ABSTRACT
Recent studies suggest an anti-inflammatory protective role for class B scavenger receptor BI (SR-BI) in endotoxin-induced inflammation and sepsis. Other data, including ours, provide evidence for an alternative role of SR-BI, facilitating bacterial and endotoxin uptake and contributing to inflammation and bacterial infection. Enhanced endotoxin susceptibility of SR-BI-deficient mice due to their anti-inflammatory glucocorticoid deficiency complicates the understanding of SR-BI’s role in endotoxemia/sepsis, calling for the use of alternative models. In this study, using human SR-BI (hSR-BI) and hSR-BII transgenic mice, we found that SR-BI and, to a lesser extent, its splicing variant SR-BII protect against LPS-induced lung damage. At 20 h after intratracheal LPS instillation, the extent of pulmonary inflammation and vascular leakage was significantly lower in hSR-BI and hSR-BII transgenic mice than in wild-type mice. Higher bronchoalveolar lavage fluid (BALF) inflammatory cell count and protein content and lung tissue neutrophil infiltration found in wild-type mice were associated with markedly (2 to 3 times) increased proinflammatory cytokine production compared to these parameters in transgenic mice following LPS administration. The markedly lower endotoxin levels detected in BALF of transgenic versus wild-type mice and the significantly increased BODIPY-LPS uptake observed in lungs of hSR-BI and hSR-BII mice 20 h after the i.t. LPS injection suggest that hSR-BI- and hSR-BII-mediated enhanced LPS clearance in the airways could represent the mechanism of their protective role against LPS-induced acute lung injury.
KEYWORDS: class B scavenger receptors, SR-BI, SR-BII, LPS, acute lung injury, proinflammatory cytokines, inflammation, lipopolysaccharide
INTRODUCTION
Class B scavenger receptor BI (SR-BI) and its splicing variant SR-BII are multifunctional receptors, mostly known for their critical role in lipoprotein metabolism (1–3). These receptors have also been implicated in host responses to various pathogen-associated molecular pattern molecules (PAMPs) (4–8) and damage-associated molecular pattern molecules (DAMPs) (6, 9). SR-BI’s role in sepsis, in particular, has recently been increasingly investigated, and several studies using SR-BI/-BII knockout mice demonstrated its protective role in cecal ligation and puncture (CLP)-induced sepsis and endotoxemia (4, 5, 7). In contrast, our recent studies utilizing gain-of-function models comprising transgenic mice with human SR-BI (hSR-BI) and hSR-BII expression revealed that both receptors are capable of mediating LPS uptake and contributing to LPS-induced proinflammatory signaling and tissue injury, thus supporting a potentially detrimental role of these receptors during endotoxemia (6, 8, 10). SR-B-deficient mice are characterized by an increased proinflammatory state and a compromised host response due to adrenal insufficiency. In addition, several metabolic and immunological disorders make this an inadequate model for sepsis-/endotoxemia-related studies (11–14). Sepsis-induced acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are significant sources of morbidity and mortality with no current treatments beyond supportive care (15–18). With the Toll-like receptor 4 (TLR-4)-mediated signaling pathway being established as the major contributor to host responses in various acute inflammation-associated syndromes, e.g., bacterial-LPS-induced systemic shock (19), sepsis (20, 21), and acute lung injury (22–24), it is likely that some key mediators contributing to ALI remain unidentified. The role of class B scavenger receptors in acute lung injury induced by pathogen infection has not been well defined thus far. Our previous study demonstrated that CD36, another class B scavenger receptor, could potentially mediate LPS-induced ALI, as peptides specifically targeting this receptor were shown to alleviate LPS-induced inflammation and lung injury in mice (25).
LPS, a major constituent of the outer membrane of Gram-negative bacteria, has been identified as a key risk factor for ALI and ARDS (18), and intratracheal (i.t.) instillation of LPS is widely used to model bacterially mediated acute lung injury. In the present study, using hSR-BI and hSR-BII transgenic mice, we assessed the potential effects of SR-BI and SR-BII human homologs in LPS-induced acute pulmonary inflammation and damage. Our findings revealed that, in contrast to the previously reported hSR-BI-/hSR-BII-dependent increase in proinflammatory response and tissue damage following intraperitoneal (i.p.) LPS administration (8), in the ALI model, both receptors contributed to markedly reduced pulmonary inflammation and lung injury. The results of this study suggest that an anti-inflammatory, protective role of hSR-BI and hSR-BII during acute lung injury could be attributed to their previously reported role in mediating LPS uptake and clearance (7, 26).
RESULTS
Tissue-specific expression of hSR-BI and hSR-BII in hSR-B transgenic mice.
Analyses of various tissues from hSR-BI and hSR-BII transgenic mice, generated using a liver-specific expression vector, revealed the highest mRNA expression of both hSR-BI and hSR-BII in the liver; it was also detected in kidney, lung, and spleen, although at levels 1 to 2 orders of magnitude lower (Fig. 1A). Correspondingly, all analyzed tissues, including, liver, kidney (8), and lung (Fig. 1B), from hSR-BI transgenic mice demonstrated 2- to 3-fold-higher protein expression of SR-BI than the tissues of wild-type animals when an antibody (Ab) recognizing both human and mouse homologs was used. While endogenous SR-BII protein was not detectable in any of the tested tissues from the wild-type mice, hSR-BII protein expression was clearly visible in all tested tissues, including lung from hSR-BII transgenic mice.
FIG 1.
Tissue-specific expression of hSR-BI and hSR-BII analyzed by qRT-PCR and Western blotting and hSR-B identification by immunofluorescence microscopy in lungs from hSR-BI and hSR-BII transgenic mice. (A) mRNA expression of hSR-BI and hSR-BII transgenes in various tissues of hSR-BI and hSR-BII transgenic mice relative to corresponding hepatic mRNA expression levels. Individual data points are plotted along with mean values ± standard deviations (SD) (n = 4 for each group). (B) Western blot analyses of hSR-BI and hSR-BII protein expression were performed in liver and lung tissue samples from WT (lanes 1 and 4), hSR-BI transgenic (lanes 2 and 5), and hSR-BII transgenic (lanes 3 and 6) mice. hSR-BI and hSR-BII protein expression was detected by using anti-human SR-BI and anti-human SR-BII custom antibodies against specific peptides from C-terminal domains. mSR-BI expression was detected by an anti-SR-BI antibody against a C-terminal peptide containing residues 450 to 509 from mouse SR-BI (catalog number NB400-101; Novus Biologicals). Protein expression of β-actin was measured as the loading control using anti-β-actin polyclonal antibody (Sigma-Aldrich). STD, standard. (C) hSR-BI and hSR-BII identification in lung epithelial cells by immunofluorescence assay. Frozen lung sections from hSR-B transgenic and WT (used as a negative control) mice were stained using anti-EpCAM antibody in combination with either anti-hSR-BI or anti-hSR-BII antibody for hSR-BI (top right) and hSR-BII (bottom right) mice, respectively, and with both anti-SR-B antibodies for WT (left images) mice, followed by incubation with the Alexa Fluor 488 (green)-/Alexa Fluor 568 (red)-conjugated secondary antibodies, according to the protocol described in Materials and Methods. Hoechst 33342 nucleic counterstain appears blue. Scale bars, 10 μM.
An immunofluorescence assay of lung tissue sections stained with antibody against the epithelial cell marker EpCAM (epithelial cell adhesion molecule) in combination with either anti-hSR-BI or anti-hSR-BII antibodies revealed a high presence of both transgene SR-Bs in alveolar epithelial cells (AECs) (Fig. 1C). These cells, lining the alveolar membrane surface, constitute physical and functional barriers that play a critical role in antimicrobial lung defense.
hSR-BI and hSR-BII mice are less susceptible to LPS-induced lung injury as assessed by inflammatory cell recruitment and vascular leakage.
To assess the potential effect of hSR-BI and hSR-BII upon LPS-induced lung injury, wild-type (WT) and hSR-BI and hSR-BII transgenic mice were infused i.t. with LPS or with phosphate-buffered saline (PBS), used as a vehicle.
Lung injury was evaluated by measurement of total cell count, total protein concentration, and concentration of polymorphonuclear (PMN) cells in bronchoalveolar lavage fluid (BALF) collected 20 h after LPS/PBS administration. In wild-type mice, an LPS challenge caused significant lung injury that was characterized by dramatic increases in both total cell and neutrophil counts in BALF, as well as by a marked increase in BALF protein content (Fig. 2). Significantly lower total and PMN cell counts and BALF protein content (P < 0.001) were observed in both groups of transgenic mice treated with LPS. A histological assessment of lung tissues from different groups of mice challenged with LPS revealed a significantly higher presence of neutrophils in lungs of wild-type animals than in lungs of hSR-BI and hSR-BII transgenic mice (Fig. 3), thus providing additional evidence of a protective role of SR-BI and SR-BII receptors in LPS-induced pulmonary injury.
FIG 2.
Cellular and protein analyses of bronchoalveolar lavage fluid after i.t. LPS challenge. WT, hSR-BI, and hSR-BII transgenic mice were infused i.t. with PBS (control; white symbols) or LPS (gray symbols), and after 20 h, the mice were sacrificed and BALF collected and analyzed for total cell count (A), PMN cell count (B), and total protein concentration (C). Individual data points are shown along with mean values ± SD (n = 4 or 5 for PBS-treated groups; n = 8 or 9 for LPS-treated groups). **, P < 0.01, and ***, P < 0.001, versus the results for WT LPS-treated mice.
FIG 3.
Histological assessment of lung injury in WT, hSR-BI, and hSR-BII transgenic mice induced by the i.t. LPS injection. Eighteen hours after the i.t. LPS (D and E) or PBS (A and B) injection, lungs were excised, fixed in 10% formalin, embedded in paraffin, sliced into 4-µm sections, and stained with H&E. Arrows indicate infiltrated neutrophils. Representative images of lung tissue from each group of mice (n = 3) are shown.
hSR-BI and hSR-BII mice are more resistant to LPS-induced pulmonary inflammation.
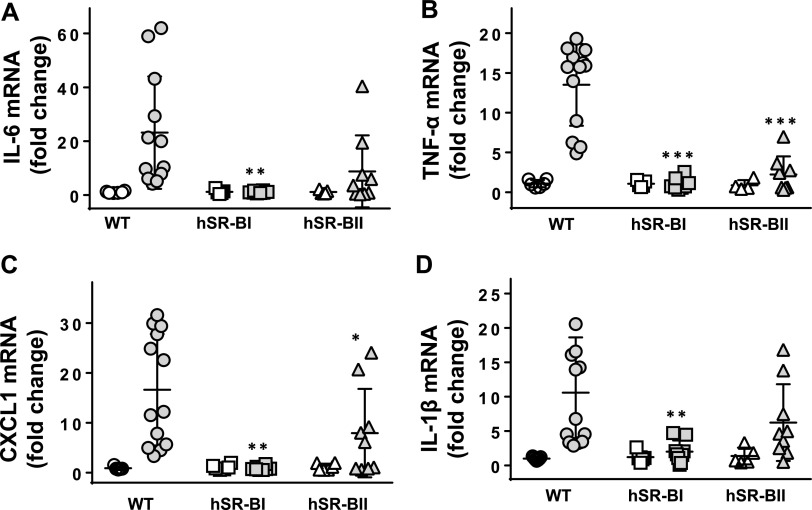
Acute lung injury is associated with significant pulmonary inflammation. To further evaluate the role of hSR-BI and hSR-BII in LPS-induced lung inflammation, the pulmonary expression levels of several proinflammatory mediators, as well as their concentrations in BALF, were assessed in all three groups of LPS-challenged mice. We have found that following LPS injection, cytokine gene expression levels were markedly elevated in lungs of wild-type mice. hSR-BII transgenic mice demonstrated lower inflammatory responses than WT mice. Extremely low (if any) inflammatory cytokine expression levels were detected in lungs of hSR-BI transgenic mice (Fig. 4). Correspondingly, following LPS treatment, the BALF levels of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), as well as the chemokine CXCL1, were markedly higher in WT mice than in hSR-BII transgenic mice, while proinflammatory markers were barely detectable in the BALF of hSR-BI transgenic mice (Fig. 5). The acute phase of lung injury is characterized by the upregulation of adhesive surface molecules like ICAM-1 by activated endothelial cells, which promotes leukocyte recruitment and inflammatory cytokine accumulation in lung tissue. Our study demonstrated markedly higher levels of pulmonary ICAM-1 mRNA expression and more pronounced upregulation of soluble ICAM-1 protein in the BALF of wild-type mice versus the BALF of hSR-BI and hSR-BII transgenic mice challenged with LPS (Fig. 6). LPS and proinflammatory cytokines have been shown to upregulate inducible nitric oxide synthase (iNOS), which contributes to the inflammatory response and host defense against infection by producing NO, a reactive molecule, high levels of which are considered to be a major cause of tissue injury and inflammation (27, 28). We observed an approximately 2-fold increase of iNOS mRNA lung expression in WT mice following i.t. LPS instillation, whereas no statistically significant changes were found in the lungs of SR-B transgenic mice challenged with LPS. These findings provide additional evidence of an anti-inflammatory role of hSR-BI and, to a lesser extent, hSR-BII in LPS-induced acute lung damage.
FIG 4.
Pulmonary gene expression of inflammatory markers in WT, hSR-BI, and hSR-BII transgenic mice challenged with intratracheal LPS. LPS (0.63 mg/kg; gray symbols) or PBS (white symbols) was infused i.t. into WT, hSR-BI, and hSR-BII transgenic mice, and 20 h later, mice were euthanized and lung tissue was collected for mRNA extraction and qRT-PCR assay as described in Materials and Methods. Expression levels of IL-6 (A), IL-1β (B), CXCL1 (C), and TNF-α (D) were normalized by GAPDH and are presented as the fold changes relative to the expression levels in the corresponding PBS-treated control. Individual data points are plotted along with mean values ± SD (n = 4 or 5 for PBS-treated groups; n = 8 to 12 for LPS-treated groups). *, P < 0.05, and **, P < 0.01, versus the results for WT LPS-treated mice.
FIG 5.
BALF levels of cytokines/chemokines in WT, hSR-BI transgenic, and hSR-BII transgenic mice infused with i.t. LPS. LPS (0.63 mg/kg; gray symbols) or PBS (white symbols) was infused i.t. into WT, hSR-BI, and hSR-BII transgenic mice, and 20 h later, mice were euthanized and BALF was collected as described in Materials and Methods. BALF levels of IL-6 (A), IL-1β (B), and CXCL1 (C) were determined using the corresponding ELISA kits. Individual data points are plotted along with mean values ± SD (n = 5 to 7 for PBS-treated groups; n = 8 to 12 for LPS-treated groups). *, P < 0.05, and **, P < 0.01, versus the results for WT LPS-treated mice.
FIG 6.
iNOS and ICAM mRNA expression in lungs and soluble ICAM-1 levels in BALF of mice following i.t. LPS challenge. LPS (0.63 mg/kg; gray symbols) or PBS (white symbols) was infused i.t. into WT, hSR-BI, and hSR-BII transgenic mice, and 20 h later, mice were euthanized and lung tissue samples and BALF were collected for further analysis of ICAM-1 (A) and iNOS (C) pulmonary expression by qRT-PCR assay and for soluble ICAM-1 protein levels (B), correspondingly. Individual data points are shown along with mean values. *, P < 0.05, and **, P < 0.01, versus the results for WT LPS-treated mice.
hSR-BI and hSR-BII promote LPS clearance in murine acute lung injury model.
Currently available experimental evidence suggests that SR-BI may exert its protective function against sepsis through detoxifying LPS. Our earlier in vitro studies demonstrated that SR-BI binds to LPS and mediates its uptake (26), and in vivo studies of others revealed that, following LPS-induced endotoxemia or CLP-induced sepsis, SR-BI-null mice display impaired LPS clearance in the circulation (7). The protective function of SR-BI/-BII during ALI could be attributed to their role in LPS uptake and clearance in airways. To test this hypothesis, we measured BALF levels of LPS in LPS-challenged mice using the LAL test. Our data revealed that hSR-BI and hSR-BII transgenic mice had significantly lower (by 80 to 90%) BALF LPS levels than wild-type mice 20 h after LPS administration (Fig. 7). These results suggest that SR-B-mediated robust LPS clearance could be the mechanism of their anti-inflammatory role during LPS-induced pulmonary injury.
FIG 7.
Endotoxin levels in BALF from mice 20 h after i.t. LPS injection. Endotoxin concentrations in BALF from LPS-challenged mice 20 h after injection were quantified using the chromogenic LAL endotoxin assay. Individual data points are plotted along with mean values ± SD (n = 13 for WT mice; n = 9 for hSR-BI and hSR-BII mice). Eu, endotoxin unit. *, P < 0.05 versus the results for WT mice.
hSR-BI and hSR-BII overexpression results in increased BP-LPS uptake in A549 human epithelial cells.
The A549 cell line transiently transfected via adenovirus type 5 (AdV5)-mediated delivery of hSR-BI or hSR-BII vectors was used to evaluate the role of hSR-BI and hSR-BII in LPS uptake in lung epithelial cells in vitro. Following a 2-h incubation at 37°C, dose-dependent BODIPY-LPS (BP-LPS) uptake was observed for all 3 cell types; however, LPS-associated fluorescence in hSR-BI- and hSR-BII-overexpressing cells was found to be 3.5 to 4.5 times higher, respectively, than in control, vector-transfected cells (Fig. 8A), suggesting a potential role of SR-Bs in LPS clearance.
FIG 8.

Dose-dependent BODIPY-LPS (BP-LPS) uptake by A549 human lung epithelial cells transfected with AdV5-hSR-BI and AdV5-hSR-BII versus AdV5-vector-transfected cells. (A) Forty-eight hours after AdV5-mediated transfections, cells were incubated with BP-LPS for 2 h, and the BP-LPS uptake was analyzed as described in Materials and Methods. The results are presented as means ± SD from triplicate measurements. Data shown are from one of three separate experiments that yielded similar results. (B) AdV5-mediated overexpression of hSR-BI and hSR-BII in A549 cells was confirmed by Western blotting using anti-hSR-BI/-BII antibody (against the hSR-BI/-BII extracellular domain). Protein expression of β-actin was assessed as the sample loading control.
BP-LPS uptake is significantly increased in lungs of SR-B transgenic mice 20 h after an i.t. LPS challenge.
To assess the SR-Bs’ role as mediators of LPS uptake and clearance in an acute lung injury model, wild-type and SR-B transgenic mice were subjected to intratracheal instillation of BP-LPS and, 20 h later, the levels of LPS-associated fluorescence in lung tissue lysates were compared between the three groups of mice. Our data reveal markedly higher BP fluorescent signals in lung lysates from hSR-BI (∼25 times higher) and hSR-BII (∼5 times higher) mice than in lung lysates from wild-type mice (Fig. 9A). Microscopy images of frozen sections from lungs of BP-LPS-challenged mice also demonstrate a significantly higher presence of BP-LPS-associated fluorescence in hSR-B transgenic mice versus wild-type mice, further supporting an SR-B-dependent mechanism of LPS clearance in lungs.
FIG 9.
BP-LPS uptake in lungs of WT, hSR-BI, and hSR-BII transgenic mice following i.t. BP-LPS infusion. After 18 h of BP-LPS i.t. infusion, lungs were collected and BP-LPS-associated fluorescence was assessed in lung tissue lysates (A) and frozen lung sections (B) for each of three groups of mice. Individual data points are plotted along with mean values ± SD (n = 3 for each group). **, P < 0.01, and ***, P < 0.001, versus the results for WT LPS-treated mice.
DISCUSSION
Studies have shown that increased blood levels of LPS due to Gram-negative bacterial infection could lead to acute lung injury, culminating in ARDS, in sepsis and endotoxemia patients (29). Bacterial LPS and related products are also known to be present in BALF from patients with ARDS (30). Currently, lung injury induction via i.t. LPS administration in rodents represents a frequently used ALI model, mimicking many features of ALI/ARDS in humans (31). Pattern recognition receptors that include TLRs and scavenger receptors class A and class B are the key players in surveillance of innate immunity, detecting components of foreign pathogens known as pathogen-associated molecular patterns (PAMPs). We suggested that in addition to TLRs, known to play a critical role in the LPS-induced innate immune host response, class B scavenger receptors, which are capable of recognizing a variety of PAMPs, including LPS, and of mediating their proinflammatory signaling (6, 8), might contribute to LPS-induced inflammation during acute lung injury. To explore the individual roles of SR-BI and SR-BII in LPS-induced lung inflammation and injury, we used hSR-BI and hSR-BII transgenic mice that, as opposed to SR-BI/-BII knockout mice, had normal immune and adrenal functions.
After 20 h of i.t. LPS administration, we compared the extent of lung damage in both groups of transgenic mice and the control group of wild-type mice by analyzing BALF recovered from their airways. Surprisingly, our results revealed that both groups of transgenic mice had markedly reduced LPS-induced lung injury, as was seen by lower levels of inflammatory cell accumulation and protein content in their lungs than in the lungs of wild-type mice. A decreased neutrophil count in the BALF of hSR-B transgenic mice was associated with reduced BALF cytokine/chemokine levels, as well as with lower pulmonary mRNA expression of proinflammatory markers. Additionally, markedly higher lung tissue expression of the adhesive surface molecule ICAM-1, as well as BALF levels of its soluble form, was found in wild-type mice than in both transgenic groups of mice. Altogether, our data demonstrating less accumulation of activated neutrophils and inflammatory cytokines/markers in the lungs of SR-B transgenic mice indicate reduced lung vascular permeability and leakage and suggest that SR-B receptors may play a protective role in LPS-induced lung injury and inflammation.
At first, this new finding seems to be inconsistent with our previous data demonstrating hSR-BI and hSR-BII as proinflammatory receptors that play a potentially deleterious role contributing to LPS-induced liver and kidney inflammation and damage in mice (8). Nevertheless, we believe that these seemingly contradictory data could be reconciled considering our previously reported data demonstrating hSR-BI involvement in bacterial phagocytosis and LPS clearance (26, 32). Taking into account these different functions of SR-BI, SR-B-mediated LPS clearance may outweigh their contribution in LPS-induced proinflammatory signaling during LPS-induced acute lung injury, resulting in lower pulmonary inflammation and injury.
To test this hypothesis, we quantified the endotoxin levels in BALF from the airways of hSR-BI, hSR-BII, and wild-type mice 20 h after i.t. LPS injection, and indeed, we found that the average LPS levels were significantly lower in the BALF of transgenic mice, ∼1 to 4% of the levels in BALF of wild-type controls. To obtain additional evidence of the SR-B-mediated pathway of LPS clearance in lungs, we looked at the role of each of the hSR-Bs in the uptake of fluorescently labeled LPS in human lung epithelial cell cultures and in an in vivo ALI model following BP-LPS i.t. infusion. The results of our in vitro experiments demonstrated markedly increased SR-B-dependent BP-LPS uptake by the lung epithelial cells overexpressing hSR-BI or hSR-BII, and at the same time, significantly higher levels of BP-LPS were found in the lungs of both groups of hSR-B transgenic mice versus the lungs of wild-type mice upon i.t. BP-LPS instillation.
Previous reports suggested that SR-BI may provide protection against sepsis through detoxifying LPS, including our earlier in vitro studies demonstrating that SR-BI binds to LPS and mediates its internalization. Intracellular LPS was found colocalized predominantly with transferrin, indicating its delivery to an endocytic recycling compartment and supporting SR-BI’s role in LPS uptake and clearance (26). In vivo studies of others revealed that, following LPS-induced endotoxemia or CLP-induced sepsis, specifically in mice deficient in hepatic SR-BI, the mice displayed impaired LPS clearance in the circulation, demonstrating a 3-fold increase in serum LPS levels compared with the levels in wild-type controls, suggesting that hepatic SR-BI is responsible for LPS clearance in sepsis (7). Recent findings of Gowdy et al. (33) have demonstrated that mice with hematopoietic cell SR-BI deficiency had higher BALF LPS levels than their SR-BI-sufficient counterparts 24 h after LPS inhalation, further supporting an important role of SR-BI in LPS clearance from the airways.
Of note, the hSR-BI-mediated anti-inflammatory and LPS-detoxifying effects in lungs that we observed in this study were more prominent than those mediated via hSR-BII. At the same time, our previous work revealed that the hSR-BII-dependent LPS-induced proinflammatory and damaging effects in liver and kidney were markedly stronger than the hSR-BI-mediated effects. It is likely that the greater contribution of hSR-BI to LPS uptake and clearance could be the result of its higher (∼80%) cell surface expression, as opposed to the predominantly (∼80%) cytosolic expression of hSR-BII (6, 34). It is not known what specific factor(s) is responsible for the functional prevalence and intracellular localization of either of these two SR-B receptors. The cell-/tissue-specific localization of these receptors could be a possible explanation.
The airways are constantly exposed to the outside environment and can be directly accessible to and influenced by the local endogenous factors. The respiratory epithelium, similar to epithelial cells of the skin and digestive systems, serves as a physical and functional barrier actively involved in the clearance of environmental agents. The alveolar epithelial cells (AECs) lining the lung lumen, together with alveolar macrophages (AMs), form the first line of defense of the body against various pathogens and other harmful substances in a dual manner: through mediating their uptake and inactivation and also via sensing their presence and signaling the host’s immune system of the impending danger. The host response to microbial challenge is integrated through pathogen recognition by different cell types within the tissue/organ. Thus, SR-Bs expressed in different types of pulmonary cells might contribute to the host response in different ways; e.g., SR-Bs expressed in AMs (or in other myeloid cells) would drive cytokine production and tissue damage, while AEC-expressed SR-Bs would mediate pathogen uptake and clearance. The cell type-selective role of SR-Bs would determine the ultimate immune response to pathogens. We suggest that in lungs, the predominant role of SR-B receptors is the role of true scavengers, protecting the body from damaging agents via their uptake and clearance, whereas the TLR4-mediated signaling pathway could be the one responsible for initiating the innate immune response toward PAMPs like those presented by bacteria (35, 36) and LPS (23, 24).
In summary, our findings reveal that the role of hSR-BI and hSR-BII in LPS-induced inflammation and tissue damage could be multifunctional and likely dependent on the specific tissue environment. Unlike the proinflammatory, potentially damaging function of these receptors found in the liver and kidney, we report here that hSR-BI and, to a lesser extent, hSR-BII expressed in the lungs play a beneficial, protective role in LPS-induced acute lung injury. Our studies suggest that to design proper SR-BI-/SR-BII-based strategies, future studies should account for various roles of these receptors in specific tissues and cell populations.
MATERIALS AND METHODS
Reagents.
LPS (Escherichia coli O55:B5) was purchased from Sigma-Aldrich (St. Louis, MO). All reagents used for RNA isolation, reverse transcription, and real-time PCR were purchased from Thermo Fisher Scientific. Enzyme-linked immunosorbent assay (ELISA) kits for quantifying mouse IL-6, TNF-α, and soluble ICAM-1 were from Thermo Fisher Scientific, and ELISA kits for mouse CXCL1 and IL-10 were from R&D Systems, Inc.
Animals.
All animal care and treatment procedures were approved by the Animal Care and Use Committees of the National Heart, Lung, and Blood Institute and the University of Maryland. Animals were handled according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (37). Male C57BL/J mice (8 to 10 weeks old), with an average weight of 20 to 25 g, were obtained from the Jackson Laboratory (Bar Harbor, ME). Human SR-BI and human SR-BII transgenic mice were generated using the liver-specific expression vector pLiv-11, which contains the human apoE promoter, as described previously (8). Animals were anesthetized by i.p. injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Bacterial LPS (0.63 mg/kg of body weight; E. coli O55:B5) or PBS was infused i.t. in a volume of 50 µl using a 20-gauge catheter.
Cell culture and BODIPY-LPS uptake assay.
Human lung epithelial cells from the A549 cell line (CCL-185; ATCC) were grown at 37°C and 5% CO2 in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum in 96-well plates, and transfections were performed at about 80% cell confluence. Cells were transiently transfected to express hSR-BI and hSR-BII using recombinant adenoviral constructs AdV5-hSRBI and AdV5-hSRBII, respectively. LPS (Escherichia coli O55:B5) was labeled using the BODIPY FL succinimidyl ester labeling kit from Thermo Fisher Scientific, following the manufacturer's suggested protocol with previously reported modifications (38). Adenovirus was added to cultured cells for 48 h, and after that, the virus-containing medium was replaced with serum-free DMEM (2% bovine serum albumin [BSA]) containing serial dilutions (starting from 100 µg/ml, step 3) of BODIPY-LPS (BP-LPS). Two hours later, the incubation medium was removed, the cells were rinsed 3 times with ice-cold PBS, and cell-associated fluorescence was measured using a Victor 3 multilabel counter (Perkin Elmer).
Bronchoalveolar lavage fluid analysis.
After 20 h, animals were sacrificed by exsanguination under anesthesia and bronchial airway lavage of the lungs was performed with 1 ml of sterile PBS while gently aspirating the fluid. The recovered BALF was used to measure total protein according to the manufacturer’s manual (bicinchoninic acid [BCA] protein assay kit; Bio-Rad). Cell pellets were examined for total cell count using a hemocytometer and differential cell count using cytocentrifugation and Diff-Quik staining (Dade Diagnostics, Deerfield, IL).
Measurement of cytokines, chemokines, and soluble ICAM-1.
The concentrations of cytokines IL-6, TNF-α, and IL-10, chemokine CXCL1/KC, and soluble ICAM-1 in mouse plasma and BALF were quantified using the corresponding ELISA kits.
Total RNA isolation and qRT-PCR analysis of proinflammatory markers in lungs.
For RNA isolation, lung tissue samples preserved in RNAlater were homogenized in TRIzol reagent using a Precellys 24 homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). RNA was isolated with the PureLink RNA minikit (Thermo Fisher Scientific) after DNase treatment. RNA (2 μg) was reverse transcribed using a TaqMan reverse transcriptase reagent kit. Real-time quantitative PCR (qRT-PCR) assays were performed with a StepOne real-time PCR system (Applied Biosystems), using 40 ng cDNA per reaction mixture. A list of TaqMan gene expression assays used in the study is shown in Table 1. Human SR-B expression was analyzed using the following custom primers: for hSR-BI, forward, 5′-GGTCCCTGTCATCTGCCAA-3′, and reverse, 5′-CTCCTTATCCTTTGAGCCCTTT-3′, and for hSR-BII, forward, 5′-TCCTGAGGACACCGTGAGC-3′, and reverse, 5′-GAGGCTCAGGCTGTGG-3′ (39).
TABLE 1.
TaqMan real-time PCR assays used in this study
| Species | Gene |
Thermo Fisher Scientific identification no. | |
|---|---|---|---|
| Name | Symbol | ||
| Mouse | Interleukin 1 beta | Il 1b | Mm00434228_m1 |
| Mouse | Interleukin 6 | Il6 | Mm00446190_m1 |
| Mouse | Chemokine (C-X-F motif) ligand 1 | Cxcl1 | Mm04207460_m1 |
| Mouse | Tumor necrosis factor | Tnfa | Mm00443258_m1 |
| Mouse | Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | Mm03302249_m1 |
| Mouse | Scavenger receptor member 1 | Scarb1 | Mm00450234_m1 |
| Mouse | Nitric oxide synthase 2 | Nos2 | Mm00440502_m1 |
| Mouse | Intercellular adhesion molecule 1 | Icam1 | Mm00516023_m1 |
Relative levels of gene expression were measured by the comparative cycle threshold (CT) method with mouse β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes as reference genes. All gene expression results were analyzed using the 2−ΔΔCT formula and are presented as normalized fold changes compared to the results for corresponding PBS-treated controls.
Endotoxin measurement in BALF.
The concentrations of LPS in BALF samples were quantified using a chromogenic Limulus amebocyte lysate (LAL) endotoxin assay kit (GenScript) according to the manufacturer’s protocol. Endotoxin concentrations (EU/ml) in the samples were determined from a standard curve using pure endotoxin standards.
Western blot assay for SR-BI/SR-BII expression.
Lung tissue samples were homogenized in tissue protein extraction reagent (Thermo Fisher Scientific) containing a 1% protease inhibitor mixture (Pierce) and subjected to Western blot analysis as described previously (8). hSR-BI and hSR-BII protein expression were detected using anti-human SR-BI or anti-human SR-BII custom antibodies (Abs) against specific peptides from C-terminal domains. The expression of mouse SR-BI (mSR-BI) was detected by anti-SR-BI Ab (against a C-terminal peptide containing residues 450 to 509; Novus Biologicals). Protein expression of β-actin was measured as the loading control using anti-β-actin antibody from Sigma. hSR-BI and hSR-BII expression in A549 cells resulting from the adenoviral transduction was confirmed by Western blot assay using anti-hSR-BI/-BII antibody (against the hSR-BI/-BII extracellular domain; BD Biosciences).
Histological assessment of lung injury.
Lungs were excised, immersed in 10% buffered formalin for ≥24 h, and then embedded in paraffin. Paraffin-embedded lung samples were sliced into 4-µm sections and mounted onto slides for hematoxylin and eosin (H&E) staining.
Immunofluorescence microscopy.
For preparing frozen sections, in order to preserve lung tissue architecture, the lungs were inflated with 1 ml of optimal cutting temperature (OCT) compound/PBS mixture (1:1) through the trachea, excised, embedded in OCT compound, and frozen in a dry ice/isopentane slurry. The OCT compound blocks were cut at 10 µm in a cryostat microtome, mounted on microscope slides, and stored at −80°C. Frozen lung sections were fixed with 3.7% formaldehyde for 10 min, washed 3 times for 5 min each with 0.5% saponin in PBS, and blocked with 5% goat serum–0.05% saponin–1% BSA–PBS for 1 h. For lung epithelial cell staining, sections were incubated overnight at 4°C with EpCAM (epithelial cell adhesion molecule) rat antibody (clone G8.8, catalog number LS-C146748; LSBio), followed by 1 h of incubation with secondary antibodies conjugated with Alexa Fluor 488 (Thermo Fisher Scientific). For hSR-BI and hSR-BII staining, sections were incubated overnight at 4°C with anti-human SR-BI or anti-human SR-BI rabbit custom antibodies produced against C-terminal-domain-specific peptides of hSR-BI (CTSAPKGSVLQEAKL; AnaSpec, San Jose, CA) and hSR-BII (CLPDSPSRQPPSPTA; Sigma, St. Louis, MO). Custom antibodies were validated in Western blotting assays using cell lysates from a HeLa cell line stably transfected with hSR-BI and hSR-BII (8). Following incubation with primary antibodies, sections were incubated for 1 h with Alexa Fluor 568 secondary antibodies (Thermo Fisher Scientific). After two washes with PBS, sections were counterstained for nuclei with Hoechst 33342 (1 μg/ml; Thermo Fisher Scientific), mounted using Vectashield antifade reagent (catalog number H-1400; Vector), and visualized using a Zeiss LSM 780 confocal microscope.
Quantification of BP-LPS uptake in lung tissue lysates.
Samples of excised lungs were weighed, quick-frozen on dry ice, and stored at −80C. After the appropriate volume of tissue extraction reagent I (Thermo Fisher Scientific) was added, samples were homogenized and centrifuged at 10,000 × g for 10 min to pellet tissue debris. The supernatants were collected, and fluorescence intensity was measured using a Victor 3 multilabel counter.
Statistical analysis.
The data are expressed as the mean value ± standard deviations (SD) for each group. Graphical and statistical analyses were performed using GraphPad Prism, version 7.02 (GraphPad, La Jolla, CA). The unpaired Student’s t test was used to determine the level of statistical significance between the sets of data. A P value of <0.05 was considered statistically significant. P values greater than 0.05 but less than 0.1 were considered a trend toward significance.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants number HL087823 and GM122940 (K.G.B.) and HL107920, HL130431, and GM114171 (A.A.B.).
Contributor Information
Irina N. Baranova, Email: ibaranova@mail.nih.gov.
Manuela Raffatellu, University of California San Diego School of Medicine.
REFERENCES
- 1.Calvo D, Gomez-Coronado D, Lasuncion MA, Vega MA. 1997. CLA-1 is an 85-kD plasma membrane glycoprotein that acts as a high-affinity receptor for both native (HDL, LDL, and VLDL) and modified (OxLDL and AcLDL) lipoproteins. Arterioscler Thromb Vasc Biol 17:2341–2349. 10.1161/01.atv.17.11.2341. [DOI] [PubMed] [Google Scholar]
- 2.Trigatti B, Rigotti A, Krieger M. 2000. The role of the high-density lipoprotein receptor SR-BI in cholesterol metabolism. Cur Opin Lipidol 11:123–131. 10.1097/00041433-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Linton MF, Tao H, Linton EF, Yancey PG. 2017. SR-BI: a multifunctional receptor in cholesterol homeostasis and atherosclerosis. Trends Endocrinol Metab 28:461–472. 10.1016/j.tem.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo L, Song Z, Li M, Wu Q, Wang D, Feng H, Bernard P, Daugherty A, Huang B, Li XA. 2009. Scavenger receptor BI protects against septic death through its role in modulating inflammatory response. J Biol Chem 284:19826–19834. 10.1074/jbc.M109.020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai L, Wang Z, Meyer JM, Ji A, van der Westhuyzen DR. 2012. Macrophage SR-BI regulates LPS-induced pro-inflammatory signaling in mice and isolated macrophages. J Lipid Res 53:1472–1481. 10.1194/jlr.M023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranova IN, Vishnyakova TG, Bocharov AV, Leelahavanichkul A, Kurlander R, Chen Z, Souza AC, Yuen PS, Star RA, Csako G, Patterson AP, Eggerman TL. 2012. Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. J Immunol 188:1371–1380. 10.4049/jimmunol.1100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo L, Zheng Z, Ai J, Huang B, Li XA. 2014. Hepatic scavenger receptor BI protects against polymicrobial-induced sepsis through promoting LPS clearance in mice. J Biol Chem 289:14666–14673. 10.1074/jbc.M113.537258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baranova IN, Souza AC, Bocharov AV, Vishnyakova TG, Hu X, Vaisman BL, Amar MJ, Chen Z, Kost Y, Remaley AT, Patterson AP, Yuen PS, Star RA, Eggerman TL. 2016. Human SR-BI and SR-BII potentiate lipopolysaccharide-induced inflammation and acute liver and kidney injury in mice. J Immunol 196:3135–3147. 10.4049/jimmunol.1501709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baranova IN, Souza ACP, Bocharov AV, Vishnyakova TG, Hu X, Vaisman BL, Amar MJ, Chen Z, Remaley AT, Patterson AP, Yuen PST, Star RA, Eggerman TL. 2017. Human SR-BII mediates SAA uptake and contributes to SAA pro-inflammatory signaling in vitro and in vivo. PLoS One 12:e0175824. 10.1371/journal.pone.0175824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leelahavanichkul A, Bocharov AV, Kurlander R, Baranova IN, Vishnyakova TG, Souza AC, Hu X, Doi K, Vaisman B, Amar M, Sviridov D, Chen Z, Remaley AT, Csako G, Patterson AP, Yuen PST, Star RA, Eggerman TL. 2012. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J Immunol 188:2749–2758. 10.4049/jimmunol.1003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. 1999. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A 96:9322–9327. 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meurs I, Hoekstra M, van Wanrooij EJA, Hildebrand RB, Kuiper J, Kuipers F, Hardeman MR, Van Berkel TJC, Van Eck M. 2005. HDL cholesterol levels are an important factor for determining the lifespan of erythrocytes. Exp Hematol 33:1309–1319. 10.1016/j.exphem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Yesilaltay A, Morales MG, Amigo L, Zanlungo S, Rigotti A, Karackattu SL, Donahee MH, Kozarsky KF, Krieger F. 2006. Effects of hepatic expression of the high-density lipoprotein receptor SR-BI on lipoprotein metabolism and female fertility. Endocrinology 147:1577–1588. 10.1210/en.2005-1286. [DOI] [PubMed] [Google Scholar]
- 14.Feng H, Guo L, Wang D, Gao H, Hou G, Zheng Z, Ai J, Foreman O, Daugherty A, Li XA. 2011. Deficiency of scavenger receptor BI leads to impaired lymphocyte homeostasis and autoimmune disorders in mice. Arterioscler Thromb Vasc Biol 31:2543–2551. 10.1161/ATVBAHA.111.234716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ragaller M, Richter T. 2010. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock 3:43–51. 10.4103/0974-2700.58663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson ER, Matthay MA. 2010. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 23:243–252. 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay MA, Ware LB, Zimmerman GA. 2012. The acute respiratory distress syndrome. J Clin Invest 122:2731–2740. 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel VJ, Roy SB, Mehta HJ, Joo M, Sadikot RT. 2018. Alternative and natural therapies for acute lung injury and acute respiratory distress syndrome. Biomed Res Int 2018:2476824. 10.1155/2018/2476824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273. 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. 2009. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A 106:2348–2352. 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng M, Scott MJ, Loughran P, Gibson G, Sodhi C, Watkins S, Hackam D, Billiar TR. 2013. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J Immunol 190:5152–5160. 10.4049/jimmunol.1300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han S, Mallampalli RK. 2015. The acute respiratory distress syndrome: from mechanism to translation. J Immunol 194:855–860. 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Wang T, Song L, Liu X. 2019. Activation of multiple Toll-like receptors serves different roles in sepsis-induced acute lung injury. Exp Ther Med 18:443–450. 10.3892/etm.2019.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang F, Fan K, Wang K, Bian C. 2018. Atractylodin attenuates lipopolysaccharide-induced acute lung injury by inhibiting NLRP3 inflammasome and TLR4 pathways. J Pharmacol Sci 136:203–211. 10.1016/j.jphs.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Bocharov AV, Wu T, Baranova IN, Birukova AA, Sviridov D, Vishnyakova TG, Remaley AT, Eggerman TL, Patterson AP, Birukov KG. 2016. Synthetic amphipathic helical peptides targeting CD36 attenuate lipopolysaccharide-induced inflammation and acute lung injury. J Immunol 197:611–619. 10.4049/jimmunol.1401028. [DOI] [PubMed] [Google Scholar]
- 26.Vishnyakova TG, Bocharov AV, Baranova IN, Chen Z, Remaley AT, Csako G, Eggerman TL, Patterson AP. 2003. Binding and internalization of lipopolysaccharide by Cla-1, a human orthologue of rodent scavenger receptor B1. J Biol Chem 278:22771–22780. 10.1074/jbc.M211032200. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan BC, McIntyreRC, Jr, Meldrum DR, Fullerton DA. 1998. L-Arginine prevents lung neutrophil accumulation and preserves pulmonary endothelial function after endotoxin. Am J Physiol 274:L337–L342. 10.1152/ajplung.1998.274.3.L337. [DOI] [PubMed] [Google Scholar]
- 28.Speyer CL, Neff TA, Warner RL, Guo RF, Sarma JV, Riedemann NC, Murpy ME, Murphy HS, Ward PA. 2003. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am J Pathol 163:2319–2328. 10.1016/S0002-9440(10)63588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brigham KL, Meyrick B. 1986. Endotoxin and lung injury. Am Rev Respir Dis 133:913–927. [PubMed] [Google Scholar]
- 30.Martin TR, Rubenfeld GD, Ruzinski JT, Goodman RB, Steinberg KP, Leturcq DJ, Moriarty AM, Raghu G, Baughman RP, Hudson LD. 1997. Relationship between soluble CD14, lipopolysaccharide binding protein, and the alveolar inflammatory response in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 155:937–944. 10.1164/ajrccm.155.3.9117029. [DOI] [PubMed] [Google Scholar]
- 31.Rittirsch D, Flierl MA, Day DE, Nadeau BA, McGuire SR, Hoesel LM, Ipaktchi K, Zetoune FS, Sarma JV, Leng L, Huber-Lang MS, Neff TA, Bucala R, Ward PA. 2008. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol 180:7664–7672. 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vishnyakova TG, Kurlander R, Bocharov AV, Baranova IN, Chen Z, Abu-Asab MS, Tsokos M, Malide D, Basso F, Remaley A, Csako G, Eggerman TL, Patterson AP. 2006. CLA-1 and its splicing variant CLA-2 mediate bacterial adhesion and cytosolic bacterial invasion in mammalian cells. Proc Natl Acad Sci U S A 103:16888–16893. 10.1073/pnas.0602126103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gowdy KM, Madenspacher JH, Azzam KM, Gabor KA, Janardhan KS, Aloor JJ, Fessler MB. 2015. Key role for scavenger receptor B-I in the integrative physiology of host defense during bacterial pneumonia. Mucosal Immunol 8:559–571. 10.1038/mi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckhardt ER, Cai L, Sun B, Webb NR, van der Westhuyzen DR. 2004. High density lipoprotein uptake by scavenger receptor SR-BII. J Biol Chem 279:14372–14381. 10.1074/jbc.M313793200. [DOI] [PubMed] [Google Scholar]
- 35.Faure K, Sawa T, Ajayi T, Fujimoto J, Moriyama K, Shime N, Wiener-Kronish JP. 2004. TLR4 signaling is essential for survival in acute lung injury induced by virulent Pseudomonas aeruginosa secreting type III secretory toxins. Respir Res 5:1. 10.1186/1465-9921-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. 2005. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun 73:1754–1763. 10.1128/IAI.73.3.1754-1763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 38.Levels JH, Abraham PR, van den Ende A, van Deventer SJ. 2001. Distribution and kinetics of lipoprotein-bound endotoxin. Infect Immun 69:2821–2828. 10.1128/IAI.69.5.2821-2828.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thilakawardhana S, Everett DM, Murdock PR, Dingwall C, Owen JS. 2005. Quantification of apolipoprotein E receptors in human brain-derived cell lines by real-time polymerase chain reaction. Neurobiol Aging 26:813–823. 10.1016/j.neurobiolaging.2004.08.004. [DOI] [PubMed] [Google Scholar]