Abstract
Background:
Current evidence suggests that women are more sensitive to the effects of cannabinoids. The aim of this study was to investigate the relevance of sex in the association of synthetic cannabinoid (SC) use with psychosis and agitation.
Methods:
A retrospective chart review was conducted for patients admitted to a psychiatric unit (2014–2016) to extract information on demographic factors, use of substances, clinical symptoms, and pharmacological treatments. Study groups were defined as SC users (anyone who reported use of SCs over the past three months), cannabis users (positive toxicology screen for delta 9-tetrahydrocannabinol [THC]), and controls (those who denied use of SCs over the past three months and had negative toxicology for THC).
Results:
Digital charts of 983 patients were reviewed. A total of 162 subjects reported use of SCs over the past three months (76% male), and 292 subjects had positive toxicology screen for THC (67% male). A total of 38.9% of SC users (n=63) had positive urine toxicology screen for THC. SC users had higher risks of psychotic presentations (adjusted odds ratios (AOR): 3.390, 95%CI. 1.390–8.267) and agitation (AOR: 4.643, 95%CI. 1.974–10.918) compared to the controls. While women had lower rates of psychosis than men in the cannabis and control groups, the rates were markedly potentiated with SC use to high levels (79%) approximately equal to that seen in men (80%). There was also a significant interaction between SC use and sex for agitation (AOR=0.308, 95%CI. 0.117–0.808). Female SC users were significantly more agitated than male SC users (73.7% vs 47.6%, respectively, p-value: 0.005).
Conclusion:
SC users are more likely than nonusers to be psychotic or agitated in an inpatient setting. The potentiated rates of psychosis and agitation with SC use in women suggest that they may have a greater sensitivity to these synthetic compounds.
Keywords: Synthetic cannabinoids, cannabis, psychosis, agitation, sex differences
Introduction
Synthetic cannabinoids (SCs), which are commonly known as K2/spice and have become popular as recreational drugs in the United States since 2009, mimic the effects of cannabis, but are much more potent and efficacious at cannabinoid receptors. Whereas Δ9-tetrahydrocannabinol (THC), the main psychoactive compound in cannabis, is a partial agonist of cannabinoid receptors, most of the SC compounds are full agonists, with higher potency and affinity to cannabinoid receptors (1). Clinical studies are limited, but severe adverse effects of SCs on mental health have been reported in case series, emergency room reports, and psychiatric inpatients. The findings indicate that psychosis and agitation are the most frequent psychiatric presentations of SC use (2–6).
Although SC use is more prevalent among men, and there is no report on the relevance of sex on the psychiatric presentation of SC use, accumulating evidence from animal and human studies suggests that females are more sensitive to some of the effects of cannabinoids. Animal studies report that female rodents are more sensitive to cannabinoid-induced locomotor suppression (7), antinociception (7–9) and hypothermia (10). Females also acquire self-administration (11) and tolerance of cannabinoids (9) more rapidly. Although human studies are limited, current evidence suggests that women may be more vulnerable. As compared to men, women have shorter time periods between first cannabis use and problematic use, known as “telescoping” (12, 13), higher subjective effects of cannabis (14), and worse cannabis withdrawal symptoms such as irritability, anger, and violent outbursts (15–17). Regarding the relationship between use of cannabinoids and psychosis (18, 19), the typical later onset of psychotic symptoms in women compared to men (20) is significantly reduced among cannabis users (21).
Becasue SCs are potent full agonists of cannabinoid receptors, the higher sensitivity of women to cannabinoids makes them more vulnerable to adverse effects of SCs. Previously, we conducted a retrospective chart review investigating the psychiatric presentations of patients admitted to a dual diagnosis unit in 1 year (March 2014 to February 2015) with use of SCs and found that they presented more frequently with psychosis and agitation compared to cannabis users (22). However, the small sample number of female SC users prevented us from exploring sex differences. In the current study, we expanded our chart review across 3 years to investigate the potential contribution of sex in the association of use of SCs with psychosis and agitation. Considering the high potency and efficacy of SCs on cannabinoid receptors and the higher sensitivity of women to the effects of cannabinoids in preclinical and clinical studies, we hypothesized that women would have more psychotic and agitated presentations associated with the use of SCs as compared to men.
Methods
Study design:
A retrospective chart review was conducted with electronic records of all patients who were admitted to a dual diagnosis psychiatric unit at Mount Sinai Beth Israel (MSBI) from January 1, 2014, to December 31, 2016. MSBI is a university-affiliated hospital in downtown Manhattan, New York City. The MSBI dual diagnosis unit mostly serves patients with a history of substance use disorder comorbid with other psychiatric conditions. For this study, we included all patients with recent (past three months) use of cannabinoids including natural (confirmed by urine toxicology) or synthetic (self-reported) cannabis. Additionally, we randomly selected a group of patients from the same unit, who denied recent use of cannabinoids and had negative toxicology for natural cannabis, as a non-cannabinoid using control group. Patients were excluded if urine toxicology screen results were not available. Patients were given a study ID number, and all extracted data was de-identified. Only the first admission information was included in the study if a patient was admitted to the unit more than once. This study was approved by the Icahn School of Medicine, MSBI Institutional Review Board.
Assessments:
Digital charts of patients were reviewed and data for specified study variables were extracted. All available data were used, including admission history and physical examination, progress notes, reports of administered medications, laboratory results, and discharge summaries. Psychotic symptoms were determined as present based on objective signs or clinical evaluations of both positive and negative symptoms (dichotomous variable). Presence of agitation was determined by whether or not a patient required pro re nata (administer when required) medication for episodes of severe agitation (dichotomous variable). Urine toxicology screening results identified current comorbid use of other substances (i.e. cocaine, opioids, benzodiazepines, phencyclidine (PCP), and amphetamines). Blood alcohol level measurement identified alcohol intoxication. Prescribed antipsychotic medications were documented from discharge summaries and were converted to Halopridol equivalent dose for both first generation (23) and second generation (24) antipsychotics. Length of hospital stay was calculated based on the number of days of inpatient hospitalization.
Data analysis:
We analyzed all data using IBM SPSS Statistics for Windows, version 23 (IBM; Armonk, New York). Study groups are identified as SC users (SC+, MJ+/−), natural cannabis/marijuana users (MJ+, SC-), and control group (MJ-, SC-) groups. Data are presented using means, percentages, and 95% CIs. Univariate analyses were performed using analysis of variance (ANOVA), t-test, and chi-square to compare the variables between study groups. When any significant differences were determined, bivariate logistic regression analyses were performed to calculate adjusted odds ratios (AORs), with psychosis and agitation as dependent variables in 2 separate models and sex, age, and use of other drugs as covariates. Sex interaction with cannabinoid use was also included in both models. Similar regression analyses were performed to calculate AORs for the effects of sex on psychosis and agitation separately in each study group (SC, MJ, and non-cannabinoid using controls), with psychosis and agitation as dependent variables and age and use of other drugs as covariates.
Results
Sociodemographic:
Digital charts of 983 patients were reviewed. Full demographic results for study groups are shown in Table 1. A total of 162 subjects reported recent use of SCs and 292 subjects had recent use of cannabis. Among SC users, 38.9% (63 individuals) had positive urine toxicology screen for natural cannabis. SC and cannabis users were significantly younger than non-cannabinoid using controls (mean age=34.88, 34.95 and 42.41 years, respectively). There were significantly more black individuals in the SC group (Table 1). When sociodemographic factors between men and women were compared, female cannabis users were significantly younger than male users (32.44 vs. 36.15 years, respectively), and the group had more black individuals (55.6% vs. 36.5%, respectively) (Table 1). There were no other significant sociodemographic differences between men and women.
Table 1.
Sociodemographic factors of Synthetic Cannabis users (SC), cannabis users (MJ) and control group
| Total | P-Value | Men | Women | P-Value | |||
|---|---|---|---|---|---|---|---|
| N=983 | SC | 162 (16.5%) | - | 124 (76.5%) | 38 (23.5%) | - | |
| MJ | 292 (29.7%) | 198 (67.8%) | 94 (32.2%) | - | |||
| Control | 529 (53.8%) | 377 (71.3%) | 152 (28.7%) | - | |||
|
Age Mean
(SD)
(n=983) |
SC | 34.88 (10.58) | <0.0001 | 34.89 (10.23) | 34.82 (11.79) | 0.968 | |
| MJ | 34.95 (11.95) | 36.15 (11.72) | 32.44 (12.09) | 0.013 | |||
| Control | 42.41 (12.73) | 42.70 (12.84) | 36.15 (11.72) | 0.401 | |||
| Ethnicity (n=954) | SC | White | 27 (17.2% | <0.001 | 20 (16.8%) | 7 (18.4%) | 0.635 |
| Black | 95 (60.5%) | 71 (59.7%) | 24 (63.2%) | ||||
| Hispanic | 30 (19.1%) | 25 (21.0%) | 5 (13.2%) | ||||
| MJ | White | 103 (36.5%) | 80 (41.7%) | 23 (25.6%) | 0.020 | ||
| Black | 120 942.6%) | 70 (36.5%) | 50 (55.6%) | ||||
| Hispanic | 52 (18.4%) | 37 (19.3%) | 15 (16.7%) | ||||
| Control | White | 186 (36.1%) | 133 (36.3%) | 53 (25.6%) | 0.723 | ||
| Black | 183 (35.5%) | 134 (36.6%) | 49 (32.9%) | ||||
| Hispanic | 135 (26.2%) | 91 (24.9%) | 44 (29.5%) | ||||
| Single/divorced (n=983) | SC | 162 (100%) | 0.423 | 124 (100%) | 38 (100%) | - | |
| MJ | 292 (100%) | 198 (100%) | 94 (100%) | - | |||
| Control | 527 (99.6%) | 376 (99.7%) | 151 (99.3%) | 0.492 | |||
| Unemployed (978) | SC | 152 (94.4%) | 0.002 | 116 (94.3%) | 36 (94.7%) | 0.641 | |
| MJ | 240 (82.8%) | 164 (83.7%) | 76 (80.9%) | 0.330 | |||
| Control | 463 (87.9%) | 327 (87.0%) | 136 (90.1%) | 0.203 | |||
Psychiatric diagnosis and use of substances:
Overall, 50.7% of our subjects (54.2% of men vs. 42% of women) were diagnosed with a psychotic disorder including schizophrenia (16.9% total, 20.0% of men vs. 9.2% of women), schizoaffective disorder (18.0% total, 18.0% of men vs. 10.0% of women), and unspecified psychotic disorder (15.8% total, 16.2% of men vs. 14.8% of women). Other main diagnoses were unspecified depressive disorder (37.3% total, 34.6% of men vs. 44.0% of women), and bipolar disorder (8.1% total, 7.6% of men vs. 9.5% of women). Regarding comorbid use of substances, cannabis was the most common drug (36.1% total, 35.3% of men vs. 38.0% of women), followed by cocaine (21.7% total, 19.9% of men vs. 26.1% of women), opioids (17.6% total, 17.7% of men vs. 17.3% of women) and self-reported use of SCs (16.6% total, 17.8% of men vs. 13.4% of women). There were no significant differences between men and women in use of any substances, except for cocaine which was more common in women (p=0.021).
Psychosis and agitation:
Psychotic presentations were significantly more frequent in SC users (79.6%), compared to cannabis users (57.5%) and the non-cannabinoid using control group (44.4%) (p <0.001). Moreover, SC users were prescribed higher doses of antipsychotic medications based on the halopridol equivalent doses of prescribed antipsychotic medications (mean = 10.72 mg) compared to cannabis users (mean = 5.15 mg) and the non-cannabinoid using control group (mean = 5.27 mg) (P < 0.001). Agitation had the same pattern, with higher presentation in SC users (53.7%), compared to cannabis users (39.7%), and the non-cannabinoids using control group (29.5%) (p < 0.001). Length of hospital stay was also significantly longer in SC users (15.19 days), but shorter in cannabis users (10.73 days) compared to controls (12.40 days) (p <0.001) (Table 2). Table 3 provides the AORs for the presence of psychotic symptoms and agitation in the whole sample. The data shows that the SC users had a significantly higher risk of psychotic presentations (AOR 3.39, p= 0.007) and agitation (4.643, p <0.001) compared to the non-cannabinoid using control group. There was a significant interaction of SC use and sex with agitation (p= 0.017).
Table 2.
Psychosis, Haldol equivalent dose of antipsychotic medications, agitation, length of hospital stay in SC users, cannabis users and control group
| Total | P-Value | Men | Women | P-Value | ||
|---|---|---|---|---|---|---|
| Psychotic presentations | SC | 129 (79.6%) | <0.001 | 99 (79.8%) | 30 (78.9%) | 1.000 |
| MJ | 168 (57.5%) | 121 (61.1%) | 47 (50.0%) | 0.077 | ||
| Control | 235 (44.4%) | 175 (46.4%) | 60 (39.5%) | 0.149 | ||
| Haldol equivalent dose of antipsychotic medications (mean, SD) | SC | 10.72 (7.77) | <0.001 | 10.25 (7.47) | 11.96 (8.67) | 0.264 |
| MJ | 5.15 (6.87) | 5.80 (7.13) | 3.79 (6.11) | 0.019 | ||
| Control | 5.27 (7.08) | 5.47 (7.25) | 4.77 (6.65) | 0.305 | ||
| Agitation (PRN+) | SC | 87 (53.7%) | <0.001 | 59 (47.6%) | 28 (73.7%) | 0.005 |
| MJ | 116 (39.7%) | 82 (41.4%) | 34 (36.2%) | 0.443 | ||
| Control | 156 (29.5%) | 118 (31.3%) | 38 (25.0%) | 0.171 | ||
| Length of hospital stay (days) (mean, SD) | SC | 15.19 (11.31) | <0.001 | 14.23 (10.91) | 18.43 (12.14) | 0.047 |
| MJ | 10.73 (8.56) | 10.91 (9.27) | 10.35 (6.85) | 0.604 | ||
| Control | 12.40 (12.11) | 12.67 (12.83) | 11.70 (10.13) | 0.405 |
Table 3:
Adjusted odds ratios (AOR) for psychosis and agitation in SC, and cannabis users compared to non-cannabinoid using control group (adjusted for age, gender and use of other drugs)
| Psychotic symptoms | Agitation | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | OR | 95%CI | P-value | |
| Non-cannabinoid using control (reference) | - | <0.001 | - | 0.001 | ||
| Cannabis users | 1.022 | 0.571–1.828 | 0.943 | 0.904 | 0.490–1.668 | 0.748 |
| SC users | 3.390 | 1.390–8.267 | 0.007 | 4.643 | 1.974–10.918 | <0.001 |
| Age | 0.987 | 0.975–1.000 | 0.044 | 0.978 | 0.966–0.990 | <0.001 |
| Sex | 1.347 | 0.867–2.092 | 0.186 | 1.144 | 0.7620–1.819 | 0.569 |
| Alcohol use | 0.256 | 0.161–0.406 | <0.001 | 0.381 | 0.232–0.627 | <0.001 |
| Cocaine use | 0.679 | 0.474–0.972 | 0.035 | 0.514 | 0.348–0.760 | 0.001 |
| Opioid use | 0.396 | 0.256–0.613 | <0.001 | 0.615 | 0.385–0.984 | 0.043 |
| Benzodiazepines use | 0.413 | 0.253–0.676 | <0.001 | 0.748 | 0.448–1.247 | 0.265 |
| Amphetamine use | 1.161 | 0.603–2.235 | 0.656 | 0.703 | 0.355–1.396 | 0.314 |
| PCP use | 0.813 | 0.295–2.240 | 0.688 | 0.303 | 0.085–1.086 | 0.067 |
| Cannabinoid use*Sex | - | 0.319 | - | 0.015 | ||
| Cannabis*Sex | 1.634 | 0.814–3.281 | 0.168 | 1.362 | 0.663–2.794 | 0.400 |
| SC*Sex | 0.888 | 0.316–2.494 | 0.821 | 0.308 | 0.117–0.808 | 0.017 |
To examine the association between use of cannabinoids with psychosis and agitation in those individuals who used both SC and cannabis compared to those who only used SC (negative urine toxicology screen), a post-hoc analysis was conducted with the subjects re-grouped into four groups: Only SC (SC+/MJ-), only cannabis (SC-/MJ+), both SC and cannabis (SC+/MJ+) and no cannabinoids (SC-/MJ-). The results demonstrated that the SC+/MJ- group more frequently presented with psychosis (84.8%), followed by SC+/MJ+ group (71.4%), the SC-/MJ+ group (57.5%), and the SC-/MJ- group (44.4%) groups (p-value<0.001). Similarly, the SC+/MJ- group required the highest dose of antipsychotic medications based on the halopridol equivalent dose (mean [SD]= 11.59 mg [9.94] mg), followed by the SC+/MJ+ group (9.37mg [7.37]), the SC-/MJ+ (5.20 [6.87]) and the SC-/MJ- (5.27 [7.08]) groups (p-value<0.001). Agitation was equally high in SC+/MJ+ (54%) and SC+/MJ- (53.5%) groups, with lower rates in other 2 groups (39.7% in SC-/MJ+, and 29.5% in the SC-/MJ- groups) (p-value<0.001). The longest hospital stay was observed in the SC+/MJ- group (mean [SD] 16.61 days [12.36] days), followed by the SC+/MJ+ group (12.92 days [9.01] days) and the SC-/MJ- group (12.39 days [12.11] days), and the shortest hospital stay was the SC-/MJ+ group (10.73 days [8.56] days) (p-value<0.001).
Sex differences in psychosis:
The presence of psychotic symptoms and the dose of prescribed antipsychotic medications were compared between men and women in each study group (Figure 1). Among cannabis users, there was a trend of less frequent psychotic presentations in women (50.0%), compared to men (61.1%) (p= 0.077) (Table 2). After control for demographic factors and use of other drugs, this difference became significant (AOR = 0.48 p= 0.009) (Table 4). In contrast, in SC users, the rate of psychosis in women was high, achieving levels similar to those of men (79.8% of men vs 78.9% of women, p= 1.000) (Table 2). There were no significant differences in the dose of prescribed antipsychotic medications (based on calculated halopridol equivalent dose) between men and women among SC users (10.25 mg in men, vs 11.96 mg in women, p=0.264) and non-cannabinoid using controls (5.47 mg in men, vs. 4.77 mg in women, p=0.305), but among cannabis users, women were prescribed lower doses of antipsychotic medications (5.80 mg in men, vs. 3.79 mg in women, p=0.019) (Table 2).
Figure 1:
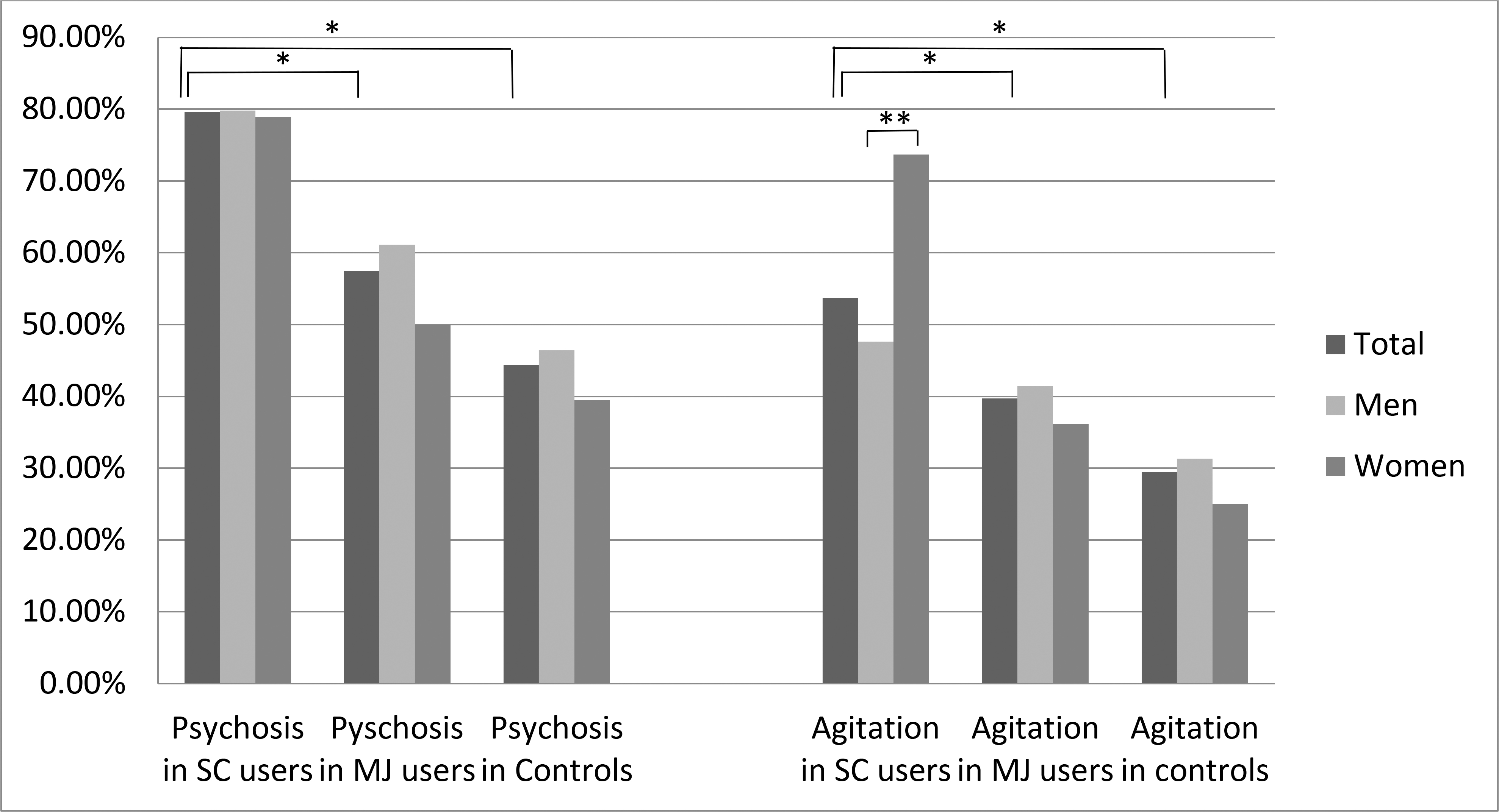
Agitation and psychosis in the whole sample, men and women (* p-value<0.001, **p-value<0.005)
Table 4:
Adjusted odds ratios (AOR) for psychosis and agitation in women compared to men SC users, cannabis users and non-cannabinoid using control group (adjusted for age, and use of other drugs)
| Psychotic symptoms | Agitation | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | OR | 95%CI | P-value | ||
| SC users |
Female
sex
(Male as reference) |
0.721 | 0.268–1.940 | 0.517 | 3.202 | 1.271–9.070 | 0.014 |
| Age | 0.974 | 0.936–1.014 | 0.206 | 0.968 | 0.935–1.003 | 0.074 | |
| Alcohol use | 1.134 | 0.127–10.118 | 0.910 | 0.282 | 0.051–1.544 | 0.144 | |
| Cocaine use | 1.274 | 0.369–4.401 | 0.701 | 0.428 | 0.146–1.255 | 0.122 | |
| Opioid use | 0.131 | 0.027–0.623 | 0.011 | 0.137 | 0.015–1.235 | 0.076 | |
| Benzodiazepines use | 0.707 | 0.134–3.731 | 0.683 | 1.350 | 0.247–7.397 | 0.729 | |
| Amphetamine use | 1.677 | 0.104–27.110 | 0.716 | 0.0 | 0.0 | - | |
| PCP use | 0.087 | 0.007–1.037 | 0.053 | 0.0 | 0.0 | - | |
| Cannabis users |
Female
sex
(Male as reference) |
0.480 | 0.277–0.833 | 0.009 | 0.684 | 0.393–1.190 | 0.179 |
| Age | 0.989 | 0.967–1.011 | 0.316 | 0.977 | 0.955–0.999 | 0.038 | |
| Alcohol use | 0.347 | 0.157–0.764 | 0.009 | 1.118 | 0.514–2.431 | 0.778 | |
| Cocaine use | 0.604 | 0.322–1.132 | 0.116 | 0.522 | 0.264–1.034 | 0.062 | |
| Opioid use | 0.470 | 0.217–1.020 | 0.056 | 0.795 | 0.355–1.780 | 0.577 | |
| Benzodiazepines use | 0.423 | 0.14–0.925 | 0.031 | 0.923 | 0.420–2.027 | 0.841 | |
| Amphetamine use | 0.813 | 0.285–2.323 | 0.700 | 1.081 | 0.386–3.028 | 0.882 | |
| PCP use | 0.811 | 0.150–4.396 | 0.808 | 0.958 | 0.166–5.522 | 0.962 | |
| Control |
Female
sex
(Male as reference) |
0.737 | 0.471–1.154 | 0.182 | 0.874 | 0.543–1.406 | 0.579 |
| Age | 0.988 | 0.972–1.004 | 0.133 | 0.980 | 0.963–0.996 | 0.018 | |
| Alcohol use | 0.174 | 0.090–0.335 | <0.001 | 0.184 | 0.084–0.402 | <0.001 | |
| Cocaine use | 0.639 | 0.395–1.034 | 0.068 | 0.517 | 0.302–0.885 | 0.016 | |
| Opioid use | 0.393 | 0.221–0.699 | 0.001 | 0.607 | 0.320–1.149 | 0.125 | |
| Benzodiazepines use | 0.371 | 0.181–0.761 | 0.007 | 0.518 | 0.231–1.159 | 0.110 | |
| Amphetamine use | 1.489 | 0.621–3.575 | 0.372 | 0.265 | 0.076–0.923 | 0.037 | |
| PCP use | 1.616 | 0.412–6.337 | 0.491 | 0.235 | 0.028–1.983 | 0.183 | |
Sex differences in agitation in study groups:
There were no significant differences in the presence of severe agitation episodes between men and women in the non-cannabinoid using control group (31.1% in men, vs 25% in women, p= 0.171), or cannabis users (41.4% of men, vs 36.2% of women, p= 0.443), but female SC users were significantly more likely to be agitated compared to male SC users (47.6% of men, vs 73.7% of women, p= 0.005) (Table 2). The higher risk of agitation in female SC users remained significant after adjustment for age and use of other drugs (AOR = 3.202, p= 0.014) (Table 4).
Sex differences in length of hospital stay:
There were no significant differences in length of hospitalization between men and women in the non-cannabinoid using group and cannabis users, but in SC users, women had significantly longer hospitalizations compared to men (14.23 days for men vs 18.43 days for women, p= 0.047) (Table 2).
Discussion
The results of this study confirmed and extended our previous findings that SC users are more likely than nonusers to have psychotic symptoms and agitation presentations (22). SC users were three times more likely to be psychotic and over four times more likely to have agitation compared to the control group. They also received higher doses of antipsychotic medications and had longer hospital stays. These findings are consistent with the literature reporting severe symptoms of psychosis and agitation as the main psychiatric presentations of SC use (2–6, 25). Our study now also demonstrates significant sex differences in the association of use of cannabinoids with psychosis and agitation. While women tended to have fewer psychotic presentations among non-cannabinoid using controls (trend-level) and cannabis users (significant) compared to men, there were equivalently high rates of psychosis in men and women among SC users. Moreover, the risk of agitation was markedly higher in women compared to men among SC users. To the best of our knowledge, this report is the first to document sex differences in the clinical presentations of SC use.
SC compounds are potent full agonists of cannabinoid receptors (26) and lack cannabidiol (CBD), which has been shown to have antipsychotic properties in clinical trials (27, 28). Our finding that SC users had a higher rate of psychotic symptoms compared to cannabis users fits in line with the accumulating evidence of a dose-response/cannabinoid receptor type 1 [CB1R] potency relationship between the use of cannabinoids and psychosis (29) and higher rates of psychosis within high-potency (higher ratio of THC to CBD) cannabis users (30).
There is limited clinical information about sex in relation to the use of cannabinoids and psychosis, except that the typically later onset of psychosis in women (20) is diminished in cannabis users (21, 31). Our results demonstrated that women with cannabis use present less frequently with psychosis compared to men in a psychiatric dual diagnosis inpatient setting. It is important to note that we have no data on the amount of cannabis used by our subjects due to the nature of retrospective chart reviews. Some studies have reported that, whereas there are no sex differences in the amount or frequency of cannabis use between male and female cannabis users in healthy individuals, among psychotic patients, men use cannabis more frequently and in larger amounts compared to women (32). Possible heavier cannabis use in male psychotic patients may explain the higher rate of psychotic presentations in male cannabis users in our inpatient sample. There were, however, no available data available on the amount and frequency of SC use in the patients.
The fact that SC use in women induced marked psychosis to the high levels seen in men might indicate a potential greater sensitivity of women to full agonist cannabinoids. Controlled human laboratory experiments are not possible to conduct with SC agents considering their health risk so evaluating aspects of metabolism and pharmacokinetics or the psychogenic effects of SCs in women versus men is difficult to do. Animal studies have, however, demonstrated that female rats acquire self-administration of synthetic cannabinoid (WIN55,212–2) to a faster extent than males (11) and administer a greater amount of the drug (33).
Our finding that patients with SC use, particularly women, have significantly more frequent presentations of agitation is of significant interest and has potential clinical importance. A possible bias towards prescribing lower doses of medications to women could be a possible explanation for the occurrence of more agitation episodes in female SC users, but our data show there is no difference in the dose of prescribed antipsychotic medications between male and female SC users. The effect of cannabinoids on agitation and aggression has been studied extensively in animal models for many decades. While some studies report that cannabis exposure decreases aggression in different animal species (34–37), other studies report an increase in aggressive behaviors (38–40) with a dose-response relationship (41). Theories to reconcile these conflicting results suggest that cannabis suppresses innate (predatory and inter-male) aggression, but increases irritable aggression and agitation in stressful situations (reviewed by Abel (42)), which has been consistently demonstrated in several studies (43–47) and is shown to be magnified by estrogen in females (48).
Similar to animal models, human studies report both decreases (49) and increases in aggressive behaviors in cannabis users (50). While cannabis may have calming effects in the general population (49), increases in aggression and agitation are observed in individuals with psychiatric vulnerability (25, 51) such as those within inpatient units (52, 53) and those with first episode psychosis (54). Similar to findings in animal models, different doses and levels of acute or chronic stress may explain these different effects in humans. In our study, cannabis use had no association with agitation. Unfortunately, our study design did not allow us to ascertain the dose of cannabis used or stress levels experienced by patients. However, SC users in our study did demonstrate significantly more agitation which was significantly greater in women compared to men. Given the enhanced pharmacological potency of SCs, our findings may support a dose-response/CB1 potency relationship between greater cannabinoid receptor agonists (SC vs THC) and agitation, particularly in women. More studies are needed to evaluate potential sex differences in relation to different doses and types of cannabinoids (e.g. partial or full cannabinoid receptor agonists, light or heavy users), on agitation and aggression and in regard to the role of stress in this association. However, our findings suggest that female SC users may need an earlier start and higher dose of pharmacological treatments in the course of their inpatient hospitalization, compared to male SC users.
Limitations
Results from retrospective chart reviews have limitations that should be considered in interpreting the findings. First, there was no standardized measure for the patients’ clinical signs and symptoms, thus we could not systematically address the severity of psychosis or agitation. However, raters used all available data to evaluate patients’ presentations and were not limited to any one specific clinical document, such as admission or discharge summaries. Moreover, to standardize our measure of agitation, we used the administration of as-needed medications given for episodes of severe agitation, which is documented precisely in patients’ charts. Second, another challenge is that standardized clinical urine toxicology reports do not test for SCs so data on SC use were based solely on patients’ self-report on their use over the past three months. In fact, these compounds vary in potency and chemical structure, making them difficult to detect even using sophisticated analytical assays. It is possible that some patients chose to withhold information about drug use from their physicians, which may cause selection bias. Nevertheless, even if patients underreported their SC use, the data still showed significant differences between those with and without a positive self-report of SC. Moreover, since the exact date of last SC use within the past three months was not available in the charts, it is not possible to differentiate acute effects of SCs from more persistent effects that may last for weeks. In addition, we did not have detailed information on the frequency and amount of use of substances including SCs and natural cannabis, which is particularly important considering the well-known dose-response relationship between use of cannabis and psychosis (29). Future studies that include a prospective study design with more detailed information obtained regarding patients’ substance use, including laboratory-verified SC consumption, will enhance interpretation of the data. Finally, our patient cohort was selected from an inpatient psychiatric unit and as such could have presented with more severe symptoms and other comorbid psychopathologies that may have influenced their hospitalization outcome. Since this population was mostly unemployed and homeless, and had histories of prior psychiatric admissions, caution should be taken in generalizing the current results to other SC users in non-psychiatric populations. Overall, despite these and other limitations, our general observations are consistent with those of other reports which suggest that SC use is associated with increased risk of psychosis and agitation, and the initial findings regarding sex differences may set the foundation for future studies.
Conclusion
Our study confirms that the use of synthetic cannabinoids is associated with higher presentation of psychosis and agitation in an inpatient population and that SC users were prescribed higher doses of antipsychotic medications and had longer hospital stays. There are significant sex differences in the association between cannabinoid use with psychosis and agitation with female SC users more frequently presenting with agitation and having longer hospital admissions, and having similar high rates of psychosis, compared to male SC users. These findings emphasize the importance of considering sex when making decisions about the diagnosis and treatment of cannabinoid users in psychiatric impatient units.
Clinical points:
Compared to men, women have higher sensitivity to synthetic cannabinoids. Synthetic cannabinoids (commonly known as K2/Spice) are similar to cannabis, but have higher potency and efficacy. No study has previously addressed potential differences in the clinical presentations of female versus male SC users.
Women who report recent use of SCs more frequently presented with agitation and needed longer hospitalizations compared to men.
Acknowledgments
Funding: YLH was supported by an internal Fund from the Icahn School of Medicine at Mount Sinai
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Anahita Bassir Nia, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, US.
Claire L. Mann, Icahn School of Medicine at Mount Sinai, New York, USA.
Sharron Spriggs, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, USA.
Daniel R. DeFrancisco, Department of Psychiatry, Mount Sinai Beth Israel, New York, US.
Steven Carbonaro, Department of Psychiatry, Mount Sinai Beth Israel, New York, US.
Lyla Parvez, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, USA.
Igor I. Galynker, Professor of Psychiatry, Icahn School of Medicine, Associate Chairman for Research, Department of Psychiatry, Mount Sinai Beth Israel, Director, Mount Sinai Beth Israel Suicide Research Laboratory, Director, Richard and Cynthia Zirinsky Center for Bipolar Disorder, New York, US.
Charles A. Perkel, Department of Psychiatry, New York Presbyterian Brooklyn Methodist Hospital, New York, Us.
Yasmin L. Hurd, Director of Addiction Institute at Mount Sinai, Ward-Coleman Chair of Translational Neuroscience, Professor of Psychiatry, Neuroscience and Pharmacological Sciences, Icahn School of Medicine, New York, US.
References
- 1.Elsohly M, Gul W, Wanas A, Radwan M. Synthetic cannabinoids: Analysis and metabolites. Life Science 2014;97:78–90. [DOI] [PubMed] [Google Scholar]
- 2.Courts J, Maskill V, Gray A, Glue P. Signs and symptoms associated with synthetic cannabinoid toxicity: systematic review. Australas Psychiatry. 2016;24:598–601. [DOI] [PubMed] [Google Scholar]
- 3.Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, MA H. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction 2013;108:534–544. [DOI] [PubMed] [Google Scholar]
- 5.Zaurova M, Hoffman R, Vlahov D, Manini A. Clinical Effects of Synthetic Cannabinoid Receptor Agonists Compared with Marijuana in Emergency Department Patients with Acute Drug Overdose. J Med Toxicol. 2016;12:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riederer A, Campleman S, Carlson R, Boyer E, Manini A, Wax P, Brent J, (ToxIC). TIC. Acute Poisonings from Synthetic Cannabinoids - 50 U.S. Toxicology Investigators Consortium Registry Sites, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craft R, Wakley A, Tsutsui K, Laggart J. Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. J Pharmacol Exp Ther. 2012;340:787–800. [DOI] [PubMed] [Google Scholar]
- 8.Tseng A, Craft R. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–47. [DOI] [PubMed] [Google Scholar]
- 9.Wakley A, Wiley J, Craft R. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2014;143:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiley J, O’connell M, Tokarz M, Wright MJ. Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. [DOI] [PubMed] [Google Scholar]
- 11.Fattore L, Spano M, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 2007;152:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SS, Secades-Villa R, Okuda M, Wang S, Perez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend. 2013;130:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers C, Gizer I, Vieten C, Gilder D, Stouffer G, Lau P, Wilhelmsen K. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol. 2015;23:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. Am J Drug Alcohol Abuse. 2010;36:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gage S, Hickman M, Zammit S. Association between cannabis and psychosis: Epidemiologic evidence. Biological Psychiatry. 2016;79:549–556. [DOI] [PubMed] [Google Scholar]
- 19.Moore T, Zammit S, Lingford-Hughes A, Barnes T, Jones P, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. [DOI] [PubMed] [Google Scholar]
- 20.Eranti SV, MacCabe JH, Bundy H, Murray RM. Gender difference in age at onset of schizophrenia: a meta-analysis. Psychol Med. 2013;43:155–167. [DOI] [PubMed] [Google Scholar]
- 21.Donoghue K, Doody GA, Murray RM, Jones PB, Morgan C, Dazzan P, Hart J, Mazzoncini R, Maccabe JH. Cannabis use, gender and age of onset of schizophrenia: data from the AESOP study. Psychiatry Res. 2014;215:528–532. [DOI] [PubMed] [Google Scholar]
- 22.Bassir Nia A, Medrano B, Perkel C, Galynker I, Hurd Y. Psychiatric comorbidity associated with synthetic cannabinoid use compared to cannabis. J Psychopharmacol. 2016;30:1321–1330. [DOI] [PubMed] [Google Scholar]
- 23.Andreasen N, Pressler M, Nopoulos P, Miller D, Ho B. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leucht S, Samara M, Heres S, Patel M, Furukawa T, Cipriani A, Geddes J, Davis J. Dose Equivalents for Second-Generation Antipsychotics: The Minimum Effective Dose Method. Schizophr Bull 2015;41:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dines A, Wood D, Galicia M, Yates C, Heyerdahl F, Hovda K, Giraudon I, Sedefov R, Euro-DEN Research Group, Dargan P. Presentations to the Emergency Department Following Cannabis use--a Multi-Centre Case Series from Ten European Countries. J Med Toxicol 2015;11:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray RM, Quigley H, Quattrone D, Englund A, Di Forti M. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuire P, Robson P, Cubala W, Vasile D, Morrison P, Barron R, Taylor A, S. W. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am J Psychiatry. 2018;175:225–231. [DOI] [PubMed] [Google Scholar]
- 28.Leweke F, Piomelli D, Pahlisch F, Muhl D, Gerth C, Hoyer C, Klosterkötter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012;20:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marconi A, Di Forti M, Lewis C, Murray R, Vassos E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr Bull 2016;42:1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques T, Handley R, Luzi S, Russo M, Paparelli A, Butt A, Stilo S, Wiffen B, Powell J, Murray R. High-potency cannabis and the risk of psychosis. Br J Psychiatry 2009;195:488–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allegri F, Belvederi Murri M, Paparelli A, Marcacci T, Braca M, Menchetti M, Michetti R, Berardi D, Tarricone I. Current cannabis use and age of psychosis onset: a gender-mediated relationship? Results from an 8-year FEP incidence study in Bologna. Psychiatry Res. 2013;210:368–370. [DOI] [PubMed] [Google Scholar]
- 32.Núñez C, Ochoa S, Huerta-Ramos E, Baños I, Barajas A, Dolz M, Sánchez B, Del Cacho N, GENIPE Group, Usall J. Differential effects of sex on substance use between first episode psychosis patients and healthy people. Compr Psychiatry 2016;69:169–178. [DOI] [PubMed] [Google Scholar]
- 33.Fattore L, Spano M, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol. 2010;160:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miczek K, Barry H. Comparison of the effects of alcohol, chlordiazepoxide, and delta9-tetrahydrocannabinol on intraspecies aggression in rats. Adv Exp Med Biol. 1977;85B:251–264. [DOI] [PubMed] [Google Scholar]
- 35.Manning F, Elsmore T. Shock-elicited fighting and delta-9-tetrahydrocannabinol. Psychopharmacologia. 1972;25:218–228. [DOI] [PubMed] [Google Scholar]
- 36.McDonough JJ, Manning F, Elsmore T. Reduction of predatory aggression of rats following administration of delta-9-tetrahydrocannabinol. Life Sci I. 1972;11:103–111. [DOI] [PubMed] [Google Scholar]
- 37.KA M. Delta9-tetrahydrocannabinol: antiaggressive effects in mice, rats, and squirrel monkeys. Science. 1978;199:1459–1461. [DOI] [PubMed] [Google Scholar]
- 38.Alves C, Carlini E. Effects of acute and chronic administration of Cannabis sative extract on the mouse-killing behavior of rats. Life Sci. 1973;13:75–85. [DOI] [PubMed] [Google Scholar]
- 39.Rosendrantz H, Sprague R, Fleischman R, Braude C. Oral delta9-tetrahydrocannabinol toxicity in rats treated for periods up to six months. Toxicol Appl Pharmacol. 1975;32:399–417. [DOI] [PubMed] [Google Scholar]
- 40.Ueki S, Fujiwara M, Ogawa N. Mouse-killing behavior (muricide) induced by delta 9-tetrahydrocannabinol in the rat. Physiol Behav. 1972;9:585–587. [DOI] [PubMed] [Google Scholar]
- 41.KA M. Mouse-killing and motor activity: effects of chronic delta9-tetrahydrocannabinol and pilocarpine. Psychopharmacology (Berl). 1976;47:59–64. [DOI] [PubMed] [Google Scholar]
- 42.EL A. Cannabis and aggression in animals. Behav Biol. 1975;14:1–20. [DOI] [PubMed] [Google Scholar]
- 43.Karniol I, Carlini E. Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia. 1973;33:53–70. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi R, Karniol I. Pharmacologic interaction between cannabinol and delta9-tetrahydrocannabinol. Psychopharmacologia. 1975;41:277–284. [DOI] [PubMed] [Google Scholar]
- 45.Carlini E, Hamaoui A, Märtz R. Factors influencing the aggressiveness elicited by marihuana in food-deprived rats. Br J Pharmacol. 1972;44:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlini E, Lindsey C, Tufik S. Cannabis, catecholamines, rapid eye movement sleep and aggressive behaviour. Br J Pharmacol. 1977;61:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musty RE LC, Carlini EA. 6-Hydroxydopamine and the aggressive behavior induced by marihuana in REM sleep-deprived rats. Psychopharmacology (Berl). 1976;48:175–179. [DOI] [PubMed] [Google Scholar]
- 48.Neto J, Nunes J, Carvalho F. The effects of chronic cannabis treatment upon brain 5-hydroxytryptamine, plasma corticosterone and aggressive behavior in female rats with different hormonal status. Psychopharmacologia. 1975;42:195–200. [DOI] [PubMed] [Google Scholar]
- 49.De Sousa Fernandes Perna E, Theunissen E, Kuypers K, Toennes S, Ramaekers J. Subjective aggression during alcohol and cannabis intoxication before and after aggression exposure. Psychopharmacology (Berl) 2016;233:3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kylie Lee K, Sukavatvibul K, Conigrave K. Cannabis use and violence in three remote Aboriginal Australian communities: Analysis of clinic presentations. Transcult Psychiatry. 2015;52:827–839. [DOI] [PubMed] [Google Scholar]
- 51.Walton M, Epstein-Ngo Q, Carter P, Zimmerman M, Blow F, Buu A, Goldstick J, Cunningham R. Marijuana use trajectories among drug-using youth presenting to an urban emergency department: Violence and social influences. Drug Alcohol Depend. 2017;173:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson J, Wu C, Winder G, Casher M, Marshall V, Bostwick J. The Effects of Cannabis on Inpatient Agitation, Aggression, and Length of Stay. J Dual Diagn 2016;12:244–251. [DOI] [PubMed] [Google Scholar]
- 53.Martinotti G, Cinosi E, Santacroce R, Papanti D, Pasquini A, Mancini V, Corbo M, Fiori F, Sarchione F, Marchetti D, Verrocchio M, Di Giannantonio M, Torrens M, Schifano F, Morlan Coarasa M, Merino Del Villar C. Substance-related psychopathology and aggressiveness in a nightlife holiday resort: Results from a pilot study in a psychiatric inpatient unit in Ibiza. Hum Psychopharmacol 32(3). 2017;32:Epub 2017 May 2030. [DOI] [PubMed] [Google Scholar]
- 54.Harris A, Large M, Redoblado-Hodge A, Nielssen O, Anderson J, Brennan J. Clinical and cognitive associations with aggression in the first episode of psychosis. Aust N Z J Psychiatry 2010;44:85–93. [DOI] [PubMed] [Google Scholar]


