ABSTRACT
Chlamydia in the genital tract is known to spread via the blood circulation system to the large intestine lumen to achieve long-lasting colonization. However, the precise pathways by which genital Chlamydia accesses the large intestine lumen remain unclear. The spleen was recently reported to be critical for chlamydial spreading. In the current study, it was found that following intravaginal inoculation with Chlamydia, mice with and without splenectomy both yielded infectious Chlamydia on rectal swabs, indicating that the spleen is not essential for genital Chlamydia to spread to the gastrointestinal tract. This conclusion was validated by the observation that intravenously inoculated Chlamydia was also detected on the rectal swabs of mice regardless of splenectomy. Careful comparison of the tissue distribution of live chlamydial organisms following intravenous inoculation revealed redundant pathways by which Chlamydia can reach the large intestine lumen. The intravenously inoculated Chlamydia was predominantly recruited to the spleen within 12 h and then detected in the stomach lumen by 24 h, in the intestinal lumen by 48 h, and on rectal swabs by 72 h. These observations suggest a potential spleen-to-stomach pathway for hematogenous Chlamydia to reach the large intestine lumen. This conclusion was supported by the observation made in mice under coprophagy-free condition. However, in the absence of spleen, hematogenous Chlamydia was predominantly recruited to the liver and then simultaneously detected in the intestinal tissue and lumen, suggesting a potential liver-to-intestine pathway for Chlamydia to reach the large intestine lumen. Thus, genital/hematogenous Chlamydia may reach the large intestine lumen via multiple redundant pathways.
KEYWORDS: Chlamydia, spreading pathways, GI tract, spleen to stomach, liver to intestine
INTRODUCTION
The mouse-adapted organism Chlamydia muridarum has been used to investigate the pathogenic mechanisms of Chlamydia trachomatis because intravaginal inoculation with C. muridarum can induce lasting pathology in the upper genital tract (1–5) similar to that observed in women with sexually transmitted infection with C. trachomatis (6–9). The C. muridarum mouse model has been useful in identifying pathogenic determinants from both C. muridarum (10–15) and the host (5, 16–19). More importantly, in vivo imaging of intravaginally inoculated C. muridarum that expresses a luciferase gene (20) revealed that genital C. muridarum spread to the gastrointestinal (GI) tract to establish long-lasting colonization (21). The chlamydial spreading from the genital tract to the GI tract has been shown to promote chlamydial pathogenicity in the upper genital tract (22, 23). Interestingly, C. trachomatis has been frequently detected in human GI tracts (24–28). However, it is unclear whether C. trachomatis in the GI tract can affect C. trachomatis pathogenicity in women’s upper genital tract.
Since genital Chlamydia spreading to the GI tract may be medically important, efforts have been made to reveal the pathways of the chlamydial spreading. We have previously shown that chlamydial spreading from the genital to the GI tract is unlikely to be caused by vaginal-anal contamination or coprophagy but is likely dependent on blood circulation (21). Indeed, intravenously inoculated C. muridarum selectively spread to the GI tract to establish long-lasting colonization in the large intestine (29). However, the precise pathways by which intravaginally or intravenously inoculated C. muridarum spreads to the mouse large intestine lumen remain unknown. A recent study revealed that the spleen plays an important role in mediating the spreading of C. muridarum from the genital tract to the GI tract (30).
The current study was designed to evaluate whether the spleen is necessary for genital C. muridarum to spread to the GI tract and to explore the potential roles of spleen-independent pathways in mediating chlamydial spreading. It was found that following intravaginal inoculation with C. muridarum, mice with and without splenectomy both produced infectious Chlamydia, detected on rectal swabs, indicating that the spleen is not essential for Chlamydia to spread from the genital tract to the GI tract. This conclusion was validated by the observation that intravenously inoculated Chlamydia was also detected on rectal swabs of mice regardless of splenectomy. Since intravenous inoculation avoids potential contamination between the genital and GI tracts, intravenous inoculation was further used to carefully compare the temporal and spatial distribution of live chlamydial organisms in mouse tissues and mucosal lumens. The comprehensive comparison revealed multiple pathways for Chlamydia to reach the large intestine lumen. In the presence of the spleen, the intravenously inoculated Chlamydia was predominantly recruited to the spleen within 12 h and then detected in the stomach lumen by 24 h, in the intestinal lumen by 48 h, and on rectal swabs by 72 h. These observations suggest a spleen-to-stomach pathway for hematogenous Chlamydia to reach the large intestine lumen. This conclusion was supported by the observation made in mice under coprophagy-free condition. Thus, although the spleen is not essential for chlamydial spreading, the spleen can promote spreading. This conclusion is further validated by the observation that the spreading of intravenously inoculated Chlamydia to the tissues and lumens of the GI tract was significantly delayed in mice with splenectomy. More importantly, in the absence of spleen, hematogenous Chlamydia was found to predominantly be recruited to the liver and then simultaneously appear in both intestinal tissues and lumens. This tissue distribution pattern suggests a potential liver-to-intestine pathway for Chlamydia to reach the large intestine lumen. Thus, it is likely that genital/hematogenous Chlamydia may be able to utilize multiple redundant host pathways to reach the large intestine lumen. This hypothesis is consistent with the concept that reaching the large intestine lumen represents a strong selection pressure, since it can allow Chlamydia to both establish long-lasting colonization in the infected host and be efficiently transmitted to other animal hosts via the oral-fecal route.
RESULTS
The spleen is not essential for genital/hematogenous Chlamydia to spread to the gastrointestinal tract.
A recent study claimed that Chlamydia infection of the spleen is a critical step for Chlamydia dissemination to the GI tract (30). Genital Chlamydia spreading to the GI tract to achieve long-lasting colonization in the GI tract is due to the fact that Chlamydia can reach the large intestine lumen, which can be monitored by detecting live chlamydial organisms on rectal swabs following intravaginal inoculation (21). In the current study, rectal swabs from mice with and without splenectomy were compared for live Chlamydia organisms for 8 weeks after intravaginal inoculation (Fig. 1). It was found that all mice regardless of splenectomy shed live chlamydial organisms for about 1 month from the genital tract and maintained long-lasting shedding of live chlamydial organisms from the GI tract. The lack of significant differences in the shedding courses from either the genital or GI tract between mice with and without splenectomy demonstrated that the spleen is not essential for either chlamydial infection in the genital tract or chlamydial spreading from the genital tract to the GI tract.
FIG 1.
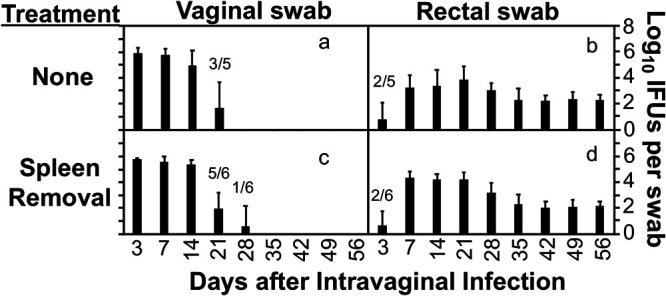
Effect of spleen removal on the spreading of genital Chlamydia to the gastrointestinal tract. Groups of female C57BL/6J mice without (a and b; n = 5) or with (c and d; n = 6) splenectomy were infected intravaginally with Chlamydia. All mice were monitored for live chlamydial organisms recovered from both vaginal (a and c) and rectal (b and d) swabs, and the titers were expressed as log10 IFU per swab on different days after intravaginal infection. Note that spleen removal failed to significantly impact either live chlamydial shedding from the genital tract or the spread of genital Chlamydia to the gastrointestinal tract. P > 0.05 (a versus c or b versus d, area under the curve, Wilcoxon rank sum). The number of mice with shedding is indicated for the points where not all mice were positive for shedding. The data were acquired from two independent experiments.
Since Chlamydia is known to spread from the genital tract to the GI tract via the blood circulatory system (21, 29–31), an intravenous inoculation approach was used to compare the chlamydial spreading to the GI tract between mice with or without splenectomy (Fig. 2). This is because intravenous inoculation avoids potential vaginal-anorectal contamination. It was found that following intravenous inoculation, live Chlamydia was detected on rectal swabs but not vaginal swabs. This finding is consistent with the previous observation that hematogenous Chlamydia can reach only the lumen of the GI tract, not the lumen of other mucosal tissues (29). More importantly, the hematogenous chlamydial organisms developed similar courses of shedding from the GI tracts of mice with and without splenectomy. This observation validated the above conclusion that the spleen is not required for Chlamydia to spread to the large intestine lumen.
FIG 2.
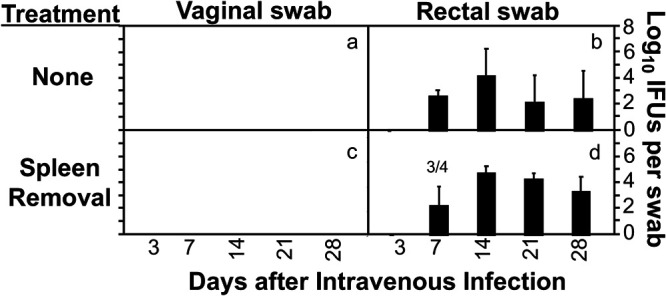
Effect of spleen removal on the spreading of hematogenous Chlamydia to the gastrointestinal tract. Groups of female C57BL/6J mice without (a and b; n = 5) or with (c and d; n = 4) splenectomy were inoculated retro-orbitally with Chlamydia. All mice were monitored for live chlamydial organism shedding from both vaginal (a and c) and rectal (b and d) swabs, and the titers were expressed as log10 IFU per swab on different days after intravaginal infection. Note that hematogenous Chlamydia was detected on the rectal swabs but not vaginal swabs of all mice regardless of splenectomy. P > 0.05 (b versus d, area under the curve, Wilcoxon rank sum). The number of mice with shedding is indicated for the points where not all mice were positive for shedding. The data were acquired from two independent experiments.
In the presence of the spleen, hematogenous Chlamydia reaches the stomach first and then the rest of the GI tract.
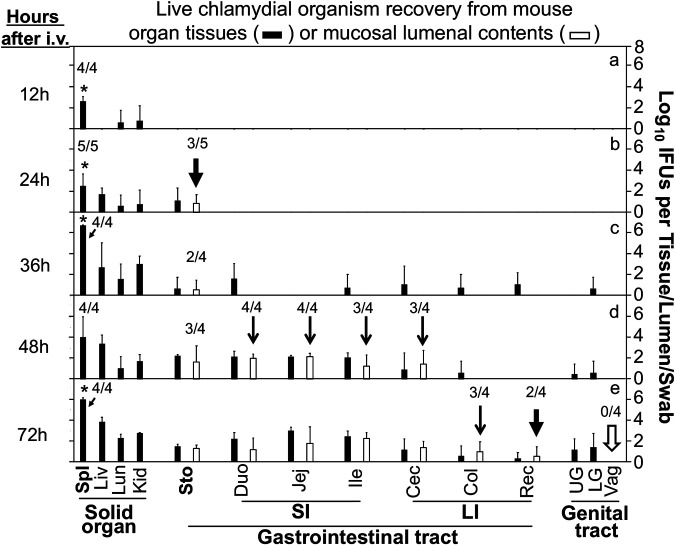
Chlamydia can survive in the stomach for only about a week and the small intestine for a month, but it can persist in the large intestine for long periods (21, 32–35). To determine how hematogenous Chlamydia gains access to the large intestine lumen, the chlamydial organism distribution in different mouse tissues was closely monitored over time starting as early as 12 h after intravenous inoculation (Fig. 3). Live Chlamydia was detected predominantly in the spleen by 12 h, the stomach tissue and lumen by 24 h, intestinal tissues by 36 h, and intestinal lumen by 48 h. By 72 h, live Chlamydia was finally detected on rectal swabs but not vaginal swabs. Although the extensive and careful titration of infectious titers of Chlamydia simultaneously from multiple tissues and luminal samples is labor-intensive, the comprehensive comparison of the tissue distribution of the live chlamydial organisms over time seems to suggest a spleen-to-stomach lumen pathway for hematogenous Chlamydia to reach the large intestine lumen.
FIG 3.
Temporal distribution of live chlamydial organisms in mouse tissues following intravenous inoculation. After retro-orbital inoculation with Chlamydia, groups of C57BL/6J mice were sacrificed at 12 h (a), 24 h (b), 36 h (c), 48 h (d), or 72 h (e) to monitor live chlamydial organisms in different tissues (solid bars) and luminal contents (open bars), including spleen (Spl), liver (Liv), lung (Lun), kidney (Kid), and gastrointestinal tract tissues and luminal contents from the stomach (Sto), small intestine (SI) (including the duodenum [Duo], jejunum [Jej], and ileum [Ile]), and large intestine (LI) (including the cecum [Cec], colon [Col], and rectum [Rec]), as well as upper genital (UP) and lower genital (LG) tract tissues and vaginal swabs (Vag). The rectum lumen was collected as rectal swabs. The infectious titers of chlamydial organisms were expressed as log10 IFU per tissue/lumen/swab. Note that hematogenous Chlamydia was detected predominantly in the spleen by 12 h, the stomach tissue and lumen (b, arrow) by 24 h, and intestinal tissues by 36 h and intestinal lumen by 48 h (d, arrows). By 72 h, live Chlamydia was first detected on the rectal swabs (e, thick arrow) but not vaginal swabs (e, open arrow). *, P < 0.05, Wilcoxon rank sum (spleen versus liver, lung, or kidney). n = 4 or 5 mice for each time point. Data are from 3 separate experiments. Due to large number of samples to be measured from each mouse, only one or two mice were processed each time. The number of mice with detectable IFU in a given tissue/sample is indicated for the samples that were important for drawing conclusions.
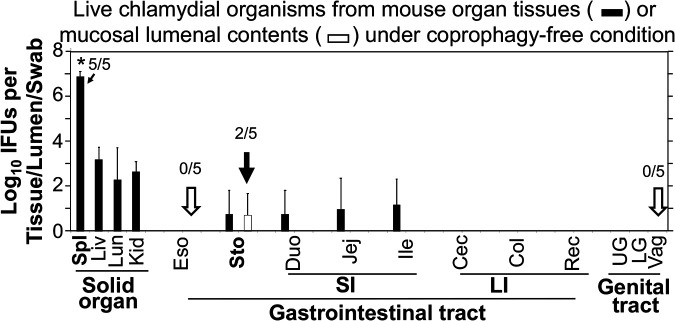
The finding that hematogenous Chlamydia reached the stomach lumen within 24 h after intravenous inoculation was surprising to us. This was unlikely to be due to oral uptake, since the intravenously inoculated Chlamydia should be restricted to the blood circulation (29) and no live chlamydial organisms were detected on rectal swabs at this time. To further exclude the possibility of coprophagy, the experiment was repeated using mice housed under coprophagy-free conditions (Fig. 4). In addition, to better determine whether the stomach luminal Chlamydia was a result of oral uptake, we also monitored live chlamydial organisms from esophagus tissue and lumen in the coprophagy-free experiment. When the chlamydial organisms were monitored in mouse tissue and luminal samples 36 h after intravenous inoculation with Chlamydia, it was found that hematogenous Chlamydia appeared predominantly in the spleen compared to the other solid organs, which is consistent with what was observed above. More importantly, when different tissues of the gastrointestinal tract were compared, live chlamydial organisms were detected in both the stomach and intestinal tissues but not the esophagus tissues, suggesting that the chlamydial organisms detected in the stomach were unlikely to be from the esophagus or oral uptake. Finally, live chlamydial organisms were also detected in the stomach lumen but not any other lumens at this time point (36 h after intravenous inoculation), which supports the concept that hematogenous Chlamydia may use the spleen-to-stomach pathway to gain access to the stomach lumen, after which the stomach luminal Chlamydia may descend to seed the rest of the GI tract.
FIG 4.
Distribution of intravenously inoculated Chlamydia in tissues of mice housed under coprophagy-free condition. After retro-orbital inoculation with Chlamydia, C57BL/6J mice (n = 5) were sacrificed at 36 h for monitoring for live Chlamydia recovery from different tissues (solid bar) and luminal contents (open bar). Abbreviations for tissues and luminal contents are as in Fig. 3, with the addition of esophagus (Eso) tissue and luminal contents (open arrow). The titers of live chlamydial organisms were expressed as log10 IFU per tissue/lumen/swab. Note that hematogenous Chlamydia was detected predominantly in the spleen and in the stomach lumen (filled arrow) but no other mucosal lumens (open arrow). *, P < 0.05, Wilcoxon rank sum (spleen versus liver, lung, or kidney). n = 5. Data are from 2 separate experiments. The number of mice with detectable IFU in a given tissue/sample is indicated for the samples that were important for drawing conclusions.
In the absence of the spleen, Chlamydia is able to spread to the large intestine lumen but without a preceding presence in the stomach.
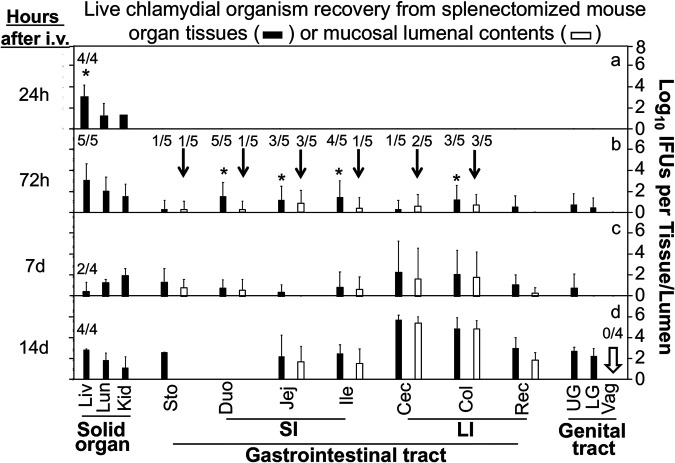
Since data in Fig. 1 and 2 demonstrated that genital or hematogenous Chlamydia can spread to the GI tract in the absence of the spleen, the next step was to examine the tissue distribution pattern of the live chlamydial organisms after intravenous inoculation. As shown in Fig. 5, by 24 h, live chlamydial organisms were predominantly detected in the livers of the splenectomized mice. There was no live Chlamydia in any GI tract tissues at this time. Only by 72 h after intravenous inoculation could significant numbers of live chlamydial organisms be detected in the GI tracts of the splenectomized mice. This represents a significant delay in the spreading of hematogenous Chlamydia to the GI tract, since, in normal mice, live chlamydial organisms were already detected in both the stomach tissue and lumen by 24 h after intravenous inoculation. Thus, the lack of a spleen can delay chlamydial spreading to the GI tract. More importantly, in the splenectomized mice, the numbers of inclusion-forming units (IFU) recovered from different intestinal tissues were significantly higher than those from the stomach, suggesting that in the absence of the spleen, hematogenous Chlamydia may spread to the GI tract via a liver-to-intestine pathway but without the need of prior presence in the stomach. Significant levels of live chlamydial organisms were simultaneously detected in the lumen of the intestine, suggesting that live chlamydial organisms may directly arrive at the intestinal lumen from the liver via the bile duct system. As a control for mucosal barrier integrity, no live chlamydial organisms were detected in the lumen of the genital tract, even on day 14 after intravenous inoculation, suggesting that the mucosal barrier function was maintained for preventing hematogenous Chlamydia from entering the lumen of extragut mucosal tissues in the splenectomized mice.
FIG 5.
Temporal distribution of intravenously inoculated Chlamydia in tissues of splenectomized mice. After retro-orbital inoculation with Chlamydia, groups of splenectomized C57BL/6J mice were sacrificed at 24 h (a), 72 h (b), 7 days (c), or 14 days (d) to monitor live chlamydial organisms in different tissues (solid bars) and luminal contents (open bars). The designations for the tissues and luminal contents are as in Fig. 3. The titers of live chlamydial organisms were expressed as log10 IFU per tissue/lumen/swab. Note that hematogenous Chlamydia was detected predominantly in the liver by 24 h (a) and different lumens of the gastrointestinal tract (b, arrows) by 72 h but not vaginal swabs even by day 14 (d, arrow). *, P < 0.05, Wilcoxon rank sum (liver versus lung or kidney in panel a or intestinal tissues versus stomach tissue in panel b). n = 4 or 5 mice for each time point. Data are from 3 separate experiments. The number of mice with detectable IFU in a given tissue/sample is indicated for the samples that were important for drawing conclusions.
DISCUSSION
Since the discovery of chlamydial spreading from the genital tract to the GI tract for long-lasting colonization (21), extensive efforts have been made to reveal the pathways. It was found that Chlamydia might spread via the blood circulation system, since the spreading was accompanied by the presence of chlamydial organisms in the blood or bacteremia and intravenously inoculated Chlamydia was localized to the GI tract (29). A recent study further reported a role of the spleen in the chlamydial spreading (30). In the current study, we present new experimental evidence to support the concept that genital/hematogenous Chlamydia may spread to the GI tract via multiple redundant pathways. First, following intravaginal inoculation, live Chlamydia was detected on the rectal swabs of mice with or without splenectomy, indicating that the spleen is not essential for genital Chlamydia to spread to the GI tract. Second, intravenously inoculated Chlamydia was also detected in the rectal swabs of mice with or without splenectomy, indicating that the spreading of hematogenous Chlamydia to the GI tract is also independent of the spleen. The hematogenous Chlamydia may enter the GI tract via a biological pathway, since the intravenously inoculated Chlamydia cannot contaminate the GI tract directly. Third, in the presence of spleen, the intravenously inoculated Chlamydia was predominantly recruited to the spleen within 12 h and then detected in the stomach lumen by 24 h, in the intestinal lumen by 48 h, and on rectal swabs by 72 h. These temporal and spatial relationships suggest a potential spleen-to-stomach pathway for hematogenous Chlamydia to reach the large intestine lumen. Fourth, the stomach luminal Chlamydia was not orally taken up by mice, since live Chlamydia was still detected in the stomach lumen but not esophagus tissue or lumens of mice housed under coprophagy-free condition. Thus, we propose a spleen-to-stomach pathway for Chlamydia to access to the GI tract. Finally, in the absence of spleen, hematogenous Chlamydia was predominantly recruited to the liver and then simultaneously detected in the intestinal lumen, suggesting a potential liver-to-intestine pathway for Chlamydia to reach the large intestine lumen. Thus, genital/hematogenous Chlamydia may have the ability to reach the large intestine lumen via multiple redundant pathways.
In normal mice, live chlamydial organisms rapidly spread to the spleen and other organs following inoculation either intravaginally (21, 36, 37) or intravenously (29). However, most chlamydial organisms are cleared within 2 weeks, while the organisms that have reached the lumen of the large intestine can maintain long-lasting colonization (21, 34, 38, 39). Chlamydia may spread from the genital tract to the GI tract via the blood circulatory system. Thus, intravenous inoculation has been used to investigate the mechanisms of chlamydial spreading to the GI tract (29). Once arriving in the GI tract, Chlamydia is restricted to the GI tract (34). The GI tract Chlamydia can be monitored by detecting live chlamydial organisms on rectal swabs (21). It was recently reported that the spleen might play a critical role in the chlamydial spreading from the genital tract to the GI tract (30). However, when mice with and without splenectomy were compared for the recoveries of live Chlamydia from the rectal swabs following intravaginal or intravenous inoculation, there was no significant difference in the time courses of live Chlamydia shedding from the GI tract between the two groups of mice (Fig. 1 and 2). Thus, the spleen is not essential for chlamydial spreading to the GI tract. This conclusion was validated by the observations made at the gastrointestinal tissue level. Live chlamydial organisms were still recovered from the large intestine lumens of the splenectomized mice. The apparent discrepancy between the report by Howe et al. (30) and our current findings might be caused by many factors, including the C. muridarum strains used for inoculating the mice and the sensitivity of the live-organism titration methods. Nevertheless, both studies have revealed an important role of the spleen in the chlamydial spreading. In the current study, it was found that the removal of the spleen delayed the spreading of the hematogenous Chlamydia to the GI tract. Live chlamydial organisms were first detected in the large intestine lumen of splenectomized mice at 72 h (Fig. 5), while the first appearance of live chlamydial organisms in the large intestine lumen of normal mice (with spleen) was at 48 h after intravenous inoculation (Fig. 3).
In the presence of the spleen, the hematogenous chlamydial organisms may use a spleen-to-stomach pathway to reach the large intestine lumen. This assumption was based on the observation that the intravenously inoculated Chlamydia was immediately enriched in the spleen, followed by the stomach lumen, after which the chlamydial organisms reached the lumen of the remaining GI tract. The detection of live chlamydial organisms in the stomach tissue and lumen by 24 h after intravenous inoculation was unexpected. This was unlikely to be due to oral uptake, since Chlamydia was delivered to mice via intravenous injection and there was no shedding of chlamydial organisms from either the GI tract or genital tract at this point in time. This conclusion was further validated using mice housed under coprophagy-free conditions. Mice that were positive for live chlamydial organisms in the stomach tissue and lumen did not display any live chlamydial organisms in the esophagus tissue or lumen, excluding the possibility of oral uptake. Once Chlamydia reached the stomach lumen, gastric Chlamydia is able to seed the rest of the GI tract (35), explaining the subsequent detection of live chlamydial organisms in the intestinal lumens and rectal swabs.
The next question is how the splenic Chlamydia reaches the stomach lumen. Intravenously injected bacteria have been shown to infect dendritic cells (DCs) in the spleen within 9 h after the injection (40), suggesting that DCs may serve as a vehicle transporting Chlamydia from the spleen to the stomach. This hypothesis is supported by the observations that Chlamydia could productively infect DCs (41). Further, depletion of CD11c+ DCs at least transiently delayed the spreading of Chlamydia to the large intestine lumen (30). The Chlamydia-laden DCs might be able to carry Chlamydia to the stomach tissue via the left gastroepiploic artery or the short gastric artery that originates from the branched ends of the splenic artery (42). The next step is for the Chlamydia-laden DCs to cross both the gastric capillary endothelial barrier and the gastric epithelial barrier to enter the stomach lumen. Although the specific mechanisms remain unknown, it is clear that once Chlamydia reaches the stomach lumen, the gastric Chlamydia is able to seed the rest GI tract downstream (35). It will be worth investigating how the intracellular Chlamydia alters the gene expression of host DCs to enable the Chlamydia-infected DCs to deliver chlamydial organisms into the stomach lumen.
In contrast, the splenectomized mice developed a different temporal and spatial distribution pattern of live chlamydial organisms following intravenous inoculation. In the absence of the spleen, most intravenously injected chlamydial organisms quickly entered the liver. Then, the organisms were simultaneously detected in multiple GI tract tissues and lumens. In particular, the yields of the live organisms recovered from different intestinal segments were significantly higher than that from the stomach. This distribution pattern appeared to suggest a liver-to-intestine pathway for the hematogenous Chlamydia to reach the large intestine lumen. Hematogenous bacteria are known to enter the bile duct via the portal-venous system (43), suggesting that the intravenously injected chlamydial organisms may also be able to use the biliary system to spread from the liver to the intestine lumen. However, many questions remain to be addressed, including whether Chlamydia enters the biliary system in free form or by hiding inside host cells and how Chlamydia survives the biliary defense system. Chlamydia is known to survive in the small intestine for about 1 month (33), which provides plenty of opportunities for small intestinal Chlamydia to seed the large intestine lumen to achieve long-lasting colonization.
We are aware of the limitations of using chlamydial temporal and spatial distribution patterns to assign chlamydial spreading pathways. This is because both the extent of blood flow and susceptibility to chlamydial infection in an organ/tissue may affect the chlamydial organism distribution. More importantly, hematogenous Chlamydia may enter the GI tract lumen by many different pathways, including the conventional circulatory system, arteriovenous anastomoses (without going through the heart), and tissue-to-tissue penetration. The chlamydial organisms may traverse from the GI tract epithelial basal/lateral side to the luminal side by either crossing the tight junction or infecting the intestinal epithelial cells from the basal or lateral sides and then releasing the progeny into the luminal side. In the latter process, it may take longer for hematogenous Chlamydia to enter the intestinal lumen, since it requires Chlamydia to complete its intracellular replication cycle in the enteric epithelial cells. Consistently, chlamydial inclusions were localized in the intestinal epithelial cells (39). Obviously, more efforts are required to illuminate the molecular and cellular pathways by which Chlamydia selectively reaches the lumen of the GI tract but not that of other mucosal tissues.
We are also aware that the knowledge obtained from the mouse model of infection with C. muridarum may not necessarily be transferable to human infection with Chlamydia trachomatis. In the murine system, mucosally inoculated Chlamydia muridarum organisms are known to spread systemically (21, 36), providing the opportunities for chlamydial organisms to reach the large intestine lumen. A spleen-dependent pathway may mediate the first wave of spreading followed by spleen-independent pathways. There may be a high selection pressure for Chlamydia muridarum to use multiple redundant pathways to reach the large intestine lumen, since reaching the large intestine lumen allows this organism to both establish long-lasting colonization in the infected hosts and be efficiently transmitted to other hosts via the fecal-oral route. However, there is no direct evidence that sexually transmitted C. trachomatis can also undergo systemic spreading. Nevertheless, both Chlamydia trachomatis and Chlamydia pneumoniae have been shown to infect human monocytes (44) and chlamydial organisms/inclusions have been isolated/detected in peripheral blood mononuclear cells from healthy blood donors (45, 46). Thus, C. trachomatis may also possess the ability to undergo systemic spreading in women (47, 48). Further, C. trachomatis has been shown to infect human enteroendocrine cells (49). C. trachomatis has been detected on rectal swabs of women who do not practice oral/anal intercourse (50), suggesting that these women may have acquired C. trachomatis in their GI tract via sexual behavior-independent pathways.
MATERIALS AND METHODS
Chlamydial organism growth.
Chlamydia muridarum clone G13.32.1, used in the current study, was derived from strain Nigg3 (GenBank accession no. CP009760.1) as described previously (14). G13.32.1 (BioProject accession no. PRJNA227769; Biosample accession no. SAMN03569056) retains the wild-type Nigg3 genome sequence; therefore, it is designated wild-type Chlamydia. Following a previously described protocol (51), chlamydial organisms were propagated in HeLa cells (human cervical carcinoma epithelial cells; ATCC catalog no. CCL2.1) and purified as elementary bodies (EBs). Aliquots of EBs were stored in sucrose-phosphate-glutamate (SPG; 220 mM sucrose, 12.5 mM phosphate, and 4 mM l-glutamic acid, pH 7.5) at −80°C until use.
Mouse infection.
Mouse experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (52). The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of the University of Texas Health Science Center at San Antonio.
EBs were used to inoculate 6- to 7-week-old female C57BL/6J mice (0006640; The Jackson Laboratory, Inc., Bar Harbor, ME.) intravaginally (21) or intravenously (29). For intravaginal inoculation, stock EBs diluted in 10 μl SPG containing 2 × 105 inclusion-forming units (IFU) were delivered to the ectocervix area using a 20-μl micropipette tip. Five days prior to inoculation, each mouse was injected subcutaneously with 2.5 mg Depo-Provera (Pharmacia Upjohn, Kalamazoo, MI) suspended in sterile phosphate-buffered saline (PBS). For intravenous inoculation, EBs diluted in 50 μl of SPG buffer containing 2 × 105 IFU were delivered to mice via retro-orbital injection as described previously (53). Briefly, mice were anesthetized with isoflurane and placed in left lateral recumbency with the head facing to the right. The mouse’s right eyeball was partially protruded from the eye socket by applying gentle pressure to the skin dorsal and ventral to the eye. A 27-gauge needle was carefully inserted, bevel down, at an angle of approximately 30°, into the medial canthus. The inoculum was then slowly and smoothly injected, after which the mouse was placed back into its cage for recovery. In some experiments, the spleen was surgically removed as described previously (54) 1 week before the inoculation. For splenectomy, mice were anesthetized with 2.5% isoflurane delivered in a stream of oxygen by a controlled precision vaporizer. The hair of the surgical site was removed, and the skin of the surgical site was disinfected three times with 70% ethanol. A 1-cm incision was made on the left posterior back of the mouse. Then, spleen was exteriorized, and the gastro-splenic ligament was cut with scissors to separate the spleen from the stomach. Splenic vessels were identified, isolated, and then ligated with 5-0 absorbable sutures. Blood vessels were transected distal to the ligature. The incision was closed by surgical clips. In some experiments, mice wearing collars were housed singly to prevent coprophagy as described previously (21).
Titrating live chlamydial organisms recovered from swabs and tissue homogenates.
Following the inoculation as described above, both vaginal and rectal swabs were taken periodically or organs/tissues were harvested (after mice were sacrificed) for titrating viable organisms as described previously (21, 29). To quantitate live chlamydial organisms in vaginal or rectal swabs, each swab was soaked in 0.5 ml of SPG and vortexed with glass beads, and the chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicate. The infected cultures were processed for immunofluorescence assay as described previously (55) and below. Inclusions were counted in five random fields per coverslip under a fluorescence microscope. For coverslips with less than 1 IFU per field, entire coverslips were counted. Coverslips showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFU per swab was calculated based on the mean number of IFU per view, the ratio of the view area to that of the well, dilution factor, and inoculation volumes. Where possible, a mean number of IFU/swab was derived from the serially diluted and duplicate samples for any given swab. The total number of IFU/swab was converted into log10 for calculating the mean and standard deviation across mice in the same group at each time point.
For quantitating live organisms from mouse organs and tissue segments, each organ or tissue segment was transferred to a tube containing 0.5 to 5 ml SPG depending the sizes of the organs. GI tract tissues include esophagus, stomach, small intestine (duodenum, jejunum, and ileum), and large intestine (cecum, colon, and rectum), while each genital tract is divided into upper (ovary, oviduct, uterine horn, uterus, endocervix) and lower (ectocervix and vagina) genital tract tissues. The organs and tissue segments were homogenized in cold SPG using a 2-ml tissue grinder (K885300-0002; Fisher Scientific, Pittsburg, PA) or an automatic homogenizer (Omni tissue homogenizer, TH115; Omni International, Kennesaw, GA). The homogenates were briefly sonicated and spun at 3,000 rpm for 5 min to pellet remaining large debris. In some experiments, the luminal contents were collected from different segments of mucosal tissues using SPG, including esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum (rectal swab), and lower genital tract (vaginal swab), before the corresponding tissue was homogenized. After sonication, the supernatants were titrated for live C. muridarum organisms on HeLa cells as described above. The results were expressed as log10 IFU per organ or tissue segment/lumen.
Immunofluorescence assay.
The immunofluorescence assay for titrating live organisms was carried out as described previously (56). A rabbit antibody (R1604, raised with purified C. muridarum EBs) was used as a primary antibody to label all C. muridarum in HeLa cells, which was visualized with a goat anti-rabbit IgG conjugated with Cy2 (green, catalog no. 111-225-144; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The DNA dye Hoechst 3328 (blue; Sigma-Aldrich, St. Louis, MO) was used to visualize nuclei. The doubly labeled samples were used for counting for C. muridarum under a fluorescence microscope (AX70; Olympus) equipped with a charge-coupled device (CCD) camera (Hamamatsu).
Statistical analyses.
All data, including the time courses of live organism shedding (IFU), were compared using area under the curve (AUC) between two groups with the Wilcoxon rank sum test (an in-house Excel sheet), while category data, including number of mice positive for live organism shedding, were analyzed using Fisher's exact test (http://vassarstats.net/tab2x2.html).
ACKNOWLEDGMENTS
This study was supported in part by U.S. NIH grants (R01AI047997, R01AI121989, and R21AI151724 to G.Z.).
Contributor Information
Dabao Xu, Email: dabaoxu@yahoo.com.
Guangming Zhong, Email: Zhongg@UTHSCSA.EDU.
Craig R. Roy, Yale University School of Medicine
REFERENCES
- 1.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison RP, Feilzer K, Tumas DB. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 4.Murthy AK, Li W, Guentzel MN, Zhong G, Arulanandam BP. 2011. Vaccination with the defined chlamydial secreted protein CPAF induces robust protection against female infertility following repeated genital chlamydial challenge. Vaccine 29:2519–2522. doi: 10.1016/j.vaccine.2011.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. 2019. Sexually transmitted disease surveillance, 2019. U.S. Department of Health and Human Services, Atlanta, GA. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/STD-Trends-508.pdf. [Google Scholar]
- 7.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, Schenken RS, Zhong G. 2010. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P. Am J Obstet Gynecol 203:494.E7–494.E14. doi: 10.1016/j.ajog.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connell CM, Ingalls RR, AndrewsCW, Jr, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 12.Zhong G. 2017. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol 25:141–152. doi: 10.1016/j.tim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, Chen C, Zhou Z, Dai J, Zhong G. 2016. The chromosome-encoded hypothetical protein TC0668 is an upper genital tract pathogenicity factor of Chlamydia muridarum. Infect Immun 84:467–479. doi: 10.1128/IAI.01171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun 83:1881–1892. doi: 10.1128/IAI.03158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Conrad T, Zhou Z, Chen J, Dutow P, Klos A, Zhong G. 2014. Complement factor C5 but not C3 contributes significantly to hydrosalpinx development in mice infected with Chlamydia muridarum. Infect Immun 82:3154–3163. doi: 10.1128/IAI.01833-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Tian Q, Wang L, Xue M, Zhong G. 2017. IL-6-mediated signaling pathways limit Chlamydia muridarum infection and exacerbate its pathogenicity in the mouse genital tract. Microbes Infect 19:536–545. doi: 10.1016/j.micinf.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun 76:515–522. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igietseme JU, Omosun Y, Partin J, Goldstein J, He Q, Joseph K, Ellerson D, Ansari U, Eko FO, Bandea C, Zhong G, Black CM. 2013. Prevention of Chlamydia-induced infertility by inhibition of local caspase activity. J Infect Dis 207:1095–1104. doi: 10.1093/infdis/jit009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell J, Huang Y, Liu Y, Schenken R, Arulanandam B, Zhong G. 2014. Bioluminescence imaging of Chlamydia muridarum ascending infection in mice. PLoS One 9:e101634. doi: 10.1371/journal.pone.0101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. doi: 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong G. 2018. Chlamydia spreading from the genital tract to the gastrointestinal tract; a two-hit hypothesis. Trends Microbiol 26:611–623. doi: 10.1016/j.tim.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian Q, Zhou Z, Wang L, Abu-Khdeir AH, Huo Z, Sun X, Zhang N, Schenken R, Wang Y, Xue M, Zhong G. 2020. Gastrointestinal coinfection promotes chlamydial pathogenicity in the genital tract. Infect Immun 88:e00905-19. doi: 10.1128/IAI.00905-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig AP, Kong FY, Yeruva L, Hocking JS, Rank RG, Wilson DP, Donovan B. 2015. Is it time to switch to doxycycline from azithromycin for treating genital chlamydial infections in women? Modelling the impact of autoinoculation from the gastrointestinal tract to the genital tract. BMC Infect Dis 15:200. doi: 10.1186/s12879-015-0939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters RP, Dubbink JH, van der Eem L, Verweij SP, Bos ML, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morre SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal Chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. doi: 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 26.Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. 2015. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 60:398–404. doi: 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 27.Musil K, Currie M, Sherley M, Martin S. 2016. Rectal chlamydia infection in women at high risk of chlamydia attending Canberra Sexual Health Centre. Int J STD AIDS 27:526–530. doi: 10.1177/0956462415586317. [DOI] [PubMed] [Google Scholar]
- 28.Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. 2014. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 41:589–591. doi: 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 29.Dai J, Zhang T, Wang L, Shao L, Zhu C, Zhang Y, Failor C, Schenken R, Baseman J, He C, Zhong G. 2016. Intravenous inoculation with Chlamydia muridarum leads to a long-lasting infection restricted to the gastrointestinal tract. Infect Immun 84:2382–2388. doi: 10.1128/IAI.00432-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howe SE, Shillova N, Konjufca V. 2019. Dissemination of Chlamydia from the reproductive tract to the gastro-intestinal tract occurs in stages and relies on Chlamydia transport by host cells. PLoS Pathog 15:e1008207. doi: 10.1371/journal.ppat.1008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z, Xie L, Wang L, Xue M, Xu D, Zhong G. 2020. Effects of immunomodulatory drug fingolimod (FTY720) on Chlamydia dissemination and pathogenesis. Infect Immun 88:e00281-20. doi: 10.1128/IAI.00281-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong G. 2021. Chlamydia overcomes multiple gastrointestinal barriers to achieve long-lasting colonization. Trends Microbiol doi: 10.1016/j.tim.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin H, He C, Koprivsek JJ, Chen J, Zhou Z, Arulanandam B, Xu Z, Tang L, Zhong G. 2019. Antigen-specific CD4(+) T cell-derived gamma interferon is both necessary and sufficient for clearing Chlamydia from the small intestine but not the large intestine. Infect Immun 87:e00055-19. doi: 10.1128/IAI.00055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Zhang Q, Zhang T, Zhang Y, Zhu C, Sun X, Zhang N, Xue M, Zhong G. 2016. The Chlamydia muridarum organisms fail to auto-inoculate the mouse genital tract after colonization in the gastrointestinal tract for 70 days. PLoS One 11:e0155880. doi: 10.1371/journal.pone.0155880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T, Huo Z, Ma J, He C, Zhong G. 2019. The plasmid-encoded pGP3 promotes Chlamydia evasion of acidic barriers in both stomach and vagina. Infect Immun 87:e00844-18. doi: 10.1128/IAI.00844-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry LL, Hughes S. 1999. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun 67:3686–3689. doi: 10.1128/IAI.67.7.3686-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li LX, McSorley SJ. 2013. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS Pathog 9:e1003707. doi: 10.1371/journal.ppat.1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. 2013. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis 68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Zhu C, Zhang T, Tian Q, Zhang N, Morrison S, Morrison R, Xue M, Zhong G. 2018. Nonpathogenic colonization with chlamydia in the gastrointestinal tract as oral vaccination for inducing transmucosal protection. Infect Immun 86:e00630-17. doi: 10.1128/IAI.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoshi T, Zinselmeyer BH, Konjufca V, Lynch JN, Zhang X, Koide Y, Miller MJ. 2008. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity 29:476–486. doi: 10.1016/j.immuni.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Rey-Ladino J, Jiang X, Gabel BR, Shen C, Brunham RC. 2007. Survival of Chlamydia muridarum within dendritic cells. Infect Immun 75:3707–3714. doi: 10.1128/IAI.01618-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Malley J, Bordoni B. 2021. Anatomy, abdomen and pelvis, stomach gastroepiploic artery. StatPearls, Treasure Island, FL. [PubMed] [Google Scholar]
- 43.Sung JY, Shaffer EA, Olson ME, Leung JW, Lam K, Costerton JW. 1991. Bacterial invasion of the biliary system by way of the portal-venous system. Hepatology 14:313–317. doi: 10.1002/hep.1840140218. [DOI] [PubMed] [Google Scholar]
- 44.Marangoni A, Bergamini C, Fato R, Cavallini C, Donati M, Nardini P, Foschi C, Cevenini R. 2014. Infection of human monocytes by Chlamydia pneumoniae and Chlamydia trachomatis: an in vitro comparative study. BMC Res Notes 7:230. doi: 10.1186/1756-0500-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaguchi H, Yamada M, Uruma T, Kanamori M, Goto H, Yamamoto Y, Kamiya S. 2004. Prevalence of viable Chlamydia pneumoniae in peripheral blood mononuclear cells of healthy blood donors. Transfusion 44:1072–1078. doi: 10.1111/j.1537-2995.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 46.Cirino F, Webley WC, West C, Croteau NL, AndrzejewskiC, Jr, Stuart ES. 2006. Detection of Chlamydia in the peripheral blood cells of normal donors using in vitro culture, immunofluorescence microscopy and flow cytometry techniques. BMC Infect Dis 6:23. doi: 10.1186/1471-2334-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dan M, Tyrrell LD, Goldsand G. 1987. Isolation of Chlamydia trachomatis from the liver of a patient with prolonged fever. Gut 28:1514–1516. doi: 10.1136/gut.28.11.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter JD, Hudson AP. 2017. Recent advances and future directions in understanding and treating Chlamydia-induced reactive arthritis. Expert Rev Clin Immunol 13:197–206. doi: 10.1080/1744666X.2017.1233816. [DOI] [PubMed] [Google Scholar]
- 49.Dlugosz A, Muschiol S, Zakikhany K, Assadi G, D'Amato M, Lindberg G. 2014. Human enteroendocrine cell responses to infection with Chlamydia trachomatis: a microarray study. Gut Pathog 6:24. doi: 10.1186/1757-4749-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foschi C, Zagarrigo M, Belletti M, Marangoni A, Re MC, Gaspari V. 2020. Genital and extra-genital Chlamydia trachomatis and Neisseria gonorrhoeae infections in young women attending a sexually transmitted infections (STI) clinic. New Microbiol 43:115–120. [PubMed] [Google Scholar]
- 51.Mukhopadhyay S, Clark AP, Sullivan ED, Miller RD, Summersgill JT. 2004. Detailed protocol for purification of Chlamydia pneumoniae elementary bodies. J Clin Microbiol 42:3288–3290. doi: 10.1128/JCM.42.7.3288-3290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 53.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. 2011. Retro-orbital injections in mice. Lab Anim (NY) 40:155–160. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li SC, Rangel AD, Kabeer MH. 2019. Precision technique for splenectomy limits mouse stress responses for accurate and realistic measurements for investigating inflammation and immunity. Bio Protoc 9:e3317. doi: 10.21769/BioProtoc.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8:e71649. doi: 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]