor volume reduction ratio after induction chemotherapy and after CRT was calculated as such: [DELTA]volume = (Pretreatment tumor volume - Posttreatment tumor volume) × 100/(Pretreatment tumor volume).
The mrTRG was also evaluated to determine the degree of tumor replacement by fibrotic or mucinous changes stroma13. Favorable mrTRG was defined as grades 1 and 2, and unfavorable mrTRG as stages 3, 4 and 513.
Histopathologic Assessment
After TME, the specimen was axially sectioned into 3- to 5-mm slices, as described by Quirke et al.43. A dedicated gastrointestinal pathologist specialized in rectal cancer in each center evaluated in a blinded manner specimens for the post-treatment yT and yN stages. Tumour regression was evaluated in pathology using the tumor regression grade (pTRG) described in the Dworak et al. study44. A favorable pTRG was defined as Dworak 3 and 4, and an unfavorable pTRG as Dworak 0, 1 and 2.
Follow-up
Patients were followed-up according to the trial rules. This comprised outpatient assessment every 4 months for 2 years, followed by assessment every 6 months for the 5 following years. Clinical follow-up included physical examinations, routine blood tests, and 6 months computed tomography of the thorax, abdomen, and pelvis. Recurrence was confirmed pathologically with findings from surgical resections, biopsies, and/or radiologic examinations. The surgical data, date of operation, procedure performed, and quality of the specimen were recorded prospectively. Date of enrollment, last follow-up, date of disease progression, and date and cause of death were also collected. The presence of distant metastatic disease or local recurrence at the time of death was recorded as rectal cancer-specific death.
Statistical Analysis
Data are expressed with medians and ranges for continuous variables and numbers and percentages for categorical variables. Associations between clinical and pathologic characteristics were evaluated using the Chi-squared or Fisher’s exact tests for categorical variables and the Kruskal-Wallis tests for continuous variables.
Disease-free survival (DFS) was defined as the time between enrollment and occurrence of the first event among local recurrence, metastasis or death whatever the cause. Patients without event at the time of the analysis were censored at the date of the last informative follow-up. DFS rates were estimated by the Kaplan-Meier method. Univariate and multivariate analyses were performed using the Cox proportional hazard’s regression model to estimate the hazard ratio and included baseline characteristics and MRI features. Comparisons were performed using the log-rank test for univariate analysis. Independent effects were evaluated from the likelihood ratio statistics. The final multivariate model was defined using backward stepwise selection (p<0.20) and a step-by-step method was used to include only the significant parameters (p<0.05). p<0.05 was considered statistically significant. All analyses were performed using the Stata software Version 13 (Texas, USA).
Results
Patients
Patients’ demographics and baseline characteristics are detailed on Supplementary Table 2.
MRI predictors of tumor response assessed by Dworak score
The Dworak score was evaluated in 117 patients (88%). Fifty-six patients (48%) showed a poor response on pathology (Dworak score 0 to 2) and 61 patients (52%) showed a good pathologic tumor response (Dworak score 3 and 4). The median initial tumor volume on MRI was larger in poor responders (50.3cm3 [range=3–387]) compared to good responders (28.3cm3 [range= 3.3–115]) (p<0.001) (Table 1).
Table 1:
Association between baseline MRI features, the Dworak score and DFS in univariate analysis
| N=133 | values | DWORAK (n=117) | DFS (n=128) | |||||
|---|---|---|---|---|---|---|---|---|
| 0–1–2 | 3–4 | P-value | HR | 95% CI | P-value | |||
|
| ||||||||
| Tumor Volume | 36 [3–387] | 50.3 [3–387] | 28.3 [3.3–115] | <0.001 | 1 | 1.00–1.00 | 0.38 | |
|
| ||||||||
| T | 3A/B | 13 (9.77%) | 3 (25%) | 9 (75%) | 1 | |||
|
| ||||||||
| 3C/D/4 | 120 (90.23%) | 53 (50.5%) | 52 (49.5%) | 0.09 | 0.69 | 0.24–1.98 | 0.51 | |
|
| ||||||||
| CRM | 0 | 44 (33.08%) | 23 (60.5%) | 15 (39.5%) | 0.06 | 1 | ||
|
| ||||||||
| 1 | 89 (66.92%) | 33 (42%) | 46 (58%) | 1.54 | 0.69–3.44 | 0.28 | ||
|
| ||||||||
|
| ||||||||
| N | 0 | 21 (15.79%) | 7 (39%) | 11 (61%) | 1 | |||
|
| ||||||||
| 1 | 112 (84.21%) | 49 (49.5%) | 50 (50.5%) | 0.41 | 1.71 | 0.52–5.64 | 0.34 | |
|
| ||||||||
| Sphincter involvement | 0 | 100 (75.19%) | 45 (52%) | 42 (48%) | 1 | |||
|
| ||||||||
| 1 | 33 (24.81%) | 11 (37%) | 19 (63%) | 0.16 | 1.80 | 0.86–3.77 | 0.13 | |
|
| ||||||||
| Tumor Location | Low | 50 (37.59%) | 17 (37%) | 29 (63%) | 1 | |||
|
| ||||||||
| Mid | 65 (48.87%) | 30 (54%) | 26 (46%) | 0.80 | 0.37–1.72 | |||
|
| ||||||||
| High | 18 (13.53%) | 9 (60%) | 6 (40) | 0.15 | 0.98 | 0.34–2.79 | 0.82 | |
|
| ||||||||
| EMVI | 0 | 59 (44.36%) | 22 (42%) | 30 (58%) | 1 | |||
|
| ||||||||
| 1 | 74 (55.64%) | 34 (52%) | 31 (48%) | 0.28 | 2.63 | 1.13–6.11 | 0.015 | |
|
| ||||||||
| Mucinous Content | 0 | 98 (73.68%) | 40 (45.5%) | 48 (54.5%) | 1 | |||
|
| ||||||||
| 1 | 35 (26.32%) | 16 (55%) | 13 (45%) | 0.36 | 1.53 | 0.73–3.20 | 0.27 | |
MRI: Magnetic Resonance Imaging; DFS: Disease-free Survival; 95% CI: 95% Confidence Interval; CRM: Circumferential Resection Margin; EMVI: Extramural venous invasion
After induction chemotherapy, a larger tumor volume on MRI (p<0.001), MRI stages T3 and T4 (p=0.008), a high rectal tumor location (p=0.009), MRI-detected EMVI (mrEMVI) (p<0.001) and a poor mrTRG (p<0.001) were associated with a poor pathologic response (Table 2).
Table 2:
Association between postinduction MRI features, the Dworak score and DFS in univariate analysis
| N=128 | Values | DWORAK (117) | DFS (n=128) | |||||
|---|---|---|---|---|---|---|---|---|
| 0–1–2 | 3–4 | P-value | HR | 95%CI | P-value | |||
|
| ||||||||
| Tumor Volume | 12 [0–217] | 16.5 [0–217] | 9.3 [0–47.6] | <0.001 | 1.01 | 1.00–1.02 | 0.019 | |
|
| ||||||||
| Volume regression | ≤60 | 51 (39.84%) | 20 (47%) | 23 (53%) | 3.15 | 1.51–6.59 | ||
|
| ||||||||
| >60 | 77 (60.16%) | 36 (51%) | 35 (49%) | 0.66 | 1 | 0.002 | ||
|
| ||||||||
| T | 0/1/2 | 39 (29.32%) | 11 (30%) | 26 (70%) | 1 | |||
|
| ||||||||
| 3/4 | 94 (70.68%) | 45 (56%) | 35 (44%) | 0.008 | 2.18 | 0.84–5.67 | 0.083 | |
|
| ||||||||
| CRM | 0 | 91 (70.0%) | 37 (45%) | 45 (55%) | 0.17 | 1 | 0.037 | |
|
| ||||||||
| 1 | 39 (30.0%) | 19 (59%) | 13 (41%) | 2.16 | 1.07–4.39 | |||
|
| ||||||||
|
| ||||||||
| N | 0 | 53 (39.85%) | 19 (39%) | 30 (61%) | 1 | |||
|
| ||||||||
| 1 | 80 (60.15%) | 37 (54%) | 31 (46%) | 0.095 | 2.0 | 0.89–4.46 | 0.077 | |
|
| ||||||||
| Sphincter involvement | 0 | 126 (95.45%) | 53 (48%) | 58 (52%) | 1 | |||
|
| ||||||||
| 1 | 6 (4.55%) | 3 (60%) | 2 (40%) | 0.67 | 1.65 | 0.39–6.93 | 0.522 | |
|
| ||||||||
| Tumor Location | Low | 44 (33.08%) | 12 (30%) | 28 (70%) | 1 | |||
|
| ||||||||
| Mid | 68 (51.13%) | 32 (53%) | 28 (47%) | 0.85 | 0.38–1.89 | |||
|
| ||||||||
| High | 21 (15.79%) | 12 (71%) | 5 (29%) | 0.009 | 1.06 | 0.39–2.92 | 0.866 | |
|
| ||||||||
| EMVI | 0 | 89 (66.92%) | 29 (37%) | 50 (63%) | 1 | |||
|
| ||||||||
| 1 | 44 (33.08%) | 27 (71%) | 11 (29%) | <0.001 | 2.25 | 1.11–4.55 | 0.0255 | |
|
| ||||||||
| Mucinous Content | 0 | 95 (71.97%) | 39 (46%) | 46 (54%) | 1 | |||
|
| ||||||||
| 1 | 37 (28.03%) | 17 (55%) | 14 (45.2%) | 0.39 | 1.92 | 0.94–3.94 | 0.080 | |
|
| ||||||||
| TRG | 1 | 1 (0.75%) | 0 | 1 (100%) | ||||
|
| ||||||||
| 2 | 41 (30.83%) | 10 (26%) | 29 (74%) | |||||
|
| ||||||||
| 3 | 31 (23.31%) | 7 (28%) | 18 (72%) | |||||
|
| ||||||||
| 4 | 51 (38.35%) | 31 (70%) | 13 (30%) | |||||
|
| ||||||||
| 5 | 9 (6.77%) | 8 (100%) | 0 | <0.001 | ||||
|
| ||||||||
| TRG 1–2/3–5 | 1–2 | 42 (31.58%) | 10 (25%) | 30 (75%) | 1 | |||
|
| ||||||||
| 3–4–5 | 91 (68.42%) | 46 (60%) | 31 (40%) | <0.001 | 2.73 | 1.05–7.11 | 0.0227 | |
Prior to surgery, a larger MRI tumor volume (p<0.001), poorer T staging (p<0.003), T3 and 4 stages (p=0.002), a high rectal tumor location (p=0.037), mrEMVI (p=0.048) and a poor mrTRG (p<0.001) were associated with poor response on pathology (Table 3).
Table 3:
Association between presurgery MRI features, the Dworak score and DFS in univariate analysis.
| N=94 | Values | DWORAK (n=81) | DFS (n=94) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 0–1–2 | 3–4 | P-value | HR | 95% CI | P-value | |||
|
| ||||||||
| Tumor Volume | 6 [0–111] | 8.5 [0–111] | 0 [0–37] | <0.001 | 1.01 | 0.99–1.03 | 0.29 | |
|
| ||||||||
| Volume regression | ≤60 | 20 (21.28%) | 9 (60%) | 6 (40%) | 2.17 | 0.80–5.88 | ||
|
| ||||||||
| >60 | 74 (78.72%) | 21 (32%) | 45 (68%) | 0.041 | 1 | 0.14 | ||
|
| ||||||||
| T | 0/1/2 | 49 (52.13%) | 10 (22%) | 35 (78%) | 1 | |||
|
| ||||||||
| 3/4 | 45 (47.87%) | 20 (56%) | 16 (44%) | 0.003 | 1.98 | 0.73–5.36 | 0.17 | |
|
| ||||||||
| CRM | 0 | 71 (82.56%) | 24 (39%) | 38 (61%) | 0.618 | 1 | 0.21 | |
|
| ||||||||
| 1 | 15 (17.44%) | 6 (46%) | 7 (54%) | 2.04 | 0.70–5.91 | |||
|
| ||||||||
|
| ||||||||
| N | 0 | 62 (65.96%) | 18 (31%) | 40 (69%) | 1 | |||
|
| ||||||||
| 1 | 32 (34.04%) | 12 (52%) | 11 (48%) | 0.076 | 1.79 | 0.69–4.63 | 0.24 | |
|
| ||||||||
| Sphincter involvement | 0 | 93 (98.94%) | NA | NA | NA | NA | NA | NA |
|
| ||||||||
| 1 | 1 (1.06%) | |||||||
|
| ||||||||
| Tumor Location | Low | 35 (38.04%) | 8 (25%) | 24 (75%) | 1 | |||
|
| ||||||||
| Mid | 44 (47.83%) | 15 (41%) | 22 (59%) | 0.59 | 0.20–1.75 | |||
|
| ||||||||
| High | 13 (14.13%) | 7 (70%) | 3 (30%) | 0.037 | 1.38 | 0.40–4.71 | 0.39 | |
|
| ||||||||
| EMVI | 0 | 78 (82.98%) | 22 (32%) | 47 (68%) | 1 | |||
|
| ||||||||
| 1 | 16 (17.02%) | 8 (67%) | 4 (33%) | 0.048 | 3.11 | 1.15–8.43 | 0.0384 | |
|
| ||||||||
| Mucinous Content | 0 | 73 (78.49%) | 21 (33%) | 42 (67%) | 1 | |||
|
| ||||||||
| 1 | 20 (21.51%) | 8 (47%) | 9 (53%) | 0.296 | 1.46 | 0.51–4.16 | 0.49 | |
|
| ||||||||
| TRG | 1 | 21 (22.34%) | 1 (5%) | 18 (95%) | ||||
|
| ||||||||
| 2 | 28 (29.79%) | 5 (19%) | 21 (81%) | |||||
|
| ||||||||
| 3 | 23 (24.47%) | 9 (56%) | 7 (44%) | |||||
|
| ||||||||
| 4 | 19 (20.21%) | 13 (72%) | 5 (28%) | |||||
|
| ||||||||
| 5 | 3 (3.19%) | 2 (100%) | 0 | <0.001 | ||||
|
| ||||||||
| TRG 1–2/3–5 | 1–2 | 49 (52.13%) | 6 (13%) | 39 (87%) | 1 | |||
|
| ||||||||
| 3–4–5 | 45 (47.87%) | 24 (67%) | 12 (33%) | <0.001 | 4.30 | 1.40–13.21 | 0.005 | |
MRI predictors of patient outcomes
After a median follow-up of 41.4 months (95% CI: 36.6–45.2), 31 of the 133 patients (23%) developed recurrence. Distant metastases were the most frequent type of relapse, reported in 22/31 patients (71%). Among them, 11 patients presented with lung metastases. Four patients (13%) had local recurrence. Among all patients with recurrence, 29 were asymptomatic, with recurrence detected by routine imaging. Two recurrences were detected in association with abnormal CEA levels.
At baseline, in univariate analysis, mrEMVI was the only prognostic factor associated with shorter DFS (HR=2.6 [1.1–6.1], p=0.0152). In multivariate analysis, a poor ECOG status (HR=17.93 [3.65–88.17], p=0.008), sphincter involvement (HR=3.30 [1.44–7.55], p=0.007) and mrEMVI (HR=3.98 [1.65–9.62], p<0.001) were independently associated with a shorter DFS (Table 3).
After induction chemotherapy, a higher tumor volume on MRI (HR=1.01 [1.00–1.02], p=0.019), tumor volume regression ≤60% (HR=3.15 [1.51–6.59], p=0.002), involvement of the CRM (HR=2.16 [1.07–4.39], p=0.037), mrEMVI (HR=2.25 [1.11–4.55], p=0.026) and a poor mrTRG (HR=2.73 [1.05–7.11], p=0.023;) were associated with shorter DFS (Table 3). In multivariate analysis, tumor volume regression ≤60% (HR=3.32 [1.50–7.32], p=0.002), and mrEMVI (HR=2.40 [1.01–5.71], p=0.04) were associated with a shorter DFS.
After completion of CRT, the absence of complete response on MRI (HR=8.5 [1.13–64.1], p=0.004), mrEMVI (HR=3.11 [1.15–8.43], p=0.04) and a poor mrTRG (HR=4.30 [1.40–13.21], p=0.005) were associated with shorter DFS, regardless of the patient arm (Table 4). In multivariate analysis, mrTRG was the only factor associated with DFS (HR=7.33 [1.49–36.03], p=0.017).
Table 4:
Multivariate analysis including all significant variables (baseline, post induction, post CRT) regarding patient outcome.
| N=128 | HR | 95% CI | P-value | |
|---|---|---|---|---|
|
| ||||
| ECOG | 0 | 1 | 0.011 | |
| 1 | 2.36 | [0.94–5.91] | ||
| 2 | 16.53 | [3.28–83.31] | ||
|
| ||||
| Arm | A surgery | 1 | 0.44 | |
| B Cap 50 then surgery | 0.21 | [0.02–2.34] | ||
| C Cap 50 then surgery | 0.45 | [0.09–2.36] | ||
| D Cap 60 then surgery | 0.31 | [0.06–1.59] | ||
|
| ||||
| Sphincter involvement (MRI 1- Baseline) | NO | 1 | [1.38–8.05] | 0.009 |
| YES | 3.33 | |||
|
| ||||
| EMVI (MRI 1- Baseline) | NO | 1 | [1.49–8.72] | 0.002 |
| YES | 3.60 | |||
|
| ||||
| Tumor volume regression (MRI2/MRI1) | ≤ 60% | 2.971 | [1.28–6.92] | 0.007 |
| > 60% | 1 | |||
An overall multivariate analysis model was created, including all significant clinical and MRI variables, adjusted according to the patient arm. In this model, an ECOG status of 2 (HR=16.53 [3.28–83.31], p=0.011), sphincter involvement at baseline (HR=3.33 [1.38–8.05], p=0.009), mrEMVI at baseline (HR=3.60 [1.49–8.72], p=0.002) and an early tumor volume regression < 60% after induction chemotherapy (HR=2.97 [1.28–6.92], p=0.007) were associated with progression (Figure 1). Patients without sphincter involvement and mrEMVI at baseline and with a tumor volume regression on T2+DWI > 60% after induction chemotherapy had only 11.5% risk of recurrence. On the contrary, patients with sphincter involvement and mrEMVI at baseline and with a tumor volume regression ≤ 60% had 57.1% risk of recurrence (Figure 1).
Figure 1.
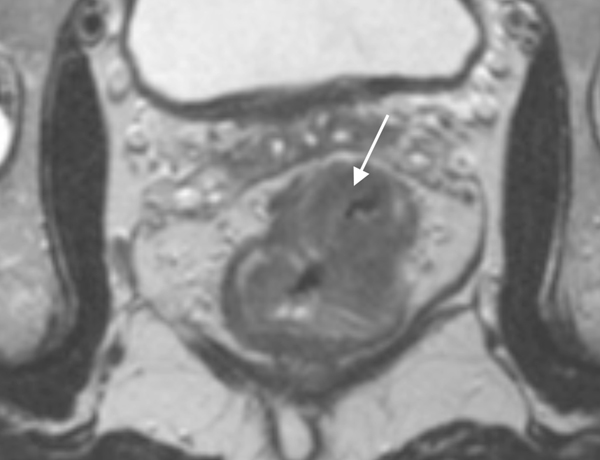
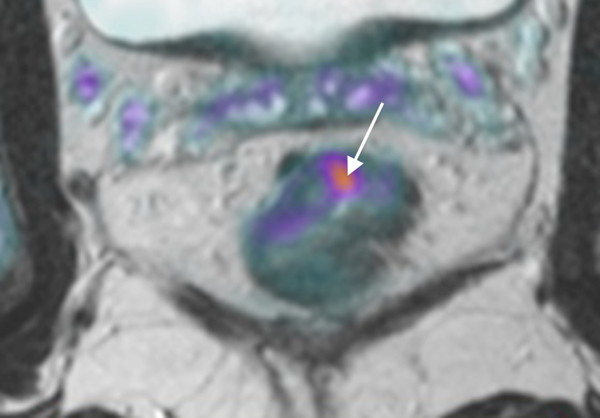
78 year-old patient with a low rectal adenocarcinoma and sphincter involvement; T2WI (a) and Fused T2WI+DWI (b) show tumor (white arrow) with adjacent extramural vascular invasion (black arrow). After induction chemotherapy (c, d), the tumor has decreased in volume, but less than 60%, and tumor deposit is still present (black arrow). The patient relapsed 14 months after surgery.
58 year-old patient with a mid-rectal adenocarcinoma. T2WI (e) and Fused T2WI+DWI (f) show a tumor (white arrow) without extramural vascular invasion. After induction chemotherapy (g, h), the tumor has largely decreased in volume, more than 60% (white arrow). Note the added value of DWI that better delineates residual tumor among therapy changes. No relapse was reported for this patient yet.
The lack of pathologic complete response on MRI was the only pathologic prognostic factor for shorter DFS (HR=8.70 [1–18–64.0], p=0.002). The pTRG (Dworak) was not associated with patient survival (HR=1.77 [0.83–3.78]).
Discussion
In the present study sphincter involvement, mrEMVI at baseline, and a tumor volume regression ≤ 60% after induction chemotherapy were associated with a higher risk of relapse. Stratifying patient treatment according to baseline EMVI status, tumor height and early tumor volume regression may thus improve patient survival.
Tumor volume reduction >60% after induction chemotherapy is an early surrogate marker for a favorable outcome. In the initial GRECCAR 4 trial, 75% tumor volume regression was chosen to define favorable response and stratify patients. This very high threshold was based on previous studies that reported that a threshold > 70% was associated with better patient outcomes after CRT12,16,18,36,45. In GRECCAR 4, most patients had a tumor volume reduction ranging between 50% and 75%, possibly because posttreatment volumes were measured after only 4 cycles of chemotherapy instead of at the end of CRT, as reported in other studies. Furthermore, contrary to other previous studies12,32,36, the “functional” residual tumor volume using the T2 and DWI combination instead of the T2 volume alone was evaluated. Volume measurements based on T2 alone often encompass fibrotic changes among the residual tumor19. The advantage of T2W-DWI volumetry is that it capitalizes on tumor biology rather than relying on only morphologic appearance that better differentiates post radiation fibrosis from residual tumor. As such, a lower threshold of 60% tumor volume reduction after induction chemotherapy was found to be an early predictor of patient outcome.
The present study found that mrEMVI at baseline is a strong predictor of DFS. This is concordant with previous studies that have found mrEMVI to be associated with poor prognosis46–53. In a large meta-analysis including 1262 patients, patients with mrEMVI developed metastases more frequently than patients with mrEMVI-negative tumors (OR=5.68, p<0.001). These findings highlight the critical role of mrEMVI evaluation in patient stratification. Despite the strong prevalence of mrEMVI and its poor prognosis, the treatment of patients with mrEMVI lacks consensus. In a survey, only 55% of surgeons and 57% of oncologists considered mrEMVI when deciding on postoperative treatment54,55. This may undertreat an MRI-identified group with higher risk of recurrence who should be considered for more aggressive therapy.
Sphincter involvement at baseline was associated with poor outcome. Low rectal tumors are associated with poorer prognosis than proximal tumors56,57. In the present study, prior to treatment, about 25% of patients showed sphincter involvement, that was reduced to 1% after neoadjuvant treatment. As such, sphincter involvement after induction chemotherapy and CRT was not associated with patient outcome. The type of surgery, i.e. intersphincteric resection in previously involved sphincter versus extralevator resection based on initial presentation of the tumor, may also contribute to the correlation between sphincter involvement and prognosis.
This study has several limitations. Beside the small sample size, radiologic evaluation was performed by one reader and reproducibility is therefore not assured. However, the volumetry technique is widely performed and reproducibility has previously been evaluated 45,58. Automated methods for volumetry evaluation with artificial intelligence may eventually overcome this limitation. The study median follow-up was 41.4 months; therefore confirmation with longer follow-up will be required. Although a dedicated gastrointestinal pathologist specialized in rectal cancer evaluated all resection specimens in a blinded manner, central pathological assessment would have been preferable.. The GRECCAR 4 clinical trial only included bulky locally-advanced tumors. The MRI prognostic parameters found in this study will therefore need to be tested on smaller tumors to demonstrate the reliability of this technique in smaller lesions.
In the era of personalized medicine, tailored treatment of LARC according to MRI parameters is essential. In this study, baseline sphincter involvement, mrEMVI presence, and early tumor volume reduction after induction chemotherapy were key predictors of shorter DFS. Further prospective studies may need to consider therapy intensification for mrEMVI-positive patients.
Supplementary Material
Acknowledgements
The GRECCAR Study Group was composed of the following, who participated in this work: Eric Rullier (Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France), Bernard Lelong (Institut Paoli-Calmettes, Marseille, France), Philippe Maingon (Centre Georges François Leclerc, Dijon, France), Jean-Jacques Tuech (Centre Hospitalier Universitaire de Rouen, Rouen, France), Denis Pezet (Centre Hospitalier Universitaire de Clermont-Ferrand, Clermont-Ferrand, France), Michel Rivoire (Centre Léon Bérard, Lyon, France), Bernard Meunier (Centre Hospitalier Universitaire de Rennes, Rennes, France), Jérome Loriau (Hôpital Saint Joseph, AP-HP, Paris, France), Alain Valverde (Groupe Hospitalier Diaconesses, Paris, France), Jean-Michel Fabre (Centre Hospitalier Universitaire Montpellier, Saint Eloi, Montpellier, France), Michel Prudhomme (Centre Hospitalier Universitaire Carémeau de Nîmes, Nîmes, France), Eddy Cotte (Hospices Civils de Lyon, Centre Hospitalier Lyon Sud, Lyon, France), Guillaume Portier (Hôpital Purpan, Centre Hospitalier Universitaire de Toulouse, Toulouse, France), Laurent Quero (Hôpital Saint Louis, AP-HP, Paris, France), and Benoit Gallix (Centre Hospitalier Universitaire de Montpellier, Montpellier, France) and from the Institut régional du Cancer de Montpellier (ICM), Montpellier, France: Claire Lemanski, Marc Ychou, Frédéric Bibeau. Dr Stéphanie Nougaret is supported by the SIRIC Montpellier Cancer Grant INCa_Inserm_DGOS_12553.
Support/Funding: French National Cancer Institute (INCa).
Grant number INCa-DGOS_5506: PHRC-K 2012-112.
List of where the study was presented, if applicable: Not applicable
Footnotes
Disclaimers: The authors claim no conflict of interest regarding this manuscript.
References
- 1.Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, et al. : Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 88:822–8, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Habr-Gama A, Sabbaga J, Gama-Rodrigues J, et al. : Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum 56:1109–17, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Pacelli F, Sanchez AM, Covino M, et al. : Improved outcomes for rectal cancer in the era of preoperative chemoradiation and tailored mesorectal excision: a series of 338 consecutive cases. Am Surg 79:151–61, 2013 [PubMed] [Google Scholar]
- 4.Valentini V, Lambin P, Myerson RJ: Is it time for tailored treatment of rectal cancer? From prescribing by consensus to prescribing by numbers. Radiother Oncol 102:1–3, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Wibe A, Law WL, Fazio V, et al. : Tailored rectal cancer treatment--a time for implementing contemporary prognostic factors? Colorectal Dis 15:1333–42, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Cho H, Kim JE, Kim KP, et al. : Phase II Study of Preoperative Capecitabine and Oxaliplatin-based Intensified Chemoradiotherapy With or Without Induction Chemotherapy in Patients With Locally Advanced Rectal Cancer and Synchronous Liver-limited Resectable Metastases. Am J Clin Oncol 39:623–629, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouanet P, Rullier E, Lelong B, et al. : Tailored Treatment Strategy for Locally Advanced Rectal Carcinoma Based on the Tumor Response to Induction Chemotherapy: Preliminary Results of the French Phase II Multicenter GRECCAR4 Trial. Dis Colon Rectum 60:653–663, 2017 [DOI] [PubMed] [Google Scholar]
- 8.van Zoggel D, Bosman SJ, Kusters M, et al. : Preliminary results of a cohort study of induction chemotherapy-based treatment for locally recurrent rectal cancer. Br J Surg, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Bujko K, Wyrwicz L, Rutkowski A, et al. : Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 27:834–42, 2016 [DOI] [PubMed] [Google Scholar]
- 10.De Felice F, Benevento I, Magnante AL, et al. : Clinical benefit of adding oxaliplatin to standard neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a meta-analysis : Oxaliplatin in neoadjuvant treatment for rectal cancer. BMC Cancer 17:325, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad P, Miraie M, Farhan F, et al. : Addition of oxaliplatin to neoadjuvant radiochemotherapy in MRI-defined T3, T4 or N+ rectal cancer: a randomized clinical trial. Asia Pac J Clin Oncol, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Nougaret S, Fujii S, Addley HC, et al. : Neoadjuvant chemotherapy evaluation by MRI volumetry in rectal cancer followed by chemoradiation and total mesorectal excision: Initial experience. J Magn Reson Imaging 38:726–32, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Patel UB, Taylor F, Blomqvist L, et al. : Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 29:3753–60, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Carbone SF, Pirtoli L, Ricci V, et al. : Diffusion-weighted MR volumetry for assessing the response of rectal cancer to combined radiation therapy with chemotherapy. Radiology 263:311, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Carbone SF, Pirtoli L, Ricci V, et al. : Assessment of response to chemoradiation therapy in rectal cancer using MR volumetry based on diffusion-weighted data sets: a preliminary report. Radiol Med 117:1112–24, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Curvo-Semedo L, Lambregts DM, Maas M, et al. : Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 260:734–43, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Ha HI, Kim AY, Yu CS, et al. : Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur Radiol 23:3345–53, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Kim YC, Lim JS, Keum KC, et al. : Comparison of diffusion-weighted MRI and MR volumetry in the evaluation of early treatment outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging 34:570–6, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Lambregts DM, Rao SX, Sassen S, et al. : MRI and Diffusion-Weighted MRI Volumetry for Identification of Complete Tumor Responders After Preoperative Chemoradiotherapy in Patients With Rectal Cancer: A Bi-institutional Validation Study. Ann Surg, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Barbaro B, Vitale R, Valentini V, et al. : Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys 83:594–9, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Bauerle T, Seyler L, Munter M, et al. : Diffusion-weighted imaging in rectal carcinoma patients without and after chemoradiotherapy: a comparative study with histology. Eur J Radiol 82:444–52, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Boone D, Taylor SA, Halligan S: Diffusion weighted MRI: overview and implications for rectal cancer management. Colorectal Dis 15:655–61, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Cai G, Xu Y, Zhu J, et al. : Diffusion-weighted magnetic resonance imaging for predicting the response of rectal cancer to neoadjuvant concurrent chemoradiation. World J Gastroenterol 19:5520–7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzik-Jurasz A, Domenig C, George M, et al. : Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 360:307–8, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Hotker AM, Schmidtmann I, Oberholzer K, et al. : Dynamic contrast enhanced-MRI in rectal cancer: Inter- and intraobserver reproducibility and the effect of slice selection on pharmacokinetic analysis. J Magn Reson Imaging 40:715–22, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Intven M, Reerink O, Philippens ME: Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J Magn Reson Imaging 41:1646–53, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Shen FU, Lu J, Chen L, et al. : Diagnostic value of dynamic contrast-enhanced magnetic resonance imaging in rectal cancer and its correlation with tumor differentiation. Mol Clin Oncol 4:500–506, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong T, Sun Y, Gollub MJ, et al. : Dynamic contrast-enhanced MRI: Use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J Magn Reson Imaging 42:673–80, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q, Liu L, Wang Q, et al. : Preoperative diagnosis and staging of rectal cancer using diffusion-weighted and water imaging combined with dynamic contrast-enhanced scanning. Oncol Lett 8:2734–2740, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birlik B, Obuz F, Elibol FD, et al. : Diffusion-weighted MRI and MR- volumetry--in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Magn Reson Imaging 33:201–12, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Intven M, Monninkhof EM, Reerink O, et al. : Combined T2w volumetry, DW-MRI and DCE-MRI for response assessment after neo-adjuvant chemoradiation in locally advanced rectal cancer. Acta Oncol 54:1729–36, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Kang JH, Kim YC, Kim H, et al. : Tumor volume changes assessed by three-dimensional magnetic resonance volumetry in rectal cancer patients after preoperative chemoradiation: the impact of the volume reduction ratio on the prediction of pathologic complete response. Int J Radiat Oncol Biol Phys 76:1018–25, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Lambregts DM, Rao SX, Sassen S, et al. : MRI and Diffusion-weighted MRI Volumetry for Identification of Complete Tumor Responders After Preoperative Chemoradiotherapy in Patients With Rectal Cancer: A Bi-institutional Validation Study. Ann Surg 262:1034–9, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Martens MH, van Heeswijk MM, van den Broek JJ, et al. : Prospective, Multicenter Validation Study of Magnetic Resonance Volumetry for Response Assessment After Preoperative Chemoradiation in Rectal Cancer: Can the Results in the Literature be Reproduced? Int J Radiat Oncol Biol Phys 93:1005–14, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Seierstad T, Hole KH, Groholt KK, et al. : MRI volumetry for prediction of tumour response to neoadjuvant chemotherapy followed by chemoradiotherapy in locally advanced rectal cancer. Br J Radiol 88:20150097, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo SG, Kim DY, Kim TH, et al. : Tumor volume reduction rate measured by magnetic resonance volumetry correlated with pathologic tumor response of preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 78:164–71, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Lee JM, Hong SH, et al. : Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 253:116–25, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Park MJ, Kim SH, Lee SJ, et al. : Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology 260:771–80, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Brown G: Thin section MRI in multidisciplinary pre-operative decision making for patients with rectal cancer. Br J Radiol 78 Spec No 2:S117–27, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Beets-Tan RG, Lambregts DM, Maas M, et al. : Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 23:2522–31, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. : Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nougaret S, Reinhold C, Mikhael HW, et al. : The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology 268:330–44, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Quirke P, Steele R, Monson J, et al. : Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 373:821–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dworak O, Keilholz L, Hoffmann A: Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12:19–23, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Nougaret S, Rouanet P, Molinari N, et al. : MR volumetric measurement of low rectal cancer helps predict tumor response and outcome after combined chemotherapy and radiation therapy. Radiology 263:409–18, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Bhangu A, Fitzgerald JE, Slesser A, et al. : Prognostic significance of extramural vascular invasion in T4 rectal cancer. Colorectal Dis 15:e665–71, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Kim YC, Kim JK, Kim MJ, et al. : Feasibility of mesorectal vascular invasion in predicting early distant metastasis in patients with stage T3 rectal cancer based on rectal MRI. Eur Radiol 26:297–305, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Siddiqui MRS, Simillis C, Hunter C, et al. : A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br J Cancer 116:1513–1519, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith NJ, Barbachano Y, Norman AR, et al. : Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 95:229–36, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Sohn B, Lim JS, Kim H, et al. : MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol 25:1347–55, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Yu J, Huang DY, Xu HX, et al. : Correlation Between Magnetic Resonance Imaging-Based Evaluation of Extramural Vascular Invasion and Prognostic Parameters of T3 Stage Rectal Cancer. J Comput Assist Tomogr 40:537–42, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Sclafani F, Brown G, Cunningham D, et al. : PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol 27:1557–65, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Patel UB, Brown G, Machado I, et al. : MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for primary rectal cancer: long-term results from the GEMCAD 0801 trial. Ann Oncol 28:344–353, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Chand M, Evans J, Swift RI, et al. : The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg 261:473–9, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Chand M, Palmer T, Blomqvist L, et al. : Evidence for radiological and histopathological prognostic importance of detecting extramural venous invasion in rectal cancer: recommendations for radiology and histopathology reporting. Colorectal Dis 17:468–73, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Gilchrist RK, David VC: A consideration of pathological factors influencing five year survival in radical resection of the large bowel and rectum for carcinoma. Ann Surg 126:421–38, 1947 [PubMed] [Google Scholar]
- 57.Freedman LS, Macaskill P, Smith AN: Multivariate analysis of prognostic factors for operable rectal cancer. Lancet 2:733–6, 1984 [DOI] [PubMed] [Google Scholar]
- 58.Nougaret S, Vargas HA, Lakhman Y, et al. : Intravoxel Incoherent Motion-derived Histogram Metrics for Assessment of Response after Combined Chemotherapy and Radiation Therapy in Rectal Cancer: Initial Experience and Comparison between Single-Section and Volumetric Analyses. Radiology 280:446–54, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.