Abstract
Context.—
Cardiac complications of immune checkpoint inhibitor therapy are rare, but reports of myocarditis are increasing. The findings have been described in case reports as lymphocytic myocarditis, but its histopathology is underreported.
Objective.—
To review the histology of myocardial biopsy–proven cases of immune checkpoint–associated myocarditis and provide immunohistochemical characterization of the inflammatory infiltrate.
Design.—
We have encountered 6 patients with biopsy-proven myocarditis in conjunction with therapy using anti-programmed death receptor-1 (PD-1)/programmed death ligand-1 (PD-L1) agents with and without cytotoxic T-lymphocyte associated protein 4 (CTLA-4) inhibitors and characterized the histopathology and immune cell profile.
Results.—
The myocarditis was multifocal/diffuse and characterized by a predominant CD163-positive histiocytic infiltrate, with an associated CD8+ and PD-1+ T-lymphocytic infiltrate, some of which were granzyme B positive. Cardiac myocytes showed immunoreactivity for PD-L1 in areas of injury, confirmed using 2 different anti-PD-L1 clones. Four of 6 patients recovered from their cardiac injury. One patient had residual tachycardiabradycardia syndrome and 1 patient expired.
Conclusions.—
The diffuse lymphohistiocytic myocarditis associated with this therapy is relatively distinctive, and this diagnosis is strongly suggested based on the histopathologic findings in the correct clinical setting.
Cardiac complications associated with the immunotherapy of advanced malignancies with anti–programmed death receptor-1 (PD-1), anti–programmed death ligand-1 (PD-L1), and anti–cytotoxic T-lymphocyte associated protein 4 (CTLA-4) targeting agents are being encountered, including myocarditis, pericardial disease, and tachyarrhythmias and bradyarrhythmias.1 The recognition of myocarditis with monotherapy as well as combination therapy as a life-threatening but potentially treatable complication has made diagnosis imperative. We report 6 cases (see supplemental digital content) demonstrating the relatively rapid progression of this complication, with integration of myocardial biopsy in the diagnostic algorithm, while characterizing the distinctive histopathology of this diffuse lymphohistiocytic myocarditis. A summary of the clinical features is provided in Table 1.
Table 1.
Summary of Clinical Features of the 6 Patients
| Patient No. | Age, y/Sex | Cancer Diagnosis | Clinical History | I/O Medication | Time to First Symptoms, da | Outcome |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 53/F | Lymphoma | DM, hypertension, dyslipidemia | Atezolizumab | 19 | Resolution, 17 d |
| 2 | 70/M | Hepatocellular | DM | Pembrolizumab | 6 | Resolution, 2 mo |
| 3 | 75/F | Endometrial carcinoma | No significant history | Tremelimumab, durvalumab | 21 | Resolution, 14 d |
| 4 | 71/M | Melanoma | No significant history | Nivolumab, ipilimumab | 7 | Death (heart failure, multisystem organ failure) |
| 5 | 75/M | Pancreatic carcinoma | DM, hypertension, dyslipidemia | Nivolumab | 38 | Resolution (partial), 20 d |
| 6 | 83/F | Melanoma | Hypothyroidism, hypertension, dyslipidemia, COPD | Nivolumab | 21 | Resolution, 7 d |
Abbreviations: COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; I/O, immune oncology.
After last dose of immune-oncology agent.
MATERIALS AND METHODS
The cases were identified via a search of CoPath for heart biopsies and cross-referenced for clinical history of malignancy, anti–PD-1/PD-L1 therapy, and anti–CTLA-4 therapy. All slides were reviewed and myocarditis confirmed. Criteria for myocarditis were as described in the Dallas criteria,2 requiring inflammation and myocyte necrosis adjacent to inflammation. In addition, the proposed criterion of more than 14 leukocytes/mm2 was also met in all cases.3 Five-micrometer sections on charged slides were evaluated by immunohistochemistry for CD163 (Leica MRQ-26, prediluted), CD3 (Leica LN10), CD8 (Leica 4B11), Granzyme B (Leica 11F1), CD4 (Leica 4B12), and CD20 (Leica MJ1), all ready-to-use antibodies, with antigen-retrieval EDTA Tris pH 9.0, 20 minutes. PD-1 (MRQ-22, Cell Marque) was also ready to use but with citrate pH 6.0 retrieval for 20 minutes, and PD-L1 antibodies (SP142, Abcam, and 22C3, Dako) were used with 1:100 and 1:50 dilution with citrate pH 6.0 for 30 minutes and Tris EDTA pH 9.0 for 20 minutes. All stains were performed on the Leica Bond III system with diaminobenzidine chromogen and scored semiquantitatively for numbers of cells staining where negative was no cells, + was rare cells, ++ multifocally staining cells, and +++ diffusely staining cells.
RESULTS
Pathologic Findings on Myocardial Biopsy
The 6 myocardial biopsies had common findings. There was significant and prominent interstitial inflammatory infiltrate composed of numerous histiocytes (Figure 1, A through C) with interspersed lymphocytes. This lymphohistiocytic infiltrate was associated with evidence of myocardial injury. This included loss of myocyte nuclei, myocytes with irregular cell membranes with moth-eaten appearance, hypereosinophilia, and shrunken and irregular cardiac myocytes, but not associated with fibrosis (Figure 1, C and D).
Figure 1.

Histology of immune checkpoint myocarditis. A, Inflammation is seen between cardiac myocytes multifocally. B, Diffuse infiltrates of mononuclear cells, many of which are histiocytic and associated with myocyte destruction. C, Higher magnification shows myocyte injury with patch of lymphocytes and histiocytes. D, Trichrome stain shows myocyte destruction in the absence of fibrosis (hematoxylin-eosin, original magnifications ×50 [A], ×100 [B], and ×150 [C]; trichrome stain, original magnification ×100 [D]).
Immunohistochemistry supported the morphologic characterization of the inflammatory infiltrate. The infiltrate was composed of numerous CD163-positive (Figure 2, A), weakly CD4-positive histiocytic cells mixed with predominantly CD8-positive T cells, many of which were PD-1 positive (Figure 2, B and C). Granzyme B–positive cells represented a subset of the CD8-positive cells (Figure 2, D). Immunohistochemistry for PD-L1 showed immunoreactivity in the distribution of the CD163-positive cells (Figure 3, A). In addition, patches of myocyte staining with PD-L1 were identified and confirmed with both SP142 and 22C3 (Figure 3, B) clones for PD-L1. Endocardial staining was also seen, not associated with injury. Areas of myocyte injury (Figure 3, C) showed immunoreactivity for PD-L1 in myocytes (Figure 3, D).
Figure 2.
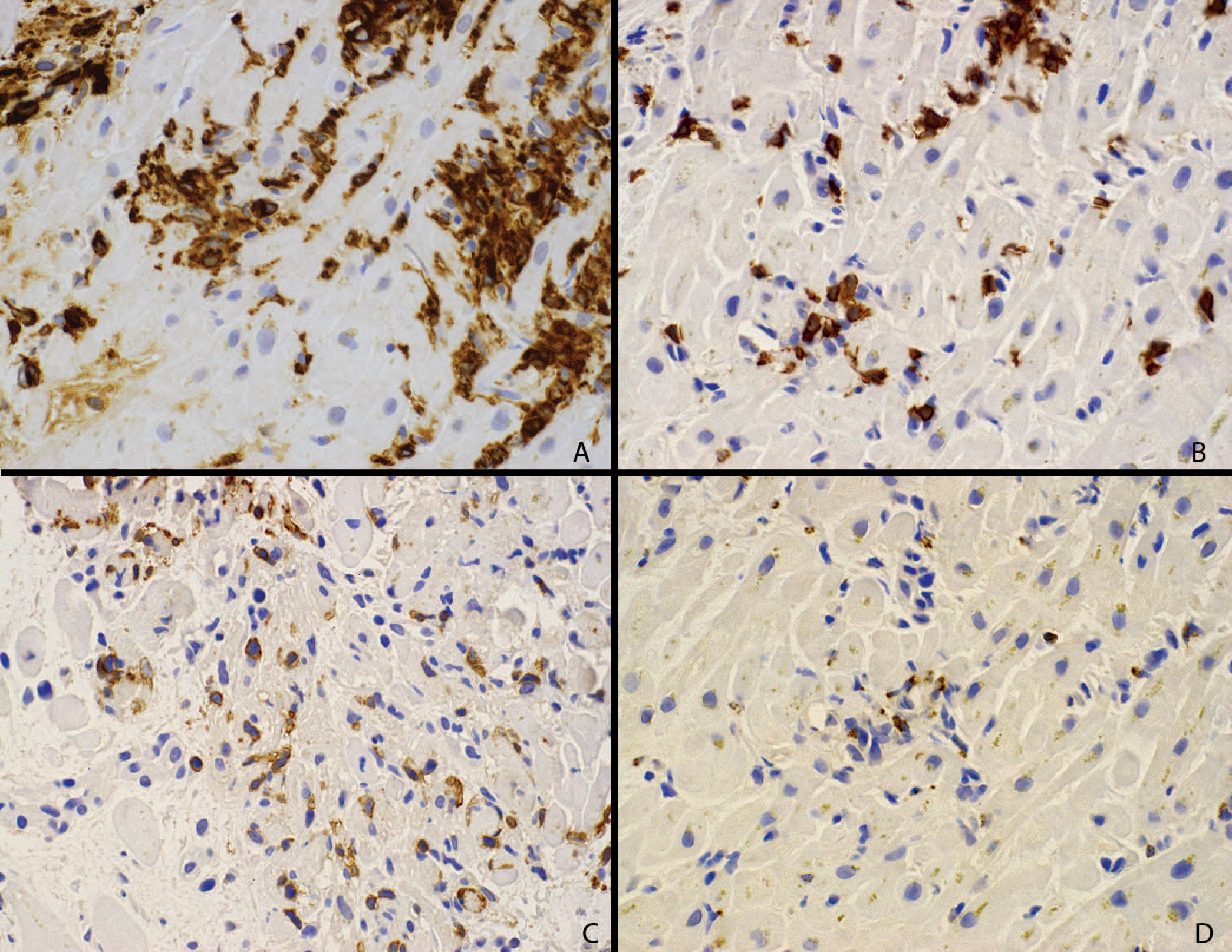
Immunohistochemical characterization of the inflammatory infiltrate. A, CD163 immunostaining confirms numerous histiocytic cells. B, Same area showing CD8-positive T cells. C, The same cells are positive for PD-1. D, Granzyme B highlights a subset of the lymphocyte population (diaminobenzidine immunohistochemistry, original magnification ×150).
Figure 3.

Myocarditis and programmed death ligand-1 (PD-L1) immunohistochemistry. A, An area of myocarditis on the right, with uninvolved myocardium on the left. B, PD-L1 immunoreactivity (SP263 clone) is seen in area of injury. Endocardium was also immunoreactive (not shown). C, High-power view of an area of myocarditis. D, The same area shows PD-L1 (SP263 clone) immunoreactivity in histiocytic cells (bottom left) and immunoreactivity of myocytes (top left and right) (hematoxylin-eosin, original magnifications ×50 [A] and ×150 [C]; diaminobenzidine immunohistochemistry, original magnifications ×50 [B] and ×150 [D]).
Table 2 summarizes the immunohistochemistry findings across the 6 biopsy cases, showing consistent CD163-positive histiocytic rich infiltrates with CD8+ PD-1+ lymphocytes, a subset of which were granzyme B positive. CD4-positive T cells were the minority of the CD3-positive population, and CD20-positive B cells were rare to absent. Of note, 2 cases also showed occasional eosinophils, and 2 cases showed rare CD56-positive cells, which were assumed to be natural killer cells.
Table 2.
Immunohistochemistry Results in Myocardial Biopsies
| Patient No. | CD163 | CD3 | CD8 | Granzyme B | CD4a | CD20 | PD-L1b | PD-L1b Myocytes | PD-1 | Other |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1 | +++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | |
| 2 | +++ | ++ | ++ | ++ | + | + | ++ | ++ | NP | Rare eos |
| 3 | +++ | ++ | ++ | ++ | ++ | − | + | ++ | ++ | Rare eos |
| 4 | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | Rare CD56+ |
| 5 | +++ | ++ | ++ | ++ | + | + | + | ++ | ++ | Rare CD56+ |
| 6 | +++ | ++ | ++ | NP | + | − | ++ | ++ | ++ | Rare CD56+ |
Abbreviations: eos, eosinophils; NP, not performed; PD-1, programmed death receptor-1; PD-L1, programmed death ligand-1; −, no cells; +, rare cells; ++, multifocally staining cells; +++, diffusely staining cells.
CD4-positive cells reflect strongly staining lymphocytes. Histiocytic cells were also CD4 positive, weakly.
This reflects results of both SP263 and SP142 clones. PD-L1 is scored in inflammatory cells and myocytes separately.
DISCUSSION
Immune-related events are known complications of immuno-oncologic therapeutic interventions, but are generally manageable. For example, several cases of ipilimumab immunotherapy complicated by inflammatory diseases, including colitis, myocarditis, and Guillain-Barre syndrome, have been reported.4 The rate of complication is by report higher with combination CTLA4- and PD-1/PD-L1–block-ing therapy, with 16.3% of patients experiencing grade 3 or grade 4 complication with nivolumab alone, 27.3% with ipilimumab alone, and 55% with combination therapy. Discontinuation of therapy because of complications occurred in 7.7% with nivolumab alone, 14.8% with ipilimumab alone, and 36.4% with combination therapy in one series and 17% with ipilimumab alone and 47% in combination treatment in another. Life-threatening complications as well as cardiac complications were rare5 in initial series, with 1 case of cardiac arrhythmia reported (no biopsy performed), not attributed to study drug.6
Emerging reports show cardiac complications other than cardiac arrest, arrhythmia, and heart failure. In a series of 8 cases demonstrating cardiac complications,7 4 patients were described as having myocarditis with lymphocytes (T cells), with 1 case showing giant cells. These reports included patients receiving ipilimumab monotherapy as well as combination ipilimumab and nivolumab therapy in the treatment of malignant melanoma. In another report, an ipilimumab-treated melanoma patient had myocarditis characterized by CD8-positive lymphocytic infiltrate and decreased FOXP3-positive cells. This patient recovered with steroid therapy and supportive care.8 Another patient9 with giant cell myocarditis was reported on ipilimumab therapy. Two cases of myocarditis and myositis in patients with malignant melanoma were reported10 following treatment with nivolumab and ipilimumab. The lymphocytic populations in these cases were CD3 positive and CD20 negative, with an admixture of CD4 and CD8 cells.10
Immune-related cardiac complications have also been reported in monotherapy with anti–PD-1 inhibitors. In a report11 of pembrolizumab-treated patients with Merkel cell carcinoma, 1 patient had myocarditis. Myocarditis in the monotherapy setting has also been reported in the treatment of non–small cell lung cancer, including both adenocarcinoma and squamous cell carcinoma12–14and melanoma,15,16 some patients with fatal outcome and others with recovery from myocarditis.
While cases of myocarditis have been described in both the monotherapy and combination-therapy settings,17 there is some suggestion that combination therapy is associated with increased risk of this complication. In one series, the rate of myocarditis was estimated at 0.09% overall, but at 0.27% in combination CTLA-4 and PD-1 blockade and 0.06% for PD-1 monotherapy. Of note, cases have been described with both PD-1 and PD-L1 blocking agents.18 In addition, combination therapy may have been associated with a greater likelihood of fatal outcome caused by myocarditis.10,19 Our 6 cases include 4 monotherapy patients, including both anti–PD-1 and anti–PD-L1 agents.
As experience with these agents expands, the reported myocardial complication rate, although low, may be higher than initially described. In a more recent series20 of 35 patients with myocarditis, the rate was estimated at 1.14%, with 81% of occurrences within 3 months of initiation of therapy. Eleven patients had biopsies, all described as lymphocytic myocarditis with T-cell infiltrates and without giant cells. Concurrent diabetes was reported as an additional risk factor for this complication, which was seen in 3 of our 6 cases.
The reported histology is often described as lymphocytic myocarditis, with the majority not having associated giant cells. Our cases suggest that the myocarditis may be more accurately described as lymphohistiocytic myocarditis, and our cases were also devoid of giant cells. In addition to histiocytic cells, the infiltrates are composed of a population of PD-1–positive, CD8-positive, granzyme-B–positive T lymphocytes.
Interestingly, the myocytes express PD-L1 in these biopsies, albeit variably and associated with areas of injury. This appears to be in keeping with prior observations. For example, PD-L1–deficient mice develop myocarditis,21 suggesting that myocyte PD-L1 may be a mechanism of avoiding self-targeting immune responses. It is hypothesized that CTLA4 blockade, by restoring T-cell proliferation, may enhance anticardiac immune responses that are thwarted by myocyte and endothelial PD-L1 expression.22 It may be that the additional blockade of the PD-L1–PD-1 axis increases the risk that such antimyocyte targeted T cells would now recognize myocytes, allowing for immune attack. Also postulated is that a common antigen to tumor and muscle may exist in this small subset of patients, and as a result combination therapy provides an opportunity for an antimyocyte immune response to occur and persist. Prior reports of a common oligoclonal T-cell immune response in cardiac and skeletal muscle lend support to this hypothesized mechanism.10 The presence of a PD-1–positive, granzyme-B–positive T-cell population in our cases, along with PD-L1–positive myocytes, supports that a self-targeting, myocyte-focused immune response is being facilitated by either PD-1 or PD-L1 blockade in spite of the myocyte PD-L1 expression.
Although the myocardial complication rate is very low, expansion of indications for immunotherapy increases our need for awareness of its possibility. Specifically, expansion of combination anti-CTLA4 and anti–PD-1/PD-L1 therapy for the common solid tumors of lung and gastrointestinal tract23,24 will require clinical and pathologic familiarity with these potential complications. Myocardial biopsy is helpful in confirming the diagnosis. Drug cessation and therapeutic intervention have reversed the cardiac complications. Further understanding of the mechanisms of this anticardiac immune response may lead to cardio-protective strategies if susceptible individuals can be successfully identified.
Supplementary Material
Acknowledgments
Supported by P30 CA008748 at Memorial Sloan Kettering Cancer Center (MSKCC). This is a cancer core grant from the National Institute of Health that covers cancer center activity at the MSKCC NCI-designated Comprehensive Cancer Center.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
Supplemental digital content is available for this article at https://meridian.allenpress.com/aplm in the November 2020 table of contents.
Contributor Information
Irina Sobol, Department of Medicine, Division of Cardiology, Weill Cornell Medicine, New York, New York.
Carol L. Chen, Department of Medicine, Division of Cardiology, Memorial Sloan Kettering Cancer Center, New York, New York.
Syed S. Mahmood, Department of Medicine, Division of Cardiology, Weill Cornell Medicine, New York, New York.
Alain C. Borczuk, Weill Cornell Pathology, New York, New York.
References
- 1.Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18(6):619–624. [DOI] [PubMed] [Google Scholar]
- 3.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5): 522–530. [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1): 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuben A, Petaccia de Macedo M, McQuade J, et al. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology. 2017;6(12):e1361097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson R, Delaune J, Szady A, Markham M. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Rep. 2016;2016. doi: 10.1136/bcr-2016-216228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matson DR, Accola MA, Rehrauer WM, Corliss RF. Fatal myocarditis following treatment with the pd-1 inhibitor nivolumab. J Forensic Sci. 2018;63(3): 954–957. [DOI] [PubMed] [Google Scholar]
- 14.Semper H, Muehlberg F, Schulz-Menger J, Allewelt M, Grohe C. Drug-induced myocarditis after nivolumab treatment in a patient with PDL1-negative squamous cell carcinoma of the lung. Lung Cancer. 2016;99:117–119. [DOI] [PubMed] [Google Scholar]
- 15.Tadokoro T, Keshino E, Makiyama A, et al. Acute lymphocytic myocarditis with anti-PD-1 antibody nivolumab. Circ Heart Fail. 2016;9(10). [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi S, Morimoto R, Okumura T, et al. Late-onset fulminant myocarditis with immune checkpoint inhibitor nivolumab. Can J Cardiol. 2018; 34(6):812.e1–812.e3. [DOI] [PubMed] [Google Scholar]
- 17.Koelzer VH, Rothschild SI, Zihler D, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors—an autopsy study. J Immunother Cancer. 2016;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood SS, Chen CL, Shapnik N, Krishnan U, Singh HS, Makker V. Myocarditis with tremelimumab plus durvalumab combination therapy for endometrial cancer: a case report. Gynecol Oncol Rep. 2018;25:74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116(18):2062–2071. [DOI] [PubMed] [Google Scholar]
- 22.Tajiri K, Aonuma K, Sekine I. Immune checkpoint inhibitor-related myocarditis. Jpn J Clin Oncol. 2018;48(1):7–12. [DOI] [PubMed] [Google Scholar]
- 23.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33(5):843–852 e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


