ABSTRACT
The Chagas disease parasite Trypanosoma cruzi must extravasate to home in on susceptible cells residing in most tissues. It remains unknown how T. cruzi undertakes this crucial step of its life cycle. We hypothesized that the pathogen exploits the endothelial cell programming leukocytes use to extravasate to sites of inflammation. Transendothelial migration (TEM) starts after inflammatory cytokines induce E-selectin expression and P-selectin translocation on endothelial cells (ECs), enabling recognition by leukocyte ligands that engender rolling cell adhesion. Here, we show that T. cruzi upregulates E- and P-selectins in cardiac ECs to which it binds in a ligand-receptor fashion, whether under static or shear flow conditions. Glycoproteins isolated from T. cruzi (TcEx) specifically recognize P-selectin in a ligand-receptor interaction. As with leukocytes, binding of P-selectin to T. cruzi or TcEx requires sialic acid and tyrosine sulfate, which are pivotal for downstream migration across ECs and extracellular matrix proteins. Additionally, soluble selectins, which bind T. cruzi, block transendothelial migration dose dependently, implying that the pathogen bears selectin-binding ligand(s) that start transmigration. Furthermore, function-blocking antibodies against E- and P-selectins, which act on endothelial cells and not T. cruzi, are exquisite in preventing TEM. Thus, our results show that selectins can function as mediators of T. cruzi transendothelial transmigration, suggesting a pathogenic mechanism that allows homing in of the parasite on targeted tissues. As selectin inhibitors are sought-after therapeutic targets for autoimmune diseases and cancer metastasis, they may similarly represent a novel strategy for Chagas disease therapy.
KEYWORDS: Chagas disease, Trypanosoma cruzi, E-selectin, P-selectin, L-selectin, transendothelial migration, sialic acid, sulfate, Chagas disease
INTRODUCTION
Trypanosoma cruzi is a protozoan parasite that causes incurable, chronic, debilitating Chagas disease (1–3). The infection typically starts after the bite of blood-sucking reduviid insects, which releases metacyclic trypomastigotes on the skin after a blood meal (4). The infection becomes patent if T. cruzi gains access to the mucosa under skin keratinocytes (Chagoma) or conjunctiva (Romaña’s sign), where the pathogen invades susceptible host cells. T. cruzi generated at those infection sites spreads to the heart, brain, and most other organs of the body to invade and thrive in parenchymal cells, for example, cardiomyocytes in the heart and astrocytes in the central nervous system. T. cruzi dissemination necessitates the circulatory system where it resides transiently (parasitemia). Similar to leukocytes or metastatic cancer cells (5, 6), trypomastigotes must traverse the vascular endothelium to reach the tissue parenchyma to home in on susceptible cells. In the absence of transendothelial migration (TEM), T. cruzi will likely degenerate and perish in the circulation, as trypomastigotes are unable to differentiate and multiply in the blood (4, 7, 8).
How T. cruzi undergoes transendothelial migration remains unknown. It is well established that pathogens exploit physiological pathways of mammalian hosts to initiate and maintain infection, such as by using heparin-binding outer membrane proteins or survival receptors for host cell entry (9–12) and by manipulating T-lymphocyte checkpoint inhibitory receptors to neutralize cytotoxicity of CD8 T cells toward cells bearing intracellular pathogens (13, 14). Thus, it seems reasonable to think that T. cruzi, to complete its life cycle in mammalian hosts, takes advantage of molecular mechanisms already in place for leukocytes to migrate through the endothelium to inflammatory sites.
Neutrophils, monocytes, and effector T cells migrate from blood vessels into sites of injury to eliminate the primary inflammatory trigger and promote tissue repair (6, 15–17). For this, leukocytes must undergo TEM, which is comprised of a cascade of discrete steps driven by complex molecular interactions between circulating immune cells and the vascular endothelium. The first step involves cell-cell recognition that reduces the speed of leukocytes on the endothelial surface at inflammatory sites (rolling), allowing subsequent adhesion, crawling, and migration across endothelial cells and extracellular matrix proteins. Rolling results from the binding of leukocytes to selectins, which are Ca2+-dependent transmembrane lectins located on the surface of the vascular endothelium, to cognate ligands present on the leukocyte surface, of which the most important is the sialomucin P-selectin glycoprotein ligand 1 (PSGL-1) (18, 19). Deficiency in rolling reduces transmigration, consequently increasing the rate of infections and tissue injury (20).
Mammalians express three selectins: E-selectin, expressed by endothelial cells after inflammatory cytokine activation, binds to ligands on most leukocytes; P-selectin, stored in α-granules of platelets and Weibel-Palade bodies of endothelial cells and quickly translocated to the cell surface upon cell activation; and L-selectin, expressed by most leukocytes, binds to ligands on some endothelial cells and most leukocytes. All three selectins bind to the tetrasaccharide sialyl Lewis-x (sLex), NeuAcα2-3Galβ1-4[Fucα1-3]GlcNAcβ1-R, and its isomer sialyl Lewis-a (sLea), (NeuAcα2-3Galβ1-3[Fucα1-4]GlcNAcβ1-R) (15, 18), located at the nonreducing end of carbohydrate chains at the tip of the PSGL-1 structure. Sialic acid (NeuAc) is essential for binding, as is fucose, which forms pivotal interactions with the Ca2+ coordination site in the lectin domain of selectins (19). The critical role of PSGL-1 recognition by E- and P-selectins is underscored in patients with leukocyte adhesion deficiency type II (LAD-II) who display increased bacterial infections of the mucosa and skin (21) due to PSGL-1 hypofucosylation resulting from a mutation in the fucose transporter gene (22).
We show here that T. cruzi strongly upregulates E- and P-selectins on endothelial cells, binds to soluble recombinant E-, P-, and L-selectins in a dose-dependent and saturable manner under static conditions, and binds to P-selectin immobilized on glass substratum subjected to hydrodynamic conditions, as visualized and quantified by video microscopy. Like leukocytes (16, 23), T. cruzi binding to P-selectin requires sialic acid and sulfate, without which T. cruzi is inefficient in undertaking the transendothelial migration process. Soluble E-, P-, and L-selectins ultimately competitively inhibit transendothelial migration of T. cruzi, as does function-blocking antibodies against E- or P-selectin. Our results support the hypothesis that T. cruzi exploits selectins as a prelude to migrating across the vascular endothelium and extracellular matrix proteins.
RESULTS
T. cruzi induces and upregulates the expression of endothelial cell selectins to which it binds in a ligand-receptor type interaction.
To begin to test the hypothesis that T. cruzi mimics known mechanisms for transendothelial migration of leukocytes, we activated ex vivo mouse cardiac ECs by brief exposure (3 h) to a low dose of T. cruzi (multiplicity of infection [MOI], 1) and then assessed selectin transcripts by transcriptome sequencing (RNA-seq). T. cruzi does induce E-selectin expression and, additionally, upregulates P-selectin by 4.4-fold relative to uninfected ECs (Fig. 1A). These RNA-seq-based results confirm a previous Northern blot-based study by Huang et al. showing that T. cruzi induces expression of E-selectin in endothelial cells (24). To gain insight into a possible mechanism of the T. cruzi-driven increase in selectin gene expression, a role normally played by inflammatory cytokines, we checked whether the pathogen exerts an upregulating effect on cytokine production by endothelial cells. We find that T. cruzi specifically and robustly augments expression of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) in cardiac endothelial cells (ECs) as well as Toll-like receptor 2 (TLR2) but not of TLR3 and TLR4 (Fig. 1B). The T. cruzi-induced increase in TLR2 in ECs could provide a mechanism of how T. cruzi increases levels of TNF-α and IL-1β (see Discussion).
FIG 1.
T. cruzi augments expression of P- and E-selectins and of inflammatory cytokines and Toll-like receptor 2 on cardiac endothelial cells and binds to P-, E-, and L-selectins dose dependently and saturable under static conditions. (A) T. cruzi selectively upregulates selectins on cardiac endothelial cells. Confluent mouse cardiac endothelial cells and human cardiomyocytes (myocytes) were incubated for 3 h with Colombiana strain trypomastigotes (MOI, 1) or control vehicle PBS; monolayers were processed for RNA-seq (in triplicates). Results are expressed as fold increase relative to uninfected (vehicle treated) cells; <LOD, below the limit of detection. (B) T. cruzi selectively augments expression of TNF-α and IL-1β in cardiac endothelial cells (ECs) and of TLR2 in cardiac ECs. The protocol used is identical to that described above. (C) Time course of T. cruzi trypomastigotes (Colombiana strain) binding to P-selectin. Parasites were incubated for the indicated times with Fc-tagged human P-selectin. T. cruzi-bound P-selectin-Fc was identified by chemiluminescence Western-blotted T. cruzi lysates using horseradish peroxidase-labeled secondary anti-Fc antibody. (D) Dose-response of T. cruzi binding to P-selectin. The protocol used here is the same as that for panel C except for keeping incubation time constant (60 min), varying the P-selectin dosage, and visualizing and quantifying T. cruzi-bound to P-selectin-Fc using the LI-COR Odyssey Western blotting system. (E) Dose-response of T. cruzi binding to E-selectin. Experimental protocol is similar to that described above. (F) Plot of T. cruzi binding to P-, E-, and L-selectins and lack of Fc-tagged human fibroblast growth factor receptor (FGFR). Panels C to E are representative of three independent experiments. (F) Data shown represent the means from three independent experiments. Error bars indicate SD.
A cell binding assay we developed earlier to study plant lectin binding to T. cruzi (25) was used to determine whether live T. cruzi specifically interacts with selectins. Exploratory results showed that the extracellular domain of E-, P-, and L-selectins, tagged with Ig Fc, bind to T. cruzi at 37°C or 4°C; however, binding in the cold showed fewer variations between samples than at 37°C (data not shown). Henceforth, all T. cruzi-binding assays were performed at 4°C. Time course shows that P-selectin binding plateaus after 30 to 60 min (Fig. 1C), and dose-response reveals that binding of P-selectin is dose dependent and saturable (Fig. 1D and F). A similar response is seen for E-selectin (Fig. 1E and F) and L-selectin (Fig. 1F). Selectin binding to T. cruzi was specific inasmuch as an unrelated receptor, fibroblast growth factor receptor (FGFR), did not detectably interact (Fig. 1F), consistent with previous results from our lab (9). Thus, cell-binding studies suggest that T. cruzi selectively recognizes both endothelial P- and E-selectins and leukocyte L-selectin. L-selectin binding is more robust, followed by P-selectin and E-selectin. It remains to be determined if these differences in selectin binding to T. cruzi mirror their role in T. cruzi extravasation or if the differences are simply a peculiarity of the cell-binding assay. These binding assays were performed under static conditions, suggesting T. cruzi can recognize selectin receptors in the endothelial cell vessel wall that can be exploited to extravasate into tissues.
T. cruzi binds P-selectin under flow conditions.
To gain insights into the possibility that circulating T. cruzi recognizes endothelial selectins under hydrodynamic conditions, we coated glass coverslips with P-selectin and subjected the selectin-coated surface to a T. cruzi-containing fluid stream. T. cruzi mobility was assessed by video microscopy. Under these conditions, the mobility of T. cruzi is significantly reduced on P-selectin surfaces relative to the bovine serum albumin (BSA) (vehicle) surface control (Fig. 2A and B), suggesting that T. cruzi flowing in the bloodstream interacts with selectins in the endothelium.
FIG 2.
T. cruzi subjected to shear flow reduces motility on P-selectin surface. (A) Visualization by video microscopy (20× objective) of T. cruzi Colombiana trypomastigotes flowing on BSA (vehicle) or human P-selectin (SELP) immobilized on glass. Lines depict the trajectory of individual trypomastigotes. (B) T. cruzi velocity quantified by Image J software. Each dot represents an individual pathogen. Data are derived from three independent experiments. Error bars indicate SD; Mann-Whitney U test was performed. ****, P < 0.001; ns, not significant. Figure S1 in the supplemental material displays T. cruzi trajectories for the duration of the video microscopy.
Molecular characteristics of P-selectin binding to T. cruzi resemble those governing interaction of selectins with leukocyte-derived PSGL-1.
P-, E-, and L-selectins have overlapping binding specificities, including recognition of sialic acid and fucose epitopes of the tetrasaccharide sialyl Lex or Lea that decorate the N-terminal tip of PSGL-1, located on the outer membrane of most leukocytes (19). Additionally, P- and L-selectins recognize a cluster of three tyrosine sulfate epitopes near the sialyl Lex or Lea of PSGL-1, substantially enhancing the affinity of the two selectins for PSGL-1 (26, 27). Because binding of selectins to their ligands requires Ca2+, metal chelation, for example, by EDTA, prevents selectin binding (28).
Those characteristics of endothelial cell-leukocyte interaction are also seen in the interaction of selectins with T. cruzi. Thus, binding of P-selectin-Fc (SELP-Fc) to T. cruzi (vehicle) is competitively inhibited by heterologous non-Fc-tagged E-selectin (SELE) (Fig. 3A), suggesting P-selectin and E-selectin bind to identical or similar carbohydrate structures on T. cruzi. In contrast, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), two endothelial cell adhesion molecules that also mediate leukocyte and cancer cell transmigration via protein-protein interaction (29, 30), do not interfere with the binding of selectin to T. cruzi (Fig. 3A), underscoring the specificity of T. cruzi recognition of selectins. Dose-response experiments reveal that SELE blocks SELP-Fc binding (dose dependently up to 100%) (Fig. 3B), suggesting that T. cruzi recognition of selectins is a ligand-receptor type of interaction.
FIG 3.
E-selectin, EDTA, plant lectins, neuraminidase, and sulfatase specifically inhibit the binding of P-selectin to T. cruzi. T. cruzi trypomastigotes (Tulahuen strain) were incubated without (vehicle) or with human SELE, ICAM-1, or VCAM-1 and then with SELP-Fc. Parasite-bound SELP-Fc was detected by chemiluminescent Western blotting using HRP-labeled anti-human Fc-specific antibodies. Band intensity was quantified using ImageJ and displayed as percent inhibition (bottom blot display). (B) Dose-response curve of T. cruzi-SELP binding inhibition by SELE. The protocol was the same as that described above. (C) T. cruzi trypomastigotes (Colombiana strain) were incubated with SELP-Fc with PBS (vehicle) or EDTA. The protocol was that described above except that SELP-Fc was detected by fluorescent Western blotting using the LI-COR Odyssey Western blotting system. (D) T. cruzi trypomastigotes (Colombiana strain) were incubated with five plant lectins having distinct sugar-binding specificities (WGA, wheat germ agglutinin; ConA, Concanavalin A; SNA, Sambuca nigra agglutinin; MAA, Maackia amurensis agglutinin; and UEA-1, Ulex europaeus agglutinin-1), followed by SELP-Fc. Results were obtained by the LI-COR Odyssey Western blotting system. (E) T. cruzi trypomastigotes (Colombiana strain) were incubated without (vehicle) or with Vibrio cholerae neuraminidase (NANAse) and P-selectin-Fc. Results were obtained by the LI-COR Odyssey Western blotting system. (F) T. cruzi trypomastigotes (Colombiana strain) were incubated without (vehicle) or with sulfatase (30 min) and P-selectin-Fc. Results were obtained by the LI-COR Odyssey Western blotting system. Panels A, B, C, E, and F are representative of three independent experiments. (D) Data shown represent the means from three independent experiments. Error bars indicate SD.
That selectins interact with T. cruzi through their carbohydrate-binding domain is also demonstrated by the inability of P-selectin to bind T. cruzi in the presence of EDTA (Fig. 3C), as Ca2+ is essential for selectin binding to carbohydrate structures. Plant lectin binding inhibition studies also corroborate the idea that binding of T. cruzi to SELP-Fc is mediated by carbohydrate-protein recognition, given the selective blockage by sialic acid-specific wheat germ agglutinin (WGA) and mannose-specific ConA (Fig. 3D). Also similarly to P-selectin binding to PSGL-1 on leukocytes (26, 27), sialic acid and sulfate determinants are essential for P-selectin recognition of T. cruzi, as removal of sialic acid and sulfate residues by treatment with V. cholerae neuraminidase (NANAse) and sulfatase completely annuls P-selectin–Fc interaction with T. cruzi (Fig. 3E and F).
Quantitation by ELISA of P-selectin binding to glycoconjugates isolated from TcEx reveals requirement for sialic acid and sulfate epitopes.
T. cruzi-derived glycoconjugates (TcEx) were isolated from ConA-Sepharose using medium conditioned from purified trypomastigotes in serum-free Dulbecco’s modified Eagle medium (DMEM) or trypomastigotes growing in serum-free DMEM overlaying infected Vero cells. Glycoconjugates from the T. cruzi extracts were isolated by affinity chromatography to ConA-Sepharose and elution with methyl-α-mannoside (see Materials and Methods). As preliminary results showed that selectin binding to TcEx purified from trypomastigotes was similar to that isolated from Vero cell overlay (data not shown), results presented here refer to a mixture of the two types of TcEx. We find that the extracellular domain of P-selectin (SELP) binds to TcEx dose dependently and in a saturable manner (Fig. 4A), akin to the binding of SELP to live T. cruzi (Fig. 1F). SELP binding to TcEx glycoproteins is specific, since SELP does not bind SHAM extract glycoproteins (derived from uninfected Vero cells) (VeroEx), and fibroblast growth factor receptor (FGFR) does not interact with TcEx (Fig. 4A). As a positive control, SELP binds to its natural ligand, PSGL-1 (Fig. 4B), and the binding is abolished by treatment with Vibrio cholerae neuraminidase or tyrosine sulfatase (Fig. 4C), similar to P-selectin binding to PSGL-1 (31). Likewise, binding of SELP to TcEx also requires sialic acid and sulfate, regardless of whether the enzymes are present during the time SELP binds to TcEx (Fig. 4D) or after the enzymes were removed from plate-bound TcEx (Fig. 4E). Thus, these results suggest that inhibition of SELP binding to TcEx results from action of the neuraminidase and sulfatase on substrates present in T. cruzi (TcEx). Additionally, both E-selectin (SELE) and L-selectin (SELL) significantly inhibit binding of P-selectin to TcEx (Fig. 4F), indicating not only that SELE and SELL bind TcEx but also that SELE and SELL recognize the same epitopes SELP interacts with in TcEx. This conclusion is in keeping with SELE inhibiting SELP binding to intact parasites (Fig. 3A). In sum, selectins binds to T. cruzi-derived soluble proteins in a specific, ligand-receptor manner, similar to their binding to intact pathogen, further suggesting that T. cruzi bears selectin ligands.
FIG 4.
Binding of P-, E-, and L-selectins to glycoproteins/mucins isolated from T. cruzi reproduces molecular characteristics of selectin binding to live parasite. (A) Ninety-six-well plates were coated with TcEx or VeroEX and incubated with the indicated amount of SELP or FGFR. The plot was made using nonlinear regression. (B) Human PSGL-1 as a positive control or TcEx as experimental subject and incubated with SELP. (C) Human PSGL-1 incubated with SELP with or without treatment with V. cholerae neuraminidase (NANAse) or tyrosine sulfatase. (D) TcEx incubated with SELP with or without V. cholerae neuraminidase (SELP + NANAse) or sulfatase (SELP + Sulfatase). (E) TcEx treated with NANAse or sulfatase, washed to remove the enzymes, and then incubated with SELP. (F) TcEx with vehicle, SELE, or SELL and subsequently with SELP. In all experiments, plate-bound P-selectin was identified and quantified using biotinylated anti-human P-selectin antibody, followed by streptavidin-HRP and TMB substrate. Results are combinations of two, three, or four independent experiments, with each point representing one well. Data are presented as the means. Error bars indicate SD. One-way ANOVA was used. *, P ≤ 0.05; **, P ≤ 0.001; ****, P ≤ 0.0001.
T. cruzi entry into human and mouse endothelial cell monolayers is competitively blocked by soluble selectins in a sialic acid-dependent manner.
Next, we sought to determine whether T. cruzi uses selectin receptors to enter endothelial cells using competitive inhibition under nontransmigrating conditions, because, if it does, it will suggest that the interaction leads to transendothelial migration. For this, we seeded primary mouse cardiac endothelial cells (mcECs) or human umbilical vein endothelial cells (HUVEC) on a flat plastic surface of 96-well plates and exposed the endothelial cells to T. cruzi trypomastigotes for a brief period of time (3 h). These assays were similar to those by Tanowitz et al. that studied endothelial responses to T. cruzi infection (32–34). We noticed that T. cruzi preferentially invades confluent mouse cardiac endothelial cells (mcECs) relative to nonconfluent cells (Fig. 5A and B), that T. cruzi entry into confluent HUVEC is blocked by soluble P-selectin (SELP) dose dependently (Fig. 5C), and that the blockage is specific given the lack of inhibitory effect by fibroblast growth factor receptor (FGFR) (Fig. 5C). Similarly, soluble E-selectin (SELE) blocked T. cruzi invasion of mouse cardiac cells (mcECs) (Fig. 5D). What is more, removal of sialic acid from T. cruzi by V. cholerae neuraminidase (NANAse) neutralizes the cell entry inhibition of SELE (Fig. 5E). Thus, these findings suggest that T. cruzi interacts with endothelial selectins and that the interaction results in pathogen entry into the endothelial cells in a sialic acid-dependent manner. However, it is not clear if T. cruzi invasion of endothelial cells seeded on a solid substratum, such as the case of the results displayed in Fig. 5, reflects transendothelial migration.
FIG 5.
T. cruzi enters confluent endothelial cells preferentially compared to multiplying subconfluent cells, and entry in confluent endothelial cells is specifically inhibited by soluble P- and E-selectins in a sialic acid-dependent manner. Confluent and approximately 60% subconfluent mouse cardiac endothelial cells were infected with trypomastigotes of T. cruzi Tulahuen or CL Brenner strain (expressing dtTomato fluorescent protein) at an MOI of 20. After 3 to 4 days, cells were fixed with methanol and stained with Diff-Quik. Percent infection was calculated by optical microscopy. Infection by the Colombiana strain gave similar results (data not shown). (B) Visualization of a representative field of a microtiter well infected with CL Brener strain T. cruzi amastigotes inside the cells, seen as red dots. (C) Confluent monolayers (in triplicates) of human umbilical vein endothelial cells (HUVECs) were exposed to T. cruzi trypomastigotes (Colombiana strain, MOI of 20) for 3 h in the presence of PBS (Veh) or the indicated concentrations of P-selectin (SELP) or fibroblast growth factor receptor (FGFR) (5 μg/ml). T. cruzi entry into HUVECs was assessed 3 days later by counting >300 endothelial cells/well containing intracellular amastigotes. (D) Experimental protocol is as described above except for the use of SELE. (E) T. cruzi was allowed to enter mcECs in the absence or presence of SELE (5 μg/ml) without (T. cruzi + SELE) or with treatment with V. cholerae neuraminidase (Tc + NANAse + SELE). Results are combinations of three independent experiments. Data are presented as the means. Error bars indicate SD. Unpaired t test (A) or one-way ANOVA (C, D, and E) were used. *, P ≤ 0.05; **, P ≤ 0.001; ***, P < 0.005; ns, not significant.
Sialic acid and sulfate epitopes are required for T. cruzi to transmigrate through human endothelial cells.
We used Boyden chambers, widely employed in TEM studies in leukocyte and cancer cell research, to assess whether selectins contribute to transendothelial cell migration by T. cruzi (Fig. 6A). In this setup, porous inserts (3.0-μm holes) are coated with extracellular matrix (ECM) proteins (collagens plus fibronectin), to which HUVEC are seeded to confluence. Red fluorescent T. cruzi trypomastigotes are resuspended in HUVEC medium and added to inserts nested on 24-well plates containing 10% FBS. As trypomastigotes (∼2 to 3 μm by 20 to 25 μm) are inherently highly mobile, instead of adhering to the outer side of the insert, as leukocytes and cancer cells do (35), T. cruzi falls into and swims in the basolateral medium after transmigration. The use of fluorescent plate readers facilitates unbiased quantitation of transmigration. We find that upwards of 50% of input parasites can migrate through the HUVEC monolayer at 4 h, although the migration extent varies with the experiment.
FIG 6.
Sialic acid and sulfate epitopes are required for T. cruzi migration through endothelial cells and extracellular matrix proteins. (A) Boyden chamber transendothelial migration setup. The upper layer of sterile 3.0-μm pore inserts was coated with extracellular matrix (ECM) collagens (gelatin, 1 mg/ml) and fibronectin (0.5 μg/ml) and placed in a 24-well plate, to which primary human vein endothelial cells (HUVEC) were seeded at a density of 5 × 104 cells/transwell and grown overnight at 37°C in endothelial cell (EC) growth medium. T. cruzi trypomastigotes (Colombiana strain) were tagged with the fluorescent CellTracker red dye, resuspended in endothelial cell medium, and added (MOI, 10) to the monolayers without or with various treatments. After interaction with cell adhesion molecules (CAMS) such as selectins, parasites traverse the endothelial cell monolayer and extracellular matrix layer, penetrate the 3-μm pores, and then fall into the basolateral medium (10% FBS–DMEM) in the lower chamber of the transwell setup. T. cruzi that transmigrated to the lower chamber was measured using BioTek Synergy HT plate reader and quantified using a calibration curve constructed with predetermined doses of red-tagged trypomastigotes. Illustration was created using www.biorender.com. (B) Sialic acid and sulfate epitopes are critical for T. cruzi transendothelial migration. T. cruzi was treated with PBS (vehicle), V. cholerae neuraminidase (NANAse), sulfatase, or α-mannosidase (α-ManAse) and the mixtures added to the 24-well plate inserts (duplicates). Transendothelial migration was assessed at 2 h and 4 h. Numbers above bars represent percent inhibition relative to vehicle-treated cells set at 100% migration for two experiments with similar results. (C) Visualization by fluorescence microscopy of T. cruzi that transmigrated HUVEC after 2 h; before visualization, parasites were pelleted by centrifuging the 24-well plates at 2,000 × g for 10 min. Results are a combination of two independent experiments. Data are presented as the means. Error bars indicate SD. One-way ANOVA was used. *, P ≤ 0.05; **, P ≤ 0.001; ***, P < 0.005; ns, not significant.
Remarkably, treatment of T. cruzi with V. cholerae neuraminidase (NANAse) or sulfatase nearly totally prevents T. cruzi from traversing the confluent HUVECs and extracellular matrix (ECM) proteins, whether transmigration was measured at 2 h or 4 h (Fig. 6B). Abrogation of T. cruzi transendothelial migration by NANAse or sulfatase is specific, inasmuch as another glycosidase, α-mannosidase (α-ManAse), does not interfere (Fig. 6B). Visualization of the differential transendothelial migration of T. cruzi by NANAse and sulfatase, and lack thereof by α-mannosidase, is displayed in Fig. 6C.
Soluble selectins block T. cruzi transendothelial migration.
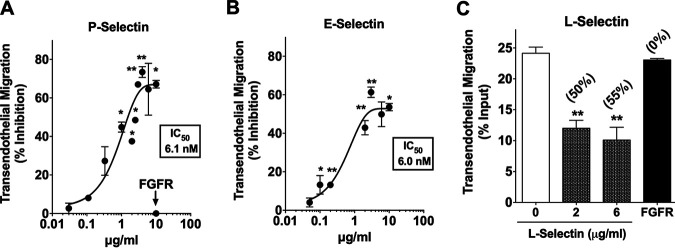
If T. cruzi exploits endothelial cell P- and E-selectins for extravasation, then water-soluble selectins, by binding to T. cruzi, should effectively compete with the binding of the pathogen to endothelium-bound selectins and, consequently, prevent transendothelial migration. Indeed, the recombinant extracellular domain of human P-selectin blocks T. cruzi transendothelial migration dose dependently and in a saturable manner, with a 50% inhibitory concentration (IC50) of 6.1 nM (Fig. 7A). Inhibition of T. cruzi transendothelial migration is specific, since fibroblast growth factor receptor (FGFR) is noninhibitory (Fig. 7A). Similarly, blockage of T. cruzi transendothelial migration by soluble human E-selectin is also dose dependent and saturable, with an IC50 of 6.0 nM, nearly equal to that of P-selectin (Fig. 7B). Noticeably, in preventing the majority of trypomastigotes from crossing the endothelial and extracellular matrix protein barrier, not all trypomastigotes are susceptible to the soluble selectin blockade (see Discussion). In addition to P- and E-selectins, soluble human L-selectin also effectively and specifically blocks transendothelial migration of T. cruzi (Fig. 7C). Although not expressed by endothelial cells, L-selectin has a sugar-binding specificity akin to that of P- and E-selectins, as it binds sialyl Lewisx or Lewisa, and, similar to P-selectin, it binds tyrosine sulfate (16, 23, 27); thus, it should competitively block T. cruzi transmigration. In sum, these results strongly support the hypothesis that T. cruzi recognition of endothelial selectins is pivotal for transendothelial migration.
FIG 7.
Soluble selectins are competitive inhibitors of T. cruzi transendothelial migration. (A, B, and C) T. cruzi trypomastigotes (Colombiana strain) were incubated with the indicated concentrations of the soluble extracellular domain of recombinant human P-selectin (A), E-selectin (B), or L-selectin (C). The mixtures were added to confluent HUVEC monolayers (in duplicates), and transendothelial migration was allowed to proceed for 2 h. Transmigrating parasites were quantified using a BioTek Synergy HT plate reader (see Fig. 6A). The plots are a composite of seven (A), four (B), and two (C) distinct experiments. Numbers in parentheses in panel C represent percent inhibition. Data are presented as the means. Error bars indicate SD. One-way ANOVA; *, P ≤ 0.05; **, P ≤ 0.001; ***, P < 0.005; ns, not significant.
Selectin blockade inhibits transendothelial migration of T. cruzi.
If selectins are enablers of T. cruzi transendothelial migration, then selectin blockade by established function-blocking antibodies, which are known to prevent migration of leukocytes, should likewise prevent T. cruzi from crossing the endothelial cell barrier. To test this prediction, we took advantage of two function-blocking antibodies that have been used by many investigators to ascertain the function of E- and P-selectin in leukocyte transendothelial migration. One of the antibodies is clone 9A9, a rat anti-mouse E-selectin IgG2b, which interferes with leukocyte transmigration under various in vivo conditions, for example, preventing T-cell rolling in postcapillary venules in the cremaster (36) and in the dermal vasculature (37). We tested whether clone 9A9 anti-E-selectin antibody prevents the migration of two biologically distinct strains of T. cruzi in the Boyden chamber assay. The Colombiana strain is cardiotropic and gives rise to slowly developing chronic infection, starting about 4 months postinfection (p.i.), as opposed to the Tulahuen strain, which quickly developed acute infection and started chronic infection within a few weeks p.i. (4). Akin to its effect on leukocyte transmigration, antibody clone 9A9 at 1 μg/ml inhibits transendothelial migration of both T. cruzi strains equally well (Fig. 8A). Dose-response experiments revealed that the inhibitory effect of clone 9A9 is specific, dose dependent, saturable (at 2 to 3 μg/ml), and a relatively potent inhibitor, as indicated by the IC50 of 1.7 nM (Fig. 8B) (see Discussion).
FIG 8.
Selectin blockade prevents transendothelial migration of T. cruzi. (A) Red-fluorescent T. cruzi trypomastigotes (Colombiana or Tulahuen strain) were mixed with vehicle (Veh) or 1 μg/ml anti-E-selectin function-blocking antibody clone 9A9, and the mixtures were added to confluent HUVEC on transwells (see Fig. 6A). After 2 h, parasites that transmigrated through the human endothelial cells and extracellular matrix proteins were quantified on a BioTek plate reader. (B) Dose-response of the inhibition of T. cruzi trypomastigote (Colombiana strain) transendothelial migration by anti-E-selectin MAb, clone 9A9 MAb (see the legend to Fig. 6A for protocol). (C) Dose-response of the inhibition of T. cruzi trypomastigote (Colombiana strain) transendothelial migration by anti-P-selectin MAb, clone AK4. (D) Red-fluorescent T. cruzi trypomastigotes (Colombiana strain) was mixed with 3 μg/ml anti-P-selectin function-blocking antibody clone AK4 or anti-E-selectin antibody clone 9A9, or with a mix of both antibodies each at 3 μg/ml, and added to confluent HUVEC monolayers. After 2 h, transmigrating parasites were quantified in the BioTek fluorescence plate reader; percent inhibition was calculated relative to parasites without antibodies set at 0% inhibition. (E) Visualization by fluorescence microscopy of T. cruzi transendothelial migration through HUVEC monolayer without (0) or with 3 μg/ml of AK4 and 3 μg/ml of 9A9 antibodies (3 + 3). Each dot or stick represents a swimming trypanosome in the basolateral medium that traversed the endothelial cell and extracellular matrix protein barrier. The data presented in panel A are a composite of two of five independent experiments (B and C). Data are presented as the means. Error bars indicate SD. One-way ANOVA was used. *, P ≤ 0.05; **, P ≤ 0.001; ns, not significant.
The other antibody we tested to determine whether it blocks T. cruzi Colombiana transendothelial migration is the anti-P-selectin antibody, clone AK4 (mouse IgG1), known to prevent leukocyte adhesive interaction with endothelial cells (38). We find that clone AK4 antibody, like anti-E-selectin antibody 9A9, competitively inhibits T. cruzi transendothelial migration dose dependently and in a saturable manner, approximately 80% inhibition at 3 to 5 μg/ml, during the 2-h observation time. Combining the anti-E-selectin and anti-P-selectin antibodies does not augment blockade of T. cruzi migration (Fig. 8D and E). The IC50 of the anti-P-selectin antibody is 2.5 nM (Fig. 8C), and, thus, is similarly as potent as the anti-E-selectin antibody clone 9A9 (Fig. 8B).
DISCUSSION
In this study, we provide evidence supporting the hypothesis that, to extravasate, T. cruzi exploits mechanisms that govern the migration of hematopoietic cells in the bloodstream en route to inflamed tissues, a phenomenon observed using three biologically distinct strains of the pathogen, suggesting that our observations apply to strains regardless of their biological activities. The first indication is that T. cruzi directly upregulates E- and P-selectins in cardiac endothelial cells (Fig. 1A). The significance of this finding is that E-selectin is normally lowly expressed in unactivated endothelial cells such that, for transendothelial migration of leukocytes to begin, E-selectin production must be induced first. P-selectin is stored inside Weibel-Palade bodies and, thus, must translocate to the endothelial surface, and its expression is further increased during transmigration. Selectin expression and translocation is accomplished by TNF-α and other inflammatory cytokines (39). It is unclear how T. cruzi upregulates E- and P-selectins, but one possibility is that the pathogen has the power to ramp up inflammatory cytokines in the absence of inflammation. T. cruzi does indeed induce upregulation of the inflammatory cytokines TNF-α and IL-1β and the innate immunity receptor TLR2 in endothelial cells in a noninflammatory setting (Fig. 1B). This result is consistent with earlier work showing that binding of T. cruzi-derived glycoinositolphospholipids to TLR2 on immune cells augments TNF-α and IL-1β (40). Hence, the possibility that T. cruzi augments selectin expression in endothelial cells by activating the TLR2 signaling pathway is consistent with parasite entry in most organs in the body before the development of inflammation that occurs during T. cruzi dissemination to systemic organs from chagoma or eye infection (4), during accidental laboratory infection of humans, for example, with contaminated needles (41, 42), or after intravenous injection in naive experimental animals (12).
The second indication is that T. cruzi does specifically bind the very selectins it upregulates on endothelial cells. This conclusion is grounded on cell binding assays (Fig. 1C to F) and enzyme-linked immunosorbent assay (ELISA) (Fig. 4). The cell-binding assay is based on a procedure we used earlier to characterize the binding of plant lectins to T. cruzi that led to identification sialic acid on the parasite (25). ELISA relies on glycoproteins isolated from materials shed from trypomastigotes suspended in serum-free DMEM or swimming on the overlay infected Vero cells (43); shed materials are solubilized in nonionic detergent, followed by affinity chromatography on ConA-Sepharose (see Materials and Methods). Cell binding assays performed under static or flow conditions revealed that the pathogen interacts with P-, E-, and L-selectin in a dose-dependent and saturable manner, indicating ligand-receptor recognition. Binding inhibition studies indicate that selectin binding is through carbohydrate-protein interaction (Fig. 3D). This conclusion is consistent with the inability of endothelial adhesion molecules ICAM-1 and VCAM-1 to compete with P-selectin binding (Fig. 3A), given that, during leukocyte migration, ICAM-1 and VCAM-1 recognize, not carbohydrate epitopes, but peptide sequences, even in heavily glycosylated mucins, such as in cancer (30, 44). Sialic acid is at least one of the carbohydrate epitopes P-selectin interacts with on T. cruzi (Fig. 3E), and sialic acid is an epitope E- and P-selectins recognize on leucocytes (28). Sulfate is another epitope P-selectin binds on T. cruzi or extract thereof (Fig. 3F and 4), similar to P-selectin binding to PSGL-1 on leukocytes (19, 27). T. cruzi interaction with P-, E-, and L-selectins occurs under static conditions and mimics recognition of selectin-expressing cells in the tissue parenchyma, and T. cruzi interacts with P-selectin under shear stress (Fig. 2), supporting the premise that circulating T. cruzi binds endothelial E- and P-selectins to initiate transendothelial migration.
The third sign of T. cruzi mimicry of transendothelial migration of leukocytes is that blockade of pathogen interaction with E- and P-selectins aborts transendothelial migration, as determined using various complementary approaches. Using a T. cruzi-endothelial cell assay in which endothelial cells are plated on an impermeable substrate that, therefore, does not simulate migration through endothelial monolayers and extracellular matrix proteins, T. cruzi enters ECs and differentiates into replicating amastigotes days after endothelial cell entry. Earlier studies by Coates et al. assessed T. cruzi migration on endothelial cells seeded on an impermeable substratum (45), akin to the one we use here. Under these conditions, T. cruzi enters confluent cardiac endothelial cells preferentially compared to nonconfluent endothelial cells (Fig. 5A and B). T. cruzi entry into ECs, whether human (HUVEC) or mouse (mcEC) endothelial cells, is P- and E-selectin dependent (Fig. 5C and D), suggesting that T. cruzi uses selectins to migrate across endothelial cells. This suggestion is supported by the requirement of T. cruzi sialic acid for selectin to prevent T. cruzi entry into endothelial cells (Fig. 5E), identical to the conclusion drawn from cell- and ELISA-based binding assays (Fig. 3 and 4). Next, we used the Boyden chamber assay that has been widely used for decades to mimic migration of leukocytes and cancer cells through the endothelium and extracellular matrix proteins (Fig. 6A). This way, we demonstrate that sialic acid and sulfate epitopes are required for T. cruzi to pierce through the endothelial cell barrier and extracellular matrix proteins (Fig. 6B and C). Not only that, we demonstrate that blockade of T. cruzi selectin ligands, using soluble selectins that bind the pathogen under static (Fig. 1C to F) or flow (Fig. 2) conditions, specifically blocks transendothelial migration (Fig. 7A to C).
Soluble P- and E-selectins block T. cruzi transendothelial migration with a very low and nearly identical IC50 of 6.1 nM and 6.0 nM (Fig. 7). This indicates that the two selectins are highly efficient in binding T. cruzi ligands to prevent initiation of the endothelial crossing process. This view is corroborated by the IC50 of anti-E- and anti-P-selectin antibodies clones 9A9 and AK4, which are 2.5- to 3.5-fold more potent than the selectins in the blockade of transendothelial migration (Fig. 8). Blockade of T. cruzi transendothelial migration, whether by soluble selectins or function-blocking antibodies, is orders of magnitude more sensitive than that in related systems, for example, in the inhibition of P-selectin binding to human monocytes by a peptide analogue, whose IC50 is 5 μM (46), and, thus, ∼2,000- and 819-fold lower affinity than soluble P-selectin and anti-P-selectin clone AK4 antibody blockade of T. cruzi transendothelial migration. However, the peptide analogue attenuates atherosclerosis in an experimental model (46). In another P-selectin-related study, the Fab antibody chimeric fragment abciximab inhibits platelet activation via GPIIb/IIIa with an IC50 of 100 nM (47), or concentration 58- and 40-fold higher than that by anti-selectin antibodies 9A9 and AK4 in preventing transendothelial cell migration of T. cruzi. Given that abciximab is a therapy used after coronary artery procedures, like angioplasty, to prevent platelet aggregation, it is tempting to speculate that, if blockade of selectin or T. cruzi-derived selectin ligands were to be used in the clinic for Chagas disease treatment, the concentration of the blockers of T. cruzi transendothelial migration should be orders of magnitude lower than that of the P-selectin peptide analogue or abciximab.
Finally, it is interesting that both anti-E-selectin (clone 9A9) and anti-P-selectin (clone AK4) antibodies prevent the majority (about 80%), but not all, of trypanosomes from migrating through endothelial cells within the 2-h time point (Fig. 8B and C). This implies that a subpopulation comprising ∼20% of the Colombiana T. cruzi trypomastigotes is resistant to the blockade by function-blocking antibodies against P- and E-selectins, including when anti-E and anti-P-selectin antibodies are combined (Fig. 8D and E). This suggestion is consistent with the inability of soluble selectins to totally abort transendothelial migration of T. cruzi (Fig. 7A and B). Thus, the possibility exists that homing of a subpopulation of T. cruzi via the vascular endothelium is not initiated by selectin recognition, as expected from the inherent heterogeneity of T. cruzi trypomastigotes, whether regarding morphology (variable proportions of slender and stumpy forms), rates of growth, length of intracellular cycle, amount of total DNA/parasite, expression of different antigens, and clone-to-clone variation in virulence (4, 48). However, it remains to be determined to what extent T. cruzi migrates across the endothelial cells using transcellular and/or paracellular routes.
In sum, our results imply that T. cruzi trypomastigotes express a yet-to-be-identified surface glycoconjugate(s) that E- and P-selectins recognize to initiate transendothelial migration. Interestingly, it has been found that L-selectin binding to T. cruzi-derived mucin, AgC10, inhibits T cell activation (49) and monocyte adherence (50), raising the possibility that T. cruzi, via L-selectin, can alter immune cell function in vivo. AgC10 could also serve as a binding partner for E- and P-selectins and affect transmigration. However, AgC10 was isolated from epimastigotes, a T. cruzi stage that replicates in reduviid insects, and is absent from infected mammalian hosts (4). Still, trypomastigotes could bear AgC10, as T. cruzi expresses hundreds of mucin genes in a stage-specific and in a stage-sharing manner, with a few them identified on a structure-based, not a function-based, approach (51–53). Our findings also raise the prospect of novel therapeutics for Chagas disease that work by preventing T. cruzi extravasation into tissues where they thrive intracellularly, an approach that will likely be helped by current drug discovery efforts for autoimmunity and cancer (54–56). Previous studies showed alteration of soluble P-selectin in patients with Chagas disease (57) and an association of soluble adhesion molecules such as VCAM-1 severity and stage of infection (58), underscoring the significance of studying the interaction of T. cruzi with adhesion molecules that mediate transendothelial migration.
MATERIALS AND METHODS
Reagents.
Human recombinant Fc-tagged P-selectin, E-selectin, and L-selectin were from R&D (137-PS-050, 724-ES-100, and 728-LS-100, respectively); non-Fc-tagged adhesion molecule E-selectin and mouse recombinant E-selectin were from BioLegend (148801); VCAM-1 and ICAM-1 were from Tonbo Biosciences (21-7178-U05, 21-7091-U050, and 21-7064-U050); PSGL-1-Fc was from Sino Biological; Fc-tagged fibroblast growth factor receptor 4 (FGFR) was from BioLegend (752504); plant lectins concanavalin A (ConA), Ulex europaeus agglutinin I (UEA 1), wheat germ agglutinin (Triticum vulgaris) (WGA), and Maackia amurensis agglutinin (MAA) were from GlycoMatrix (22070010-3, 21510130-1, 21510011-1, and 21510007-1, respectively); Vibrio cholerae neuraminidase (NANAse) was from Millipore Sigma (1080725001); Aerobacter aerogenes tyrosine O-sulfatase was from Millipore Sigma (S1629); α-mannosidase from Canavalia ensiformis (jackbean) was from Millipore Sigma (M7257); and human anti–E-selectin (clone 9A9) antibody was a gift from Francis W. Lucinskas, Harvard Medical School.
Parasites.
T. cruzi strains (Colombiana, Tulahuen, and CL-Brener) were obtained from Vero cells as previously described (9, 12). Trypomastigotes were harvested 3 to 4 days after the start of infection, purified by differential centrifugation mobility, and then resuspended in phosphate-buffered saline (PBS) or culture medium containing BSA or not. The CL Brener strain expresses dtTomato fluorescent protein and was a gift from Rick Tarleton via Roberto Docampo, University of Georgia (59).
TcEx.
TcEx was prepared by slightly modifying a procedure we used to isolate T. cruzi neuraminidase/trans-sialidase from trypomastigote-shed proteins (43). In short, conditioned medium was obtained by incubating T. cruzi trypomastigotes (Colombiana strain) (108/ml) in serum-free DMEM at 37°C for 5 to 8 h, clearing the conditioned medium of debris by centrifugation (2,000 × g, 10 min), and filtration on 0.22-μm membranes. Conditioned medium was stored at −20°C until use. After defrosting, conditioned medium was recentrifuged and refiltered, concentrated 50- to 70-fold on 10-kDa Amicon filters (number UFC901008), solubilized in 0.5% octyl-glucoside, and applied to a protein G-Sepharose column to remove residual immunoglobulin contaminants. Effluent glycoproteins were adsorbed to ConA-Sepharose and eluted with 0.2 M methyl-α-mannoside (MαM). MαM eluate, named TcEx, was equilibrated in PBS (pH 7.2) by multiple rounds of concentrations/dilutions in Amicon 10-kDa filters and concentrated 10× relative to the volume applied to ConA-Sepharose or 500- to 700-fold relative to the original 1× conditioned medium. TcEx was used after preparation or stored at −20°C after sterilization on 0.22-μm filters. We also obtained T. cruzi conditioned medium from medium conditioned in T. cruzi-infected Vero cell monolayers for 1 to 2 days in serum-free DMEM, and isolation of TcEx proceeded the same way as that for medium conditioned from trypomastigotes only. Protein concentration in TcEx was measured using a Pierce bicinchoninic acid (BCA) protein assay (23225; Thermo Fisher).
Isolation of primary cardiac endothelial cells.
Endothelial cells were isolated from the hearts of female C57BL/6J mice (2 to 6 months old; Jackson Laboratory). The mice were euthanized by CO2 inhalation, and their hearts were removed. To generate single-cell suspensions, hearts were processed with a gentle magnetic-activated cell sorting (MACS) dissociator (Miltenyi Biotech) per the manufacturer’s protocol and were incubated for 30 min at 37°C in Hanks balanced salt solution (HBSS) buffer containing type-2 collagenase (10,000 U/ml). Cells were plated in 0.2% gelatin-coated 6-well plates for 1 h to allow fibroblast adherence. Subsequently, unbound cells were collected and endothelial cells were positively selected by MACS using CD31-coated beads with (CD31 MicroBeads number 130-097-418; Miltenyi Biotec). The cells were grown in endothelial cell growth medium (CCM027; R&D).
Human-induced pluripotent stem cell-derived cardiomyocytes were purchased from Cellular Dynamics International (CMC-100-012-001) and grown by following the recommendations of the manufacturer.
RNA-seq.
mRNA from mouse cardiac endothelial cells and from human cardiomyocytes was isolated using TRIzol reagent, and RNA sequencing was performed in duplicate samples and triplicate biological replicates at the Tufts University Core Facility using the Illumina NextSeq 500 system.
ELISA.
Ninety-six-well plates (three to six wells per point) were coated with 5 μg/ml TcEx or PSGL1 (13863-H08H; Sino Biological) overnight at 4°C on a shaker. Nonspecific binding was blocked with 1% bovine serum albumin (BSA) for 1 h at room temperature (RT), followed by either (i) 1 μg/ml P-selectin (SELP), with or without treatment with Vibrio cholerae neuraminidase (NANAse) or sulfatase, or (ii) NANAse or sulfatase followed by 1 μg/ml of SELP. Subsequently, wells were incubated with 1 μg/ml of biotin anti-human SELP antibody (304914; BioLegend) for 1 h at RT. All reaction volumes were 100 μl in PBS, and plates were washed after each step with PBS–0.05% Tween 20. Binding of α-SELP was detected by using streptavidin-conjugated horseradish peroxidase (HRP) (1:200 dilution in PBS) for 1 h and then 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (34029; Thermo Fisher Scientific), and the reaction was stopped with 1 N HCl. The absorbance at 450 nm was measured in a plate reader (Biotek Microplate Synergy HT, VT, USA).
T. cruzi-selectin binding assay.
T. cruzi trypomastigotes were incubated with the indicated amounts of cell adhesion proteins in 0.2% BSA in DMEM (106/point) for 1 h at 4°C. Subsequently, they were washed 3 times (1,500 × g for 10 min) in 0.1% BSA in PBS or DMEM to remove unbound ligand. To visualize T. cruzi-bound selectin, parasites were resuspended in SDS sample buffer, run on SDS-PAGE, and transferred to a polyvinylidene membrane (PVDF; 1704156; Bio-Rad).
Fluorescent imaging.
The PVDF membrane was blocked with 5% BSA and probed for 1 h with 680RD goat anti-human IgG fluorescent antibody at RT (P/N 925–68078; LI-COR). Washing was done by following the manufacturer’s instructions and imaged and quantified using the Odyssey CLx infrared imaging system.
Chemiluminescent imaging.
The PVDF membrane was blocked with 5% milk for 1 h at RT and probed for 1 h with goat anti-human antibody in 5% milk at RT. The membrane was visualized using a charge-coupled device image sensor (Gel Doc XR+ system; Bio-Rad). Band intensity was quantified using ImageJ.
Flow chamber real-time video microscopy.
T. cruzi Colombiana trypomastigotes interactions with immobilized human P-selectin (10 μg/ml) or the control (BSA) were observed by video microscopy (×20 magnification) in a parallel plate apparatus using Nikon NIS-Elements software. T. cruzi was introduced as a bolus (9 × 105 in 150 μl) under shear stress and interacted with the adhesion molecule without shear for 3 min. Subsequently, a picture of the same field of view was taken every 5 s for 5 min (61 frames for each video). The velocity of T. cruzi on immobilized adhesion molecules was measured by tracking each T. cruzi organism for the duration of the 61 frames by using ImageJ.
T. cruzi infection of endothelial cells was performed as previously described (9). HUVECs (passage 2 to 12; CRL-1730; ATCC) or primary mouse cardiac endothelial cells were plated in 96-well plates at a density of either 4,000 cells/well or 35,000 cells/well and grown for 4 days to ensure confluence. T. cruzi trypomastigotes were incubated for 1 h with the indicated amounts of human recombinant proteins and enzymes at RT in 0.1% BSA in DMEM. Subsequently, they were washed 3× by centrifugation (1,500 × g for 10 min) to remove the unbound protein and added to the endothelial cells at an MOI of 20 for 3 h at 37°C. Following washing, to remove noninvaded parasites, cells were cultured for 3 or 4 days to allow T. cruzi intracellular replication. Cells were fixed with methanol and stained with hematoxylin and eosin (H&E; 22-122911; Fisher Scientific). Percent infection was calculated by optical microscopy by counting >300 cells/well.
TEM of T. cruzi.
The upper layer of sterile 3.0-μm-pore-size cell culture inserts (Falcon transwells, no. 353096) were coated with 0.1% gelatin plus 0.5 μg/ml fibronectin and placed in a 24-well plate. Subsequently, HUVEC, passage 2 to 12 (CRL-1730; ATCC) were plated at a density of 4 × 103 cells/insert and allowed to attach overnight. T. cruzi (Colombiana strain) was tagged with the fluorescent CellTracker red CMTPX dye (C34552; Thermo Fisher), incubated with vehicle PBS, NANAse (3,750 mU/ml), sulfatase (35 mU/ml), or α-mannosidase (3,750 mU/ml) for 30 min at RT, and added to the transwells at an MOI of 10. Ten percent FBS-DMEM was added to the lower chamber of the transwell, and transmigration was allowed to proceed for various times. HUVEC monolayer integrity was assessed by microscopy in H&E-stained wells (22-122911; Fisher Scientific). The 24-well plates were centrifuged (1,000 × g, 3 min), and the fluorescent signal intensity of the transmigrated parasites was measured with a BioTek Synergy HT plate reader.
Statistical analysis.
Statistical analyses were performed with GraphPad Prism 7.0 (GraphPad Software, Inc.) with two or three biological replicates. Statistical significance was determined with unpaired t test or Mann-Whitney U test when comparing two groups or one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison or Tukey’s posttest when comparing three or more groups. Data are expressed as the means ± standard deviations (SD) unless otherwise noted, and a P value of <0.05 was considered statistically significant. ns, P > 0.05; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
Data availability.
The raw data and analyzed data from this study were deposited in the NCBI GEO database under accession number GSE178073.
ACKNOWLEDGMENTS
We thank the staff of the 5th and 8th floors of the Jaharis building at Tufts Medical School for their technical support and many useful discussions.
This work was supported by grants from the National Institutes of Health (R01s AI113075 and AI09738 to M.A.P. and R21 AI142037 to M.A.P. and P.A.).
We have no conflict of interest to declare.
Contributor Information
Mercio A. Perrin, Email: Mercio.Perrin@tufts.edu.
Jeroen P. J. Saeij, UC Davis School of Veterinary Medicine
REFERENCES
- 1.Perez-Molina JA, Molina I. 2018. Chagas disease. Lancet 391:82–94. 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- 2.RassiA, Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.Prata A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 1:92–100. 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 4.Brener Z. 1973. Biology of Trypanosoma cruzii. Annu Rev Microbiol 27:347–382. 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- 5.Borsig L. 2018. Selectins in cancer immunity. Glycobiology 28:648–655. 10.1093/glycob/cwx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnoor M, Alcaide P, Voisin MB, van Buul JD. 2016. Recruitment of immune cells into inflamed tissues: consequences for endothelial barrier integrity and tissue functionality. Mediators Inflamm 2016:1561368. 10.1155/2016/1561368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caradonna KL, Burleigh BA. 2011. Mechanisms of host cell invasion by Trypanosoma cruzi. Adv Parasitol 76:33–61. 10.1016/B978-0-12-385895-5.00002-5. [DOI] [PubMed] [Google Scholar]
- 8.Nagajyothi F, Machado FS, Burleigh BA, Jelicks LA, Scherer PE, Mukherjee S, Lisanti MP, Weiss LM, Garg NJ, Tanowitz HB. 2012. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol 14:634–643. 10.1111/j.1462-5822.2012.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Melo-Jorge M, PereiraPerrin M. 2007. The Chagas' disease parasite Trypanosoma cruzi exploits nerve growth factor receptor TrkA to infect mammalian hosts. Cell Host Microbe 1:251–261. 10.1016/j.chom.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Weinkauf C, Salvador R, PereiraPerrin M. 2011. Neurotrophin receptor TrkC is an entry receptor for Trypanosoma cruzi in neural, glial, and epithelial cells. Infect Immun 79:4081–4087. 10.1128/IAI.05403-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega-Barria E, Pereira ME. 1991. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell 67:411–421. 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- 12.Ledoux T, Aridgides D, Salvador R, Ngwenyama N, Panagiotidou S, Alcaide P, Blanton RM, Perrin MA. 2019. Trypanosoma cruzi neurotrophic factor facilitates cardiac repair in a mouse model of chronic Chagas disease. J Pharmacol Exp Ther 368:11–20. 10.1124/jpet.118.251900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLane LM, Abdel-Hakeem MS, Wherry EJ. 2019. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 37:457–495. 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, Araki K, Ahmed R. 2018. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med 69:301–318. 10.1146/annurev-med-012017-043208. [DOI] [PubMed] [Google Scholar]
- 15.Vestweber D, Blanks JE. 1999. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 79:181–213. 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 16.McEver RP. 2015. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res 107:331–339. 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley K, Kansas GS. 2004. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol 4:325–335. 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 18.Zarbock A, Ley K, McEver RP, Hidalgo A. 2011. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118:6743–6751. 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings RD. 1999. Structure and function of the selectin ligand PSGL-1. Braz J Med Biol Res 32:519–528. 10.1590/s0100-879x1999000500004. [DOI] [PubMed] [Google Scholar]
- 20.Impellizzeri D, Cuzzocrea S. 2014. Targeting selectins for the treatment of inflammatory diseases. Expert Opin Ther Targets 18:55–67. 10.1517/14728222.2013.841140. [DOI] [PubMed] [Google Scholar]
- 21.Wild MK, Luhn K, Marquardt T, Vestweber D. 2002. Leukocyte adhesion deficiency II: therapy and genetic defect. Cells Tissues Organs 172:161–173. 10.1159/000066968. [DOI] [PubMed] [Google Scholar]
- 22.Luhn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. 2001. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet 28:69–72. 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- 23.McEver RP, Cummings RD. 1997. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 100:485–491. 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, Calderon TM, Berman JW, Braunstein VL, Weiss LM, Wittner M, Tanowitz HB. 1999. Infection of endothelial cells with Trypanosoma cruzi activates NF-kappaB and induces vascular adhesion molecule expression. Infect Immun 67:5434–5440. 10.1128/IAI.67.10.5434-5440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira ME, Loures MA, Villalta F, Andrade AF. 1980. Lectin receptors as markers for Trypanosoma cruzi. Developmental stages and a study of the interaction of wheat germ agglutinin with sialic acid residues on epimastigote cells. J Exp Med 152:1375–1392. 10.1084/jem.152.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppanen A, Yago T, Otto VI, McEver RP, Cummings RD. 2003. Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J Biol Chem 278:26391–26400. 10.1074/jbc.M303551200. [DOI] [PubMed] [Google Scholar]
- 27.Leppanen A, White SP, Helin J, McEver RP, Cummings RD. 2000. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem 275:39569–39578. 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- 28.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. 1992. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol 118:445–456. 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walling BL, Kim M. 2018. LFA-1 in T cell migration and differentiation. Front Immunol 9:e00952. 10.3389/fimmu.2018.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong DH, Kim YK, Kim MR, Jang JH, Lee S. 2018. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int J Mol Sci 19:1057. 10.3390/ijms19041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP. 1996. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem 271:6342–6348. 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- 32.Tanowitz HB, Gumprecht JP, Spurr D, Calderon TM, Ventura MC, Raventos-Suarez C, Kellie S, Factor SM, Hatcher VB, Wittner M. 1992. Cytokine gene expression of endothelial cells infected with Trypanosoma cruzi. J Infect Dis 166:598–603. 10.1093/infdis/166.3.598. [DOI] [PubMed] [Google Scholar]
- 33.Morris SA, Tanowitz H, Makman M, Hatcher VB, Bilezikian JP, Wittner M. 1992. Trypanosoma cruzi: alteration of cAMP metabolism following infection of human endothelial cells. Exp Parasitol 74:69–76. 10.1016/0014-4894(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee S, Huang H, Petkova SB, Albanese C, Pestell RG, Braunstein VL, Christ GJ, Wittner M, Lisanti MP, Berman JW, Weiss LM, Tanowitz HB. 2004. Trypanosoma cruzi infection activates extracellular signal-regulated kinase in cultured endothelial and smooth muscle cells. Infect Immun 72:5274–5282. 10.1128/IAI.72.9.5274-5282.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mastyugin V, McWhinnie E, Labow M, Buxton F. 2004. A quantitative high-throughput endothelial cell migration assay. J Biomol Screen 9:712–718. 10.1177/1087057104269495. [DOI] [PubMed] [Google Scholar]
- 36.Velazquez F, Grodecki-Pena A, Knapp A, Salvador AM, Nevers T, Croce KJ, Alcaide P. 2016. CD43 functions as an E-selectin ligand for Th17 cells in vitro and is required for rolling on the vascular endothelium and Th17 cell recruitment during inflammation in vivo. J Immunol 196:1305–1316. 10.4049/jimmunol.1501171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nacher M, Blazquez AB, Shao B, Matesanz A, Prophete C, Berin MC, Frenette PS, Hidalgo A. 2011. Physiological contribution of CD44 as a ligand for E-selectin during inflammatory T-cell recruitment. Am J Pathol 178:2437–2446. 10.1016/j.ajpath.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner MP, Lucas CM, Burns GF, Chesterman CN, Berndt MC. 1991. GMP-140 binding to neutrophils is inhibited by sulfated glycans. J Biol Chem 266:5371–5374. 10.1016/S0021-9258(19)67603-9. [DOI] [PubMed] [Google Scholar]
- 39.Schnoor M, Alcaide P, Voisin MB, van Buul JD. 2015. Crossing the vascular wall: common and unique mechanisms exploited by different leukocyte subsets during extravasation. Mediators Inflamm 2015:946509. 10.1155/2015/946509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos MA, Gazzinelli RT. 2004. Trypanosoma cruzi and its components as exogenous mediators of inflammation recognized through Toll-like receptors. Mediators Inflamm 13:139–143. 10.1080/09511920410001713565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herwaldt BL. 2001. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev 14:659–688. 10.1128/CMR.14.3.659-688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofflin JM, Sadler RH, Araujo FG, Page WE, Remington JS. 1987. Laboratory-acquired Chagas disease. Trans R Soc Trop Med Hyg 81:437–440. 10.1016/0035-9203(87)90162-3. [DOI] [PubMed] [Google Scholar]
- 43.Scudder P, Doom JP, Chuenkova M, Manger ID, Pereira ME. 1993. Enzymatic characterization of beta-D-galactoside alpha 2,3-trans-sialidase from Trypanosoma cruzi. J Biol Chem 268:9886–9891. 10.1016/S0021-9258(18)98428-0. [DOI] [PubMed] [Google Scholar]
- 44.Rahn JJ, Chow JW, Horne GJ, Mah BK, Emerman JT, Hoffman P, Hugh JC. 2005. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis 22:475–483. 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 45.Coates BM, Sullivan DP, Makanji MY, Du NY, Olson CL, Muller WA, Engman DM, Epting CL. 2013. Endothelial transmigration by Trypanosoma cruzi. PLoS One 8:e81187. 10.1371/journal.pone.0081187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye Z, Zhang S, Liu Y, Wang S, Zhang J, Huang R. 2019. A peptide analogue of selectin ligands attenuated atherosclerosis by inhibiting monocyte activation. Mediators Inflamm 2019:8709583. 10.1155/2019/8709583. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Caron A, Theoret JF, Mousa SA, Merhi Y. 2002. Anti-platelet effects of GPIIb/IIIa and P-selectin antagonism, platelet activation, and binding to neutrophils. J Cardiovasc Pharmacol 40:296–306. 10.1097/00005344-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Dvorak JA. 1984. The natural heterogeneity of Trypanosoma cruzi: biological and medical implications. J Cell Biochem 24:357–371. 10.1002/jcb.240240406. [DOI] [PubMed] [Google Scholar]
- 49.Alcaide P, Fresno M. 2004. The Trypanosoma cruzi membrane mucin AgC10 inhibits T cell activation and IL-2 transcription through L-selectin. Int Immunol 16:1365–1375. 10.1093/intimm/dxh138. [DOI] [PubMed] [Google Scholar]
- 50.Alcaide P, Lim YC, Luscinskas FW, Fresno M. 2010. Mucin AgC10 from Trypanosoma cruzi interferes with L-selectin-mediated monocyte adhesion. Infect Immun 78:1260–1268. 10.1128/IAI.00794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frasch AC. 2000. Functional diversity in the trans-sialidase and mucin families in Trypanosoma cruzi. Parasitol Today 16:282–286. 10.1016/s0169-4758(00)01698-7. [DOI] [PubMed] [Google Scholar]
- 52.Acosta-Serrano A, Almeida IC, Freitas-Junior LH, Yoshida N, Schenkman S. 2001. The mucin-like glycoprotein super-family of Trypanosoma cruzi: structure and biological roles. Mol Biochem Parasitol 114:143–150. 10.1016/s0166-6851(01)00245-6. [DOI] [PubMed] [Google Scholar]
- 53.Todeschini AR, de Almeida EG, Agrellos OA, Jones C, Previato JO, Mendonca-Previato L. 2009. Alpha-N-acetylglucosamine-linked O-glycans of sialoglycoproteins (Tc-mucins) from Trypanosoma cruzi Colombiana strain. Mem Inst Oswaldo Cruz 104(Suppl 1):270–274. 10.1590/s0074-02762009000900035. [DOI] [PubMed] [Google Scholar]
- 54.Patel MS, Miranda-Nieves D, Chen J, Haller CA, Chaikof EL. 2017. Targeting P-selectin glycoprotein ligand-1/P-selectin interactions as a novel therapy for metabolic syndrome. Transl Res 183:1–13. 10.1016/j.trsl.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. 2007. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets 11:1473–1491. 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Natoni A, Macauley MS, O'Dwyer ME. 2016. Targeting selectins and their ligands in cancer. Front Oncol 6:93. 10.3389/fonc.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laucella SA, Segura EL, Riarte A, Sosa ES. 1999. Soluble platelet selectin (sP-selectin) and soluble vascular cell adhesion molecule-1 (sVCAM-1) decrease during therapy with benznidazole in children with indeterminate form of Chagas' disease. Clin Exp Immunol 118:423–427. 10.1046/j.1365-2249.1999.01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laucella S, De Titto EH, Segura EL, Orn A, Rottenberg ME. 1996. Soluble cell adhesion molecules in human Chagas' disease: association with disease severity and stage of infection. Am J Trop Med Hyg 55:629–634. 10.4269/ajtmh.1996.55.629. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez A, Tarleton RL. 2012. Transgenic parasites accelerate drug discovery. Trends Parasitol 28:90–92. 10.1016/j.pt.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data and analyzed data from this study were deposited in the NCBI GEO database under accession number GSE178073.