Abstract
Background & Aims:
Gastrointestinal side effects are common during oral immunotherapy (OIT) and eosinophilic esophagitis (EoE) is a potential complication. We aimed to characterize eosinophilic gastrointestinal responses to peanut OIT, in which peanut protein is given orally, with incremental increases in dose over time.
Methods:
Twenty adults with IgE-mediated peanut allergy were randomly assigned to groups given peanut OIT (n=15) or placebo (n=5); 1 additional subject withdrew before randomization. Serial gastrointestinal biopsies were collected at baseline (n=21, 0 weeks), following dose escalation (n=10, 52 weeks), and during the maintenance phase (n=11, 104 weeks). Endoscopic findings were characterized using the EoE endoscopic reference score. Biopsies were assessed for eosinophils per high-power field (eos/hpf) and other pathology features using EoE histologic scoring system scores. We performed immunohistochemical analyses of eosinophil peroxidase deposition, quantified using automated image analysis.
Results:
At baseline, no subjects reported current gastrointestinal symptoms. However, 3 of the 21 subjects (14%) had esophageal peak eosinophil counts ≥15 eos/hpf and all subjects had dilated intercellular spaces (DIS). OIT induced or exacerbated esophageal eosinophilia (EE) at 52 weeks in most subjects (peak eosinophil counts >5 eos/hpf in 6 of 7 patients [86%]; peak eosinophil counts ≥15 eos/hpf in 4 of 7 patients [57%]). One subject met clinicopathologic criteria for EoE and withdrew; no significant changes in esophageal peak eosinophil counts were observed in the placebo group. EE in the OIT group corresponded with significant increases in EoE histologic scoring system scores and deposition of eosinophil peroxidase. In 4 of 6 participants (67%), OIT-induced EE and gastrointestinal eosinophilia resolved by the end of the maintenance phase. Gastrointestinal symptoms were not clearly associated with EE or gastrointestinal eosinophilia.
Conclusions:
In this pilot study, we found that peanut OIT-induced EE and gastrointestinal eosinophilia are usually transient and are not always associated with gastrointestinal symptoms. Clinicaltrials.gov no: NCT02103270
Keywords: EREFS, EoEHSS, Food Allergy, Inflammation
Introduction
IgE-mediated food allergy is an increasingly common and potentially life-threatening disease. Currently, the mainstay of treatment is food avoidance and management of acute allergic reactions. The unpredictable nature of these reactions has generated significant interest in proactive treatments that attempt to achieve tolerance or desensitization to food triggers through gradual antigen exposure.1 Several multi-center clinical trials have investigated oral immunotherapy (OIT) as a promising treatment for peanut allergy;2–6 notwithstanding, questions regarding its safety remain.7 Among potential adverse events, gastrointestinal side effects (e.g. abdominal pain, nausea, and vomiting) are the most common (affecting approximately 30% of OIT subjects) and are often the rationale for treatment discontinuation.8 Another major concern is the development of eosinophilic esophagitis (EoE) during OIT.9
EoE is a chronic antigen-mediated disease characterized by eosinophilic inflammation of the esophageal tissue resulting in esophageal dysfunction. If left untreated, eosinophilic inflammation may result in tissue fibrosis leading to esophageal narrowing, stricture formation, and food impaction.10 The incidence of EoE during OIT has been estimated at 2.7%.9 This is likely an underestimate as patients with gastrointestinal symptoms during clinical trials of OIT are not systematically evaluated with endoscopy. Some have estimated the incidence to be as high as 8–14% when gastrointestinal symptoms and dropout rates are used as alternative measures.8
There are three possible explanations for the occurrence of EoE during OIT: (1) subclinical disease exists prior to initiating OIT; (2) EoE develops irrespective of OIT; and (3) EoE is induced by OIT. Previously, we addressed the first of these scenarios by performing endoscopies in 21 peanut-allergic adult subjects prior to initiating OIT.11 We found increased eosinophils (>5 eosinophils per high-power field (eos/hpf)) in 24% (5/21) of subjects, 3 of whom (14%, 3/21) had ≥15 eos/hpf. Tissue eosinophilia in some subjects was also accompanied by mild endoscopic and histologic findings characteristic of EoE. Of note, these patients did not meet the clinicopathologic diagnostic criteria for EoE given the absence of symptoms of esophageal dysfunction. This study addresses scenarios 2 and 3 by performing serial endoscopic biopsies in the same subjects receiving peanut OIT vs. placebo.
Methods
Study population
Participants in this sub-study were recruited from a larger phase II, randomized, double-blind, placebo-controlled (DBPC), clinical trial. The Peanut Oral Immunotherapy: Safety, Efficacy and Discovery (POISED) Clinical Trial at the Sean N. Parker Center for Allergy and Asthma Research at Stanford University was conducted from April 2014 to March 2016 (clinicaltrials.gov; NCT02103270).11, 12 Both the parent trial and this sub-study were authorized by the Stanford University Institutional Review Board (Stanford, CA); this sub-study included a separate data safety monitoring board. Inclusion and exclusion criteria were described previously.11 Briefly, subjects >18 years old with IgE-mediated peanut allergy but no history of eosinophilic gastrointestinal disease (EGID) were recruited. Peanut allergy was confirmed by a DBPC food challenge and a comprehensive gastrointestinal symptom questionnaire (published previously11) was administered to assess clinical symptoms at baseline and during the trial. Subjects were withdrawn from the study if they met clinicopathologic criteria for EGID.
Endoscopic and histopathologic evaluation
Subjects underwent esophagogastroduodenoscopy (EGD) at three time points: baseline, 52 weeks (optional), and 104 weeks. Endoscopic biopsies were obtained from the proximal esophagus (PE), middle esophagus (ME), distal esophagus (DE), gastric antrum and proximal duodenum. Four passes were performed at each location and one biopsy was obtained with each pass. Three esophageal biopsies (1 proximal, 1 mid, 1 distal) were analyzed by histology in order to conserve samples for future mechanistic studies. Endoscopic findings were scored by a gastroenterologist using the EoE Endoscopic Reference Score (EREFS).13 Esophageal biopsy sections were evaluated using the EoE Histologic Scoring System (EoEHSS)14 and eosinophil counts were assessed using H&E stains. Eosinophilic inflammation was further assessed by automated analysis of EPX immunohistochemistry.11 Additional details regarding the dosing of OIT, symptoms questionnaires, evaluation of gastrointestinal pathology and statistical methods are found in the Supplemental Methods. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Participant overview and clinical outcomes
Baseline characteristics of participants in the peanut OIT and placebo arms were similar (Table 1). One subject withdrew prior to randomization. The majority of participants underwent scheduled study EGDs (Figure 1). Eight subjects (40%) passed the desensitization challenge at week 104; all of them were from the peanut OIT group. Seven of 20 subjects (6/15 in peanut OIT group, 1/5 in the placebo group) dropped out prior to week 104. Those subjects that withdrew from the active treatment group were deemed desensitization failures. Of note, one subject in the peanut OIT group withdrew at week 78, after developing EoE (participant #11).
Table 1.
Baseline characteristics
| Characteristic | Total (n = 20*) | Treatment | |
|---|---|---|---|
| Peanut (n=15) | Placebo (n=5) | ||
| Age at baseline (years), median (IQR) | 26.5 (23.5, 33.5) | 26 (23, 31) | 31 (24, 47) |
| Males, n (%) | 15 (75%) | 11 (73%) | 4 (90%) |
| White, n (%) | 12 (60%) | 8 (53%) | 4 (80%) |
| Atopic conditions, n (%) | |||
| Asthma | 16 (80%) | 12 (80%) | 4 (80%) |
| Allergic rhinitis | 17 (85%) | 12 (80%) | 5 (100%) |
| Atopic dermatitis | 11 (55%) | 8 (53%) | 3 (60%) |
| Other food allergies | 11 (55%) | 6 (40%) | 5 (100%) |
| Total serum IgE (kU/L), median (IQR) | 253.0 (88.5, 461.3) | 201.0 (88.0, 398.5) | 335.0 (242.0, 458.0) |
| Peanut-specific IgE (kU/L), median (IQR) | 6.6 (2.5, 70.8) | 6.3 (2.1, 57.5) | 26.1 (3.1, 163.0) |
| Peanut-specific IgG4 (ng/L), median (IQR) | 0.3 (0.1, 0.4) | 0.2 (0.1, 0.4) | 0.3 (0.2, 0.3) |
| Peanut skin prick test wheal diameter (mm), median (IQR) | 13.5 (7.8, 17.8) | 16.0 (8.6, 19.9) | 9.0 (7.0, 11.5) |
| Absolute eosinophil counts (cells/L), median (IQR) | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.3) | 0.1 (0.1, 0.3) |
IQR = interquartile range.
Excluded 1 participant who was not randomized into the POISED parent study.
Figure 1. CONSORT diagram.
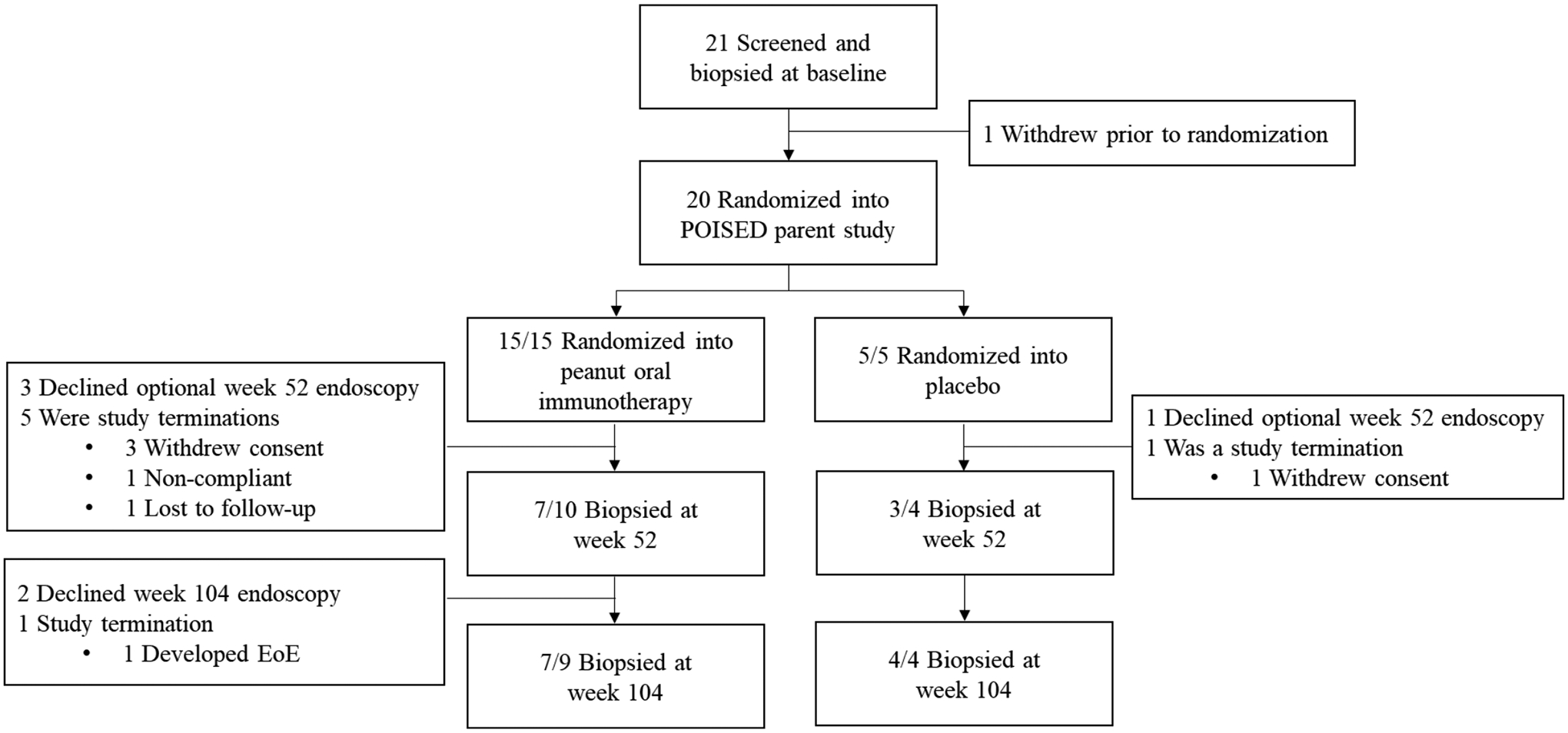
Denominators indicate the number of subjects still enrolled in the trial at each time point.
Gastrointestinal eosinophil counts and EPX levels
Longitudinal eosinophil counts for peanut and placebo groups over study time, by site, are presented in Figure 2. Eosinophilic responses in the esophagus were most common in the DE. There was no significant change in tissue eosinophil counts in the placebo group. One subject in the placebo group had increased eosinophils (11 eos/hpf) in the ME at baseline, but tissue eosinophilia resolved by week 104. In contrast, OIT induced or increased esophageal eosinophilia (EE) (peak eosinophil counts (PEC) >5 eos/hpf) in a majority of peanut OIT subjects from baseline to week 52 across the 3 esophageal sites (6/7 subjects (85.7%) with biopsies at 0 and 52 weeks. Increases from baseline were statistically significant in the PE (p=0.022), ME (p=0.0064), and DE (p=0.019). Four (57%) had ≥15 eos/hpf which resolved by week 104 in 2/4 subjects. Among those that did not resolve, one subject failed the desensitization challenge and had marked EE (60 eos/hpf) (participant #6). Another subject (participant #11) met clinicopathologic criteria for EoE (PEC = 38 eos/hpf, dysphagia, and food impaction) prompting withdrawal at week 78 for safety concerns. Histology and EPX immunostaining of the DE in this subject at baseline and week 52 are depicted in Figure 3.
Figure 2.
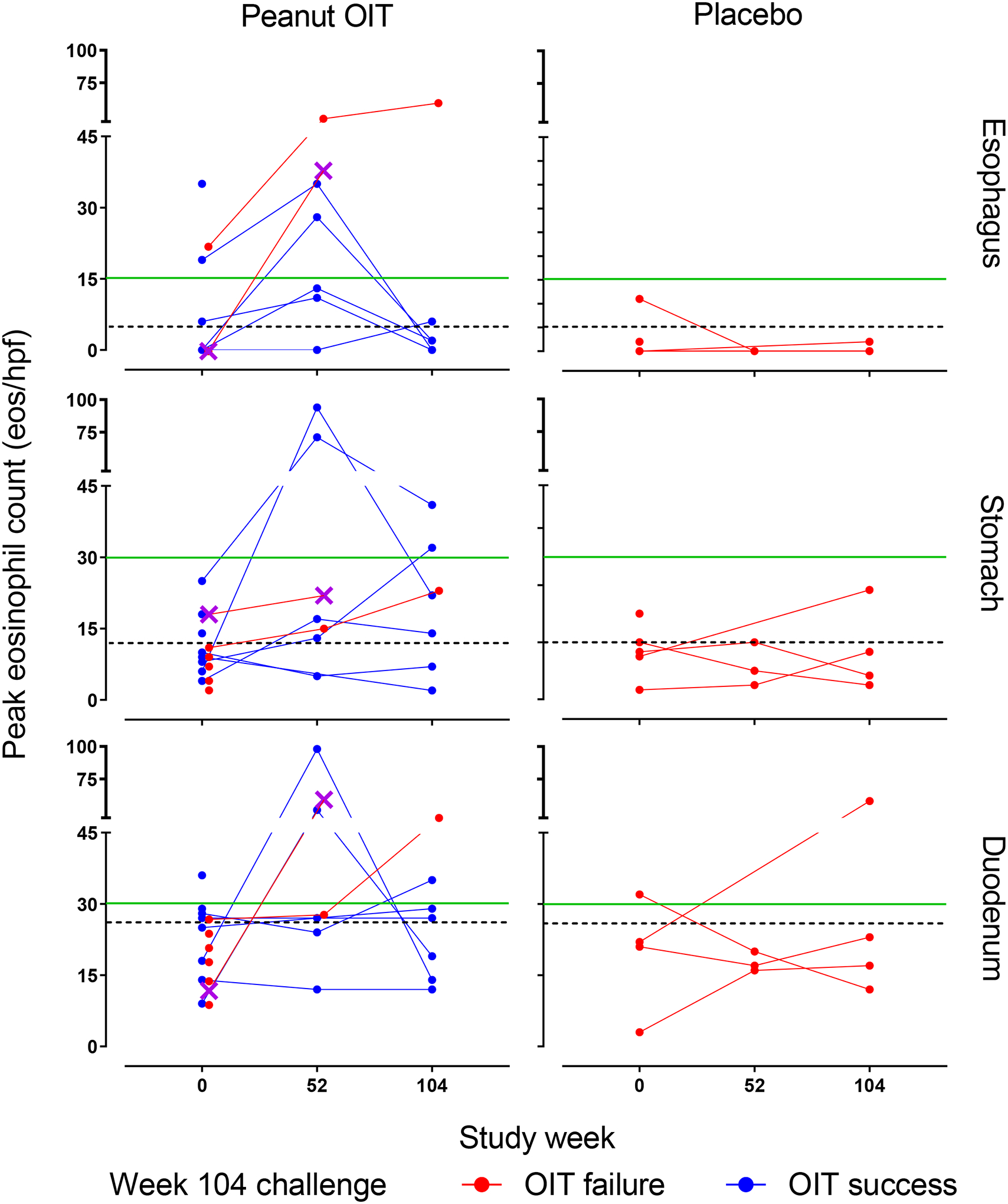
PEC over time by treatment arm, biopsy site, and week 104 challenge outcome. PEC at week 0 (peanut OIT 15, placebo 5), week 52 (peanut OIT 7, placebo 3), and week 104 (peanut OIT 7, placebo 4). Horizontal dashed black lines indicate upper limit of normal in the esophagus (5 eos/hpf), gastric antrum (12 eos/hpf), and duodenum (26 eos/hpf). Horizontal green line indicates histologic threshold for eosinophilic gastrointestinal disease (≥15 for esophagus, ≥30 for stomach and duodenum). Purple X denotes participant #11, who developed eosinophilic esophagitis, eos/hpf, eosinophils per high-power field; OIT, oral immunotherapy; PEC, peak eosinophil count.
Figure 3. Histology and EPX immunohistochemistry in a subject with OIT-induced EoE.
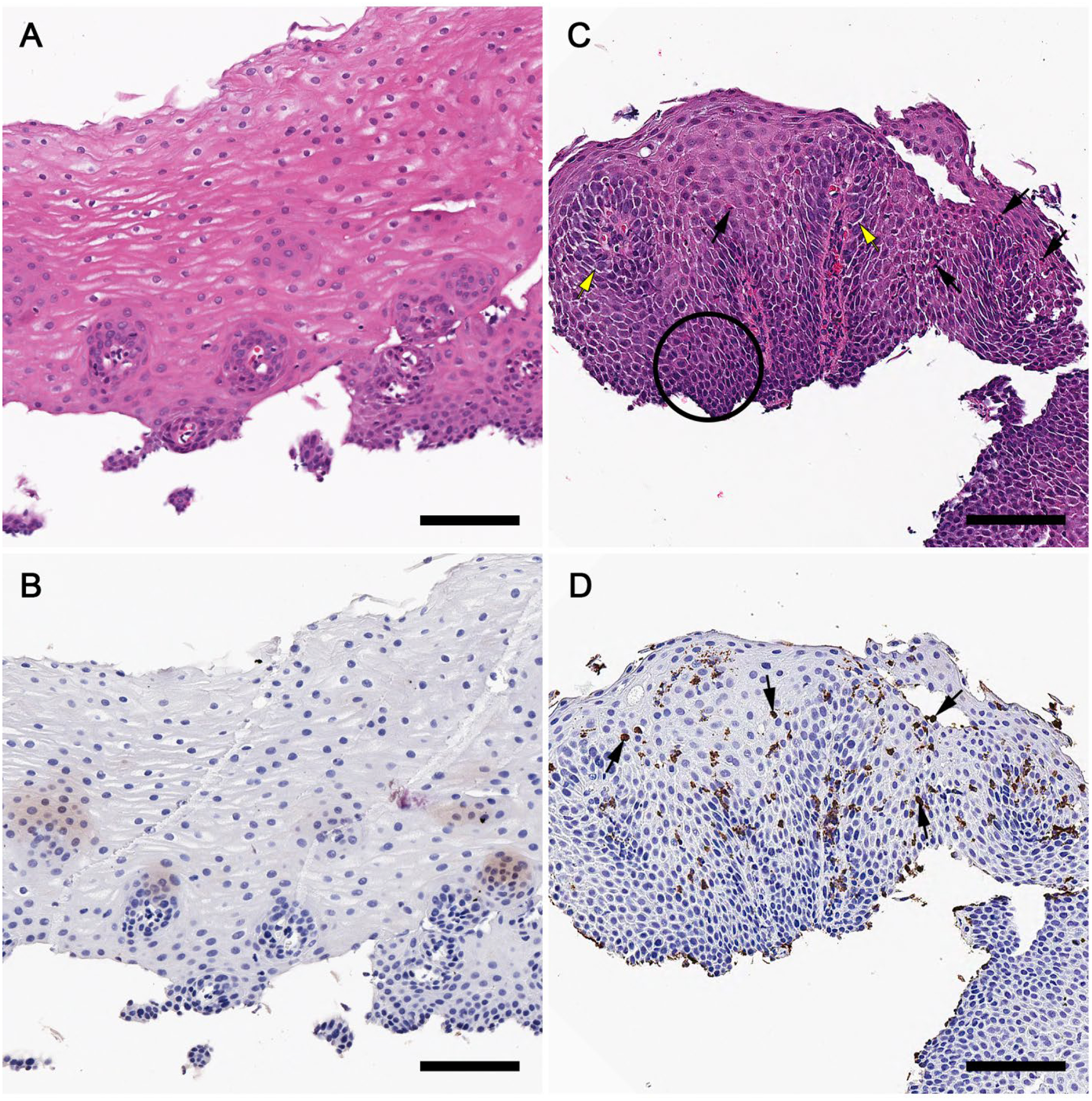
Hematoxylin and eosin (H&E) stains of distal esophageal (DE) biopsies from participant #11 at baseline (A) and week 52 (C). Corresponding EPX stains shown are shown below (B and D). EI (black arrows, C and D), BZH (black circle, C), and DIS (yellow arrows, C) are notable at 52 weeks. A marked increase in EI and degranulation is seen (C and D) compared to baseline (A and B). Scale bar=100 microns.
Gastrointestinal eosinophilia (GE) during peanut OIT was not limited to the esophageal mucosa. At baseline, 9/21 (42%) subjects showed gastric (>12 eos/hpf) and/or duodenal eosinophilia (>26 eos/hpf), though none of the participants met histologic criteria for eosinophilic gastritis (>30 eos/hpf in 5 hpf) or duodenitis (>30 eos/hpf in 3 hpf) based on eosinophil counts. However, at 52 weeks, eosinophil counts increased significantly in the stomach (p=0.034) and 2/7 participants in the active treatment arm exceeded thresholds for eosinophilic gastroenteritis and eosinophilic duodenitis. Another subject had tissue eosinophil counts consistent with eosinophilic duodenitis alone and all subjects receiving peanut OIT had either gastric (> 12 eos/hpf) or duodenal (>26 eos/hpf) eosinophilia. The proportion of subjects exceeding EGID thresholds is detailed in Table S1. Of the three subjects on placebo with endoscopic biopsies at 52 weeks, none had gastric or duodenal eosinophilia. For most subjects with longitudinal specimens, tissue eosinophilia was transient, with counts decreasing by week 104. EPX staining of tissue biopsy sections showed similar trends to the tissue eosinophilia observed by H&E staining (Figure S1). Changes in EPX/mm2 from baseline to week 52 were significant in the PE (p=0.011), DE (p=0.0067), ME (p=0.026), and stomach (p=0.038). Please see the Supplemental Results for additional observations regarding trends in EPX staining and biomarkers of EE.
Gastrointestinal symptoms
Most subjects were asymptomatic at baseline (Figure 4). The most common gastrointestinal symptom within the 4 weeks prior to the baseline endoscopy was abdominal pain. Five of 20 subjects (25%) reported infrequent (<3 times per month) abdominal pain prior to the study and 2 subjects (10%) endorsed infrequent abdominal pain at the time of EGD. Of the three subjects with baseline esophageal PEC ≥15 eos/hpf, none reported gastrointestinal symptoms. Seven subjects (47%) in the active treatment arm were deemed desensitization failures (6/15 withdrew). Only subject #11, who developed EoE, withdrew due to OIT-induced gastrointestinal side effects.
Figure 4. Frequency of gastrointestinal symptom questionnaire results.
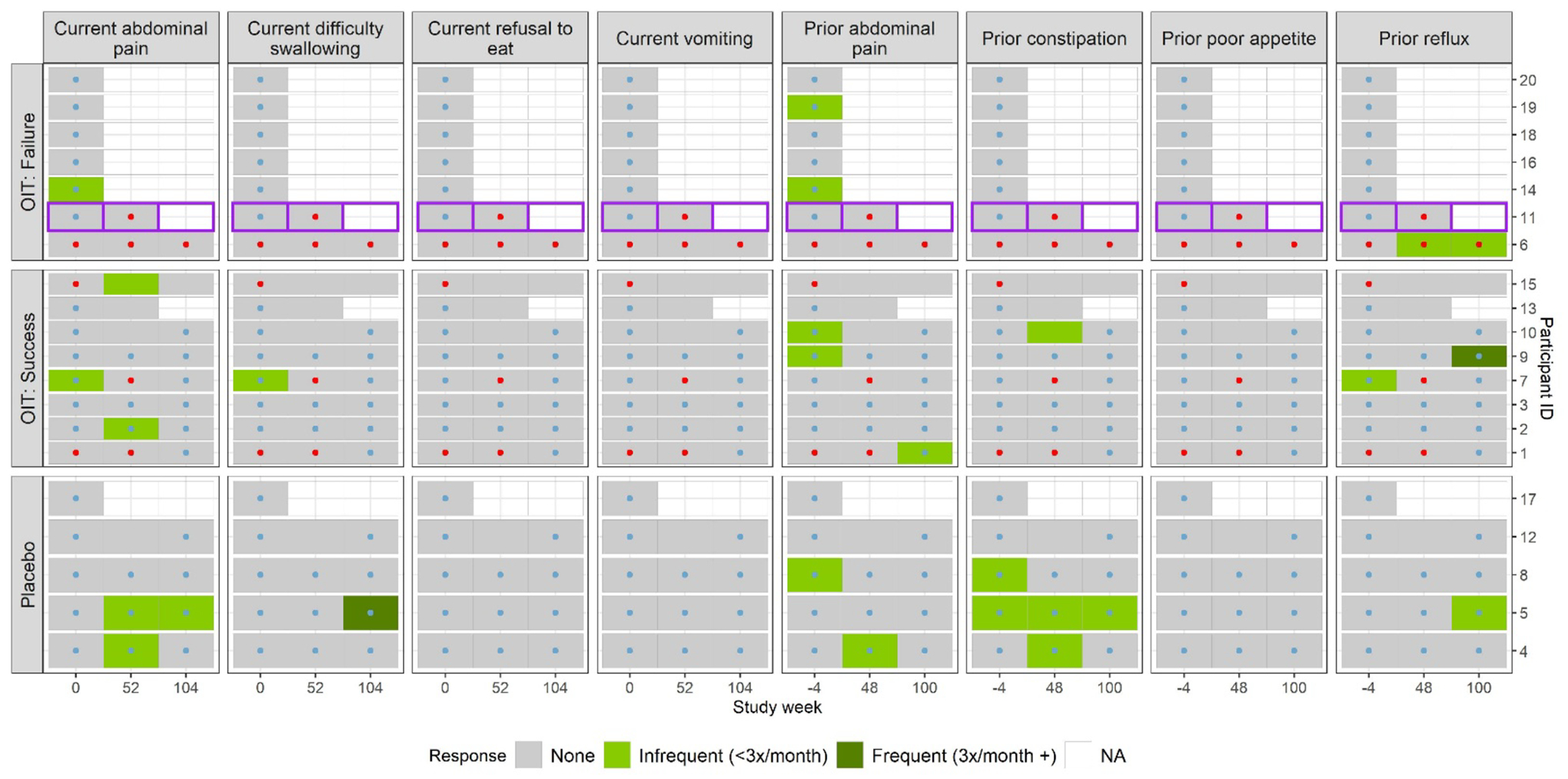
Questionnaire results over time by treatment arm and week 104 challenge outcome for prior (4 weeks prior to the relevant endoscopy) and current (at time of the study visit) gastrointestinal symptoms. Red dots denote that the participant had a PEC ≥ 15 (eos/hpf) in at least 1/3 esophageal sites in that study week; blue dots denote that the participant had a PEC <15 (eos/hpf) in all three esophageal sites in that study week. The participant who developed EoE (participant #11) is outlined in purple. This subject developed dysphagia and food impaction 6 months after the EGD at 52 weeks. NA signifies that the patient did not answer the questionnaire.
Among subjects with follow up through week 52, 7/10 (70%) subjects in the active treatment group reported gastrointestinal symptoms during the trial compared to 3/4 (75%) in the placebo group. Symptoms reported during the trial were generally mild (Figure S2). Of the eight instances (five different subjects) where EE was noted during the study, only three were associated with gastrointestinal symptoms (2 different subjects). Subjects exceeding thresholds for GE or EGID did not receive treatment with proton pump inhibitors, swallowed steroids or diet modification, with exception of the one subject that developed EoE.
Endoscopic findings
Mild edema and furrows were the most common endoscopic findings in the esophagus (Figure S3). Nine subjects in the active treatment group (60%) with EE did not have gross abnormalities on EGD. One subject in the placebo group had grade 1 edema and furrows at baseline. This subject developed rings and had persistent grade 1 edema at weeks 52 and 104. Four subjects in the active treatment group developed mild endoscopic findings (edema, rings, exudates, and furrows) which were not present at baseline. Endoscopic gross images of the subject that developed EoE had grade 1 edema, furrows, rings, and exudates are shown in Figure S4. None of the subjects developed strictures. Relevant endoscopic findings in the stomach/duodenum are detailed in the Supplemental Results.
Histopathology
The most common histopathological findings observed were dilated intercellular spaces (DIS), basal zone hyperplasia (BZH), and eosinophil infiltration (EI) (Table S2a–b, Figures S5–S6). Every peanut allergic subject had evidence of DIS at baseline and this abnormality persisted during the study across treatment groups (Figures S5–S7). Lamina propria fibrosis (LPF) could not be assessed in a majority of the biopsies due to inadequate tissue sampling. Subjects in the placebo group did not show eosinophilic abscess (EA), eosinophil surface layering (SL), surface epithelial alteration (SEA), or dyskeratotic epithelial cells (DEC). BZH was noted in five peanut OIT subjects vs. none in the placebo arm. Esophageal biopsies from two peanut OIT subjects showed EA and SL during active treatment. Among subjects receiving peanut OIT, the cumulative EoEHSS scores at ME significantly increased over the study period for both grade (p=0.007) and stage (p=0.010) (Figure 5). Generally, EoEHSS scores showed a transient increase from baseline to week 52 with resolution by 104 weeks. Esophageal biopsies from the two subjects in the treatment failure group with follow up through at least 52 weeks displayed some of the highest EoEHSS scores.
Figure 5. EoEHSS scores by study week, treatment arm, and esophageal biopsy site, and grade/stage.
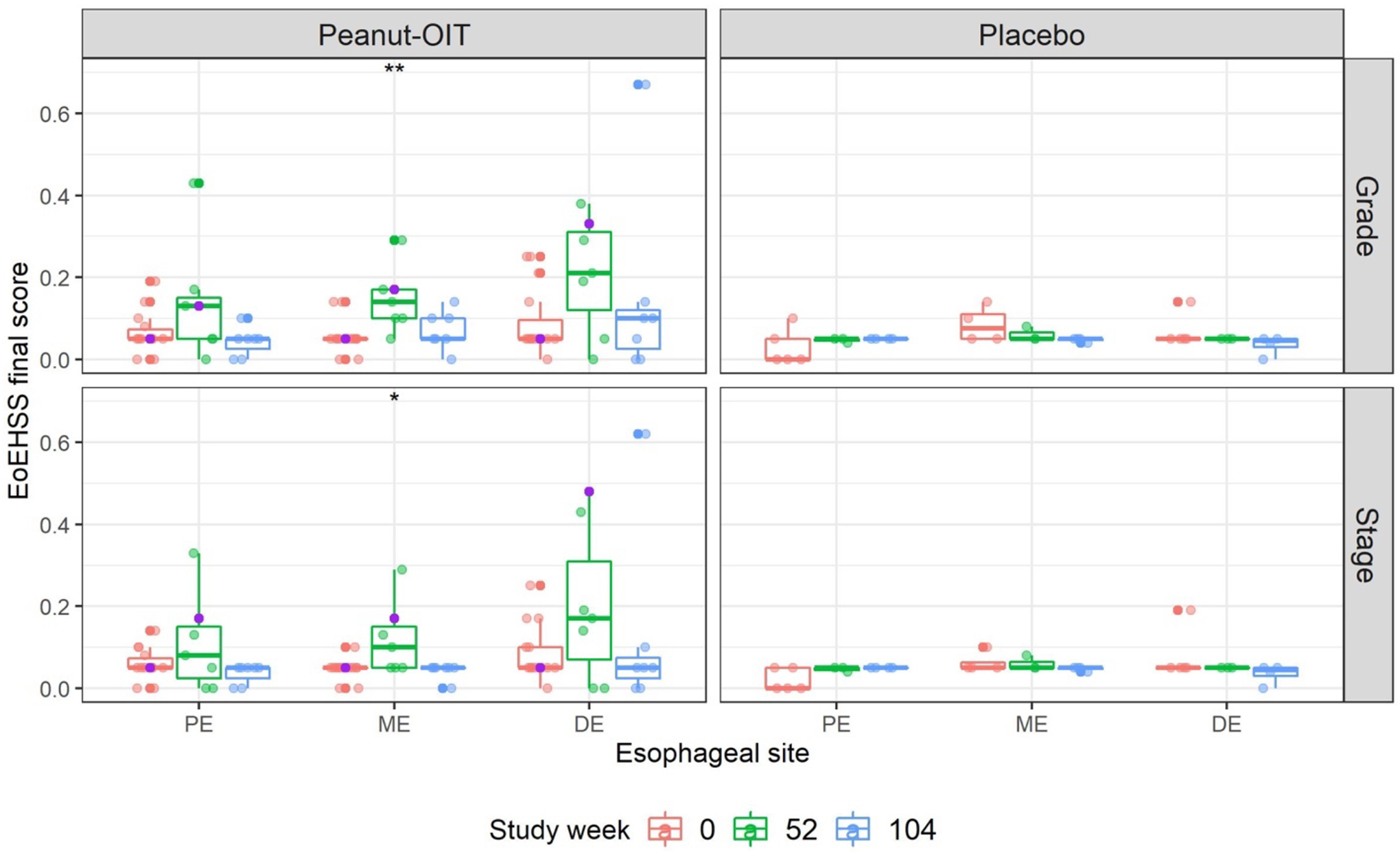
Boxplots of EoEHSS final score by treatment arm, esophageal biopsy site and study week. Participant #11, who developed EoE, is identified by the purple points. The Kruskal-Wallis rank sum test was used to determine whether differences in score across study week were present within each treatment, score type, and esophageal site. ** p=0.007, * p=0.010.
Discussion
This is the first study to systematically and prospectively evaluate eosinophilic gastrointestinal responses to OIT. Here, we elaborate on our previous report that adults with IgE-mediated peanut allergy may have asymptomatic EE accompanied by mild endoscopic and histologic findings seen in EoE.11 We observed that OIT induces EE in a majority of OIT subjects. EE induced by OIT at 52 weeks was accompanied by increases in EoE EREFS and EoEHSS scores that resolved in the majority of patients by week 104. Importantly, EE did not occur in subjects receiving placebo, suggesting active treatment with OIT induces eosinophilic responses. The endoscopic and histologic changes induced by OIT are usually mild but identical to those seen in EoE; indeed, one subject developed EE and symptoms of esophageal dysfunction (i.e. food impaction) and was diagnosed with EoE. This suggests that OIT-induced EE and EoE share a common pathophysiology and may only be distinguished by clinical symptoms and the extent or chronicity of disease pathology.
Considerable overlap exists between IgE-mediated food allergy and EoE. First, the two diseases are often associated.15 Indeed, the risk of EoE in patients with IgE-mediated food allergy is 118 times that of the general population (4.7% vs. 0.04%).16 Second, the foods that trigger EoE are similar to those that elicit IgE-mediated reactions.16 Finally, EoE may be induced by OIT and patients with EoE may develop IgE-mediated food allergy de novo during periods of food elimination.17 Taken together, these observations suggest that the two diseases sit at opposite ends of the same disease spectrum where food avoidance predisposes to IgE-mediated food allergy and chronic antigen exposure leads to EoE in susceptible patients.
Previous reports have documented eosinophilic gastrointestinal complications during food immunotherapy.9 A recent systematic review by Petroni et al.8 suggests the overall occurrence of biopsy-confirmed OIT-induced EoE is 5.3%. Generally, EoE resolves following withdrawal of OIT.9 Goldberg et al.18 recently found that patients with OIT-induced gastrointestinal symptoms and peripheral blood eosinophilia had higher absolute eosinophil counts (AEC) at baseline and escalated dosing faster during OIT. Importantly, OIT induced peripheral blood eosinophilia in subjects with and without gastrointestinal symptoms. Consistent with our observations of gastrointestinal tissue eosinophils, increases in AEC were transient in a majority of subjects; and, in most, dose reduction or temporary symptom treatment allowed for symptomatic patients to continue or resume OIT.
Our study suggests dissociation between OIT-induced gastrointestinal symptoms and the presence of EE/GE in some subjects. For example, 9/20 subjects (45%) crossed histologic thresholds for EGID over a 2-year study period and only 3/9 (33%) of these subjects reported clinical symptoms at the time of endoscopy. In contrast, 20% of subjects reported gastrointestinal symptoms (20% abdominal pain, 5% dysphagia) during OIT. This observation suggests tissue eosinophils alone may not be the primary driver of OIT-induced adverse gastrointestinal side effects. There are several lines of evidence to support this notion: (1) eosinophils are downstream of other effector cells (e.g. basophils and mast cells) in the inflammatory cascade and may be recruited later19; (2) clinical symptoms in EoE are not reliable indicators of tissue eosinophilia20; and (3) an EoE-like disease without tissue eosinophilia may occur in EoE kindreds.21
Major questions remain as to the roles of tissue eosinophils in OIT. In our study, EE was transient in most subjects and it resolved during maintenance OIT. IgE-mediated stimulation of effectors cells (e.g. basophils and mast cells) through antigen exposure may induce tissue eosinophil recruitment22 which diminishes as these cells are desensitized. The transient nature of EE induced by OIT, despite continued therapy, may also suggest that eosinophils initially play a homeostatic role in desensitization or oral tolerance.23 Eosinophils have recently been shown to participate in several immunoregulatory functions including suppression of T cell proliferation24 and IgA production25, 26. Eosinophils also influence Treg and dendritic cell development in gut-associated tissues.25, 27 One finding present in the esophageal mucosa of every peanut allergic subject at baseline was DIS; a feature also present in other conditions associated with EE including EoE, GERD, and celiac disease28–32. The mechanism of DIS formation in the esophageal epithelium of patients with EoE has recently been elucidated.33 EE in patients with food allergy may increase local IL-13 production and upregulate sodium-hydrogen exchanger member 3 (NHE3) to induce DIS formation as a compensatory response to tissue acidification. We speculate that eosinophils may participate in a homeostatic attempt to restore immunologic tolerance that is dysregulated in the context of an over exuberant Th2 response.
This study has certain limitations. First, this cohort is small, has only three time points, and several subjects withdrew or elected not to undergo endoscopy at 52 and 104 weeks (due to time constraints of the parent study, not adverse events). Second, only three esophageal biopsies were analyzed (PE, ME, and DE). EE may be patchy and 5 esophageal biopsies are recommended for EoE diagnosis34, therefore, this study may underestimate the extent of tissue EI. Larger studies of OIT patients undergoing serial endoscopies during OIT would be costly and difficult to justify in the absence of clinical symptoms. Notwithstanding the reduced number of patients with follow up biopsies and the number of biopsies taken, we noted consistent trends among subjects receiving peanut OIT vs. placebo and do not have reason to suspect that subjects with missing data would exhibit markedly different responses. Third, symptoms were not assessed using a validated patient reported outcome measure for EoE. Finally, our histopathologic evaluation was limited to conventional histology and EPX immunohistochemistry. We acknowledge other cells types including, but not limited to, T cells, B cells, mast cells, and basophils may play critical roles that contribute to OIT-induced gastrointestinal responses. Analysis of these other cell populations and their products is ongoing.
In summary, this is the first longitudinal placebo-controlled study to examine serial endoscopic biopsies during peanut OIT. We confirmed that subclinical disease exists prior to initiation of OIT (scenario 1). Indeed, all of the peanut allergic subjects in our cohort had signs of epithelial barrier dysfunction in the esophagus manifested by DIS at baseline and, in some instances, EE. While the number of placebo subjects in this study is small, our findings suggest that the occurrence of EoE during OIT is dependent on antigen exposure (scenario 2) as OIT increased GE in a majority of peanut OIT subjects (scenario 3) and in contrast to almost none of the subjects receiving placebo. Interestingly, for most, tissue eosinophilia was transient despite continuation of peanut OIT; however, in at least one subject, it resulted in a clinical diagnosis of EoE. We speculate that continuous eosinophil recruitment to the gastrointestinal tract (particularly the esophagus) reflects an inability to compensate for barrier dysfunction and that those subjects with the greatest degree of epithelial barrier impairment are most likely to develop EoE during OIT.35 Future mechanistic studies are needed to identify biomarkers of epithelial barrier dysfunction useful for predicting incident EGIDs during OIT.
Supplementary Material
Need to Know.
Background:
Gastrointestinal side effects are common during oral immunotherapy (OIT) and eosinophilic esophagitis (EoE) is a potential complication
Findings:
In a pilot study of 20 adults with IgE-mediated peanut allergy, peanut OIT induced esophageal eosinophilia, gastrointestinal eosinophilia, and, less frequently, EoE. However, OIT-induced esophageal eosinophilia or gastrointestinal eosinophilia was transient and was not always associated with gastrointestinal symptoms. One participant developed EoE which resolved with OIT discontinuation and PPI therapy
Implications for patient care:
Oral immunotherapy for peanut allergy can induce transient esophageal eosinophilia, gastrointestinal eosinophilia, and, less frequently, EoE.
Acknowledgements:
The authors would like to thank the patients who participated in this study. The authors also acknowledge Stephen Galli, MD, PhD, Holden Maecker, PhD, James J. Lee, PhD, and Kari Nadeau, MD, PhD for their contributions to the study and to the manuscript. The authors thank Vanitha Sampath who provided editorial assistance as a medical writer funded by Stanford University.
Funding:
NIH grant U19Al104209, Donald and Kathy Levin Family Foundation, Mayo Clinic Foundation, and the Sean N. Parker Center for Allergy and Asthma Research at Stanford University.
Conflicts of interest:
BLW reports grants from Arizona Biomedical Research Consortium and Phoenix Children’s Hospital Foundation. SDB reports grants from NIH (U19AI104209 and R01AI125567) during the conduct of the study and personal fees from Regeneron/Sanofi, outside the submitted work. SBS reports grant support from NIH and is involved in clinical trials with Regeneron, Aimmune Therapeutics, DBV Technologies, Adare Pharmaceuticals, Sanofi and Novartis. RSC is a consultant for Alladapt and reports grants from NIAID, CoFAR, Aimmune Therapeutics, DBV Technologies, Astellas, AnaptysBio, Novartis, and Regeneron. All other authors declare that they have no competing interest.
Abbreviations:
- AE
adverse effect
- AEC
absolute eosinophil count
- BZH
basal zone hyperplasia
- DBPC
double-blind placebo-controlled
- DE
distal esophagus
- DIS
dilated intercellular spaces
- DEC
dyskeratotic epithelial cells
- EA
eosinophilic abscess
- EE
esophageal eosinophilia
- EEsAI
eosinophilic esophagitis symptom activity index
- EGD
esophagogastroduodenoscopy
- EGID
eosinophilic gastrointestinal disease
- EI
eosinophil infiltration
- EoE
eosinophilic esophagitis
- eos/hpf
eosinophils per high-power field
- EoEHSS
eosinophilic esophagitis histologic scoring system
- EPX
eosinophil peroxidase
- EREFS
endoscopic reference score
- FeNO
fractional exhaled nitric oxide
- GE
gastrointestinal eosinophilia
- HPF
high-power field
- LPF
lamina propria fibrosis
- ME
middle esophagus
- OIT
oral immunotherapy
- PE
proximal esophagus
- PEC
peak eosinophil count
- SEA
surface epithelial alteration
- SL
eosinophil surface layering
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Burks AW, Sampson HA, Plaut M, et al. Treatment for food allergy. J Allergy Clin Immunol. 2018;141(1):1–9. [DOI] [PubMed] [Google Scholar]
- 2.Vickery BP, Berglund JP, Burk CM, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. 2017;139(1):173–81 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andorf S, Purington N, Block WM, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2018;3(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Investigators PGoC, Vickery BP, Vereda A, et al. AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med. 2018;379(21):1991–2001. [DOI] [PubMed] [Google Scholar]
- 7.Chu DK, Wood RA, French S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393(10187):2222–32. [DOI] [PubMed] [Google Scholar]
- 8.Petroni D, Spergel JM. Eosinophilic esophagitis and symptoms possibly related to eosinophilic esophagitis in oral immunotherapy. Ann Allergy Asthma Immunol. 2018;120(3):237–40 e4. [DOI] [PubMed] [Google Scholar]
- 9.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113(6):624–9. [DOI] [PubMed] [Google Scholar]
- 10.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–6 e2. [DOI] [PubMed] [Google Scholar]
- 11.Wright BL, Fernandez-Becker NQ, Kambham N, et al. Baseline Gastrointestinal Eosinophilia Is Common in Oral Immunotherapy Subjects With IgE-Mediated Peanut Allergy. Front Immunol. 2018;9:2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinthrajah RS, Purington N, Andorf S, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019;394(10207):1437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–95. [DOI] [PubMed] [Google Scholar]
- 14.Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus. 2017;30(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelz BJ, Wechsler JB, Amsden K, et al. IgE-associated food allergy alters the presentation of paediatric eosinophilic esophagitis. Clin Exp Allergy. 2016;46(11):1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract. 2017;5(2):369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill DA, Shuker M, Cianferoni A, et al. The development of IgE-mediated immediate hypersensitivity after the diagnosis of eosinophilic esophagitis to the same food. J Allergy Clin Immunol Pract. 2015;3(1):123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg MR, Nachshon L, Levy MB, et al. Risk factors and treatment outcomes for Oral Immunotherapy-Induced Gastrointestinal Symptoms and Eosinophilic Responses (OITIGER). J Allergy Clin Immunol Pract. 2019. [DOI] [PubMed] [Google Scholar]
- 19.Noti M, Wojno ED, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19(8):1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults With Eosinophilic Esophagitis. Gastroenterology. 2016;150(3):581–90 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straumann A, Blanchard C, Radonjic-Hoesli S, et al. A new eosinophilic esophagitis (EoE)-like disease without tissue eosinophilia found in EoE families. Allergy. 2016;71(6):889–900. [DOI] [PubMed] [Google Scholar]
- 22.Cheng LE, Sullivan BM, Retana LE, et al. IgE-activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Exp Med. 2015;212(4):513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JJ, Jacobsen EA, McGarry MP, et al. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40(4):563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingblom C, Andersson J, Andersson K, et al. Regulatory Eosinophils Suppress T Cells Partly through Galectin-10. J Immunol. 2017;198(12):4672–81. [DOI] [PubMed] [Google Scholar]
- 25.Chu VT, Beller A, Rausch S, et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40(4):582–93. [DOI] [PubMed] [Google Scholar]
- 26.Jung Y, Wen T, Mingler MK, et al. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8(4):930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu DK, Jimenez-Saiz R, Verschoor CP, et al. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J Exp Med. 2014;211(8):1657–72. Epub 2014/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravelli A, Villanacci V, Cadei M, et al. Dilated intercellular spaces in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2014;59(5):589–93. [DOI] [PubMed] [Google Scholar]
- 29.Caviglia R, Ribolsi M, Maggiano N, et al. Dilated intercellular spaces of esophageal epithelium in nonerosive reflux disease patients with physiological esophageal acid exposure. Am J Gastroenterol. 2005;100(3):543–8. [DOI] [PubMed] [Google Scholar]
- 30.Pinto-Sanchez MI, Nachman FD, Fuxman C, et al. Altered Esophageal Mucosal Structure in Patients with Celiac Disease. Can J Gastroenterol Hepatol. 2016;2016:1980686. Epub 2016/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ari A, Morgenstern S, Chodick G, et al. Oesophageal eosinophilia in children with coeliac disease. Arch Dis Child. 2017;102(9):825–9. [DOI] [PubMed] [Google Scholar]
- 32.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102(6):1301–6. [DOI] [PubMed] [Google Scholar]
- 33.Zeng C, Vanoni S, Wu D, et al. Solute carrier family 9, subfamily A, member 3 (SLC9A3)/sodium-hydrogen exchanger member 3 (NHE3) dysregulation and dilated intercellular spaces in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(6):1843–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen JA, Lager DJ, Lewin M, et al. The optimal number of biopsy fragments to establish a morphologic diagnosis of eosinophilic esophagitis. Am J Gastroenterol. 2014;109(4):515–20. [DOI] [PubMed] [Google Scholar]
- 35.Rochman M, Azouz NP, Rothenberg ME. Epithelial origin of eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


