ABSTRACT
In the Gram-positive pathogen Staphylococcus aureus, pore-forming toxins (PFTs), such as leukocidins and hemolysins, play prominent roles in staphylococcal pathogenesis by killing host immune cells and red blood cells (RBCs). However, it remains unknown which combination of toxin antigens would induce the broadest protective immune response against those toxins. In this study, by targeting six major staphylococcal PFTs (i.e., gamma-hemolysin AB [HlgAB], gamma-hemolysin CB [HlgCB], leukocidin AB [LukAB], leukocidin ED [LukED], Panton-Valentine leukocidin [LukSF-PV], and alpha-hemolysin [Hla]), we generated 10 recombinant toxins or toxin subunits, 3 toxoids, and their rabbit antibodies. Using the cytolytic assay for RBCs and polymorphonuclear cells (PMNs), we determined the best combination of toxin antibodies conferring the broadest protection against those staphylococcal PFTs. Although anti-HlgA IgG (HlgA-IgG) showed low cross-reactivity to other toxin components, it was essential to protect rabbit and human RBCs and human PMNs. For the protection of rabbit RBCs, HlaH35L toxoid-IgG was also required, whereas for human PMNs, LukS-IgG and LukAE323AB-IgG were essential too. When the toxin/toxoid antigens HlgA, LukS-PV, HlaH35L, and LukAE323AB were used to immunize rabbits, they increased rabbit survival; however, they did not block staphylococcal abscess formation in kidneys. Based on these results, we proposed that the combination of HlgA, LukS, HlaH35L, and LukAE323AB is the optimal vaccine component to protect human RBCs and PMNs from staphylococcal PFTs. We also concluded that a successful S. aureus vaccine requires not only those toxin antigens but also other antigens that can induce immune responses blocking staphylococcal colonization.
KEYWORDS: Staphylococcus aureus, immunization, neutralizing antibodies, toxins, vaccines
INTRODUCTION
Staphylococcus aureus is a Gram-positive pathogen that causes diverse diseases in humans, ranging from skin and soft tissue infections to life-threatening diseases, including sepsis, pneumonia, endocarditis, and toxic shock syndrome (1, 2). This versatility of S. aureus as a pathogen is, at least in part, due to the production of numerous virulence factors. Of those, exotoxins play an important role in evasion defense against the host immune system by killing host immune cells. For example, the pore-forming toxins (PFTs), such as bicomponent leukocidins and alpha-hemolysin (Hla), kill phagocytic cells and red blood cells (RBCs) (3, 4).
S. aureus produces five bicomponent leukocidins: gamma-hemolysin AB (HlgAB), gamma-hemolysin CB (HlgCB), leukocidin AB (LukAB = LukGH), leukocidin ED (LukED), and Panton-Valentine leukocidin (LukSF-PV, henceforth LukSF). These leukocidins are composed of an S subunit (HlgA, HlgC, LukA, LukE, and LukS) and an F subunit (HlgB, LukB, LukD, and LukF). Of those, HlgAB, HlgCB, LukED, and LukSF share significant sequence homology among their S and F subunits (3). The S and F subunits of those four leukocidins are produced as a monomer, and the S subunits bind to the host cell receptor first during host attack; then, the F subunits bind to the S subunit and induce oligomerization and pore formation (5, 6). In contrast, LukAB shows low sequence homology to other PFTs and is produced as a LukAB dimer. LukA and LukB are individually unstable, and dimerization is required for protein stability. During infection, LukA in the LukAB dimer binds to host receptors, and then toxin oligomerization and pore formation proceed (7).
Due to their prominent roles in killing host immune and red blood cells (RBCs), these toxins have been a target of vaccine development and antibody production for passive immunization. For example, Hla toxoid and LukS were used to develop a staphylococcal vaccine (8). Hla and leukocidins were used to generate neutralizing monoclonal antibodies (MAbs) for passive immunization (9, 10). Recently, the MedImmune group reported that administration of two antitoxin MAbs, MEDI4893 and SAN177 or SAN481, provided excellent protection in rabbits against staphylococcal pneumonia (11, 12). MEDI4893 neutralizes Hla, whereas both SAN177 and SAN481 neutralize HlgAB, HlgCB, LukED, and LukSF by binding to the F subunits of the leukocidins (i.e., HlgB, LukD, and LukF) (13). On the other hand, the MAb named ASN100, which is comprised of the MAbs ASN-1 and ASN-2, is known to neutralize all six PFTs (10, 14).
For the generation of an effective vaccine that can protect hosts from staphylococcal toxins, it is imperative to identify the toxin antigens that can provide broad neutralization activity. However, to our knowledge, such toxin components have not yet been systemically determined. Therefore, in this study, we generated 10 recombinant toxins or toxin subunits (HlgA, HlgB, HlgC, LukE, LukD, LukS, LukF, LukA, LukB, and Hla) and 3 toxoids (HlaH35L, LukST244A, and LukAE323A) as well as their rabbit antibodies. Using those toxin/toxoid proteins and their antibodies, we identified the optimal combination of antibodies to protect human RBCs and polymorphonuclear cells (PMNs) against the six staphylococcal PFTs.
RESULTS
Purified recombinant staphylococcal PFTs are functional.
Individual toxin components were expressed as a His6-tagged protein via a plasmid vector in Escherichia coli and were purified by nickel (Ni) column chromatography (Fig. 1A and B; see also Fig. S1A in the supplemental material). Since dimerization of LukAB is required for protein stability and activity, LukA and LukB were coexpressed from a plasmid and purified together as a dimer (Fig. 1A). In each toxin subunit, the His6 tag was attached to either the N terminus (HlgB and LukA) or C terminus (LukS, LukF, LukE, LukD, LukB, HlgA, HlgC, and Hla) (Fig. S1A). We also generated a nontoxic mutant protein for Hla (HlaH35L), LukS (LukST244A), and LukA (LukAE323A) (Fig. 1B; see also Fig. S1A) (15, 16). To assess the functionality of the recombinant toxin components, we added a mix of an equal amount of S and F subunits (LukED, LukSF, HlgAB, and HlgCB) or toxin dimer (LukAB) to human PMNs (hPMNs) and measured their cytotoxicity. All five leukotoxins efficiently killed hPMNs (Fig. 1C), demonstrating the functionality of those recombinant toxins. On the other hand, when mutant forms of LukS and LukA (i.e., LukST244A and LukAE323A) were used, no killing was observed. The functionality of Hla was examined by a rabbit RBC (rRBC) lysis assay. The wild-type Hla efficiently lysed rRBCs, whereas the mutant HlaH35L did not (Fig. 1D), confirming the functionality of the recombinant Hla. Based on these results, we concluded that the purified recombinant toxin components are all functional and suitable for further experiments.
FIG 1.

Purification of the recombinant pore-forming toxins and toxoids. (A, B) SDS-PAGE analysis of the purified toxin components. Each protein (A, 5 μg; B, 3 μg) was subjected to 15% SDS-PAGE under reducing conditions. LukA and LukB were coexpressed and copurified. (C) Leukotoxin activity of the purified bicomponent leukocidins. Except for LukAB and Hla (each 1 μg), all other bicomponent toxins were reconstituted by mixing equal amounts (0.5 μg) of S and F subunits. Surviving hPMNs were counted after staining with trypan blue under a microscope. (D) Hemolytic activity of the purified recombinant alpha-hemolysin (Hla). Hla or HlaH35L toxoid (1 μg each) was mixed with rabbit red blood cells and incubated. For a positive control, rabbit red blood cells (rRBCs) were mixed with Triton X-100 (0.05% final concentration) and incubated. After centrifugation, the supernatant was collected, and its absorbance at 405 nm was measured. All assays were carried out at least three times independently, and the results were presented as the mean ± SD.
Antibodies of bicomponent toxin subunits show various degrees of cross-reactivity.
Except for LukAB, the other four bicomponent leukotoxins (i.e., HlgAB, HlgCB, LukSF, and LukED) share significant sequence homologies (5). Therefore, antibodies generated with these toxin components are expected to show cross-reactivity. To evaluate antibody cross-reactivity, we generated antitoxin polyclonal antibodies by immunizing rabbits with seven toxin subunits (HlgA, HlgB, HlgC, LukS, LukF, LukE, and LukD), LukAB, LukAE323AB, and HlaH35L. Then, antitoxin IgGs were purified by toxin-coupled affinity chromatography, where each toxin antigen was conjugated to sepharose columns (Fig. S2). Finally, anti-His6 IgGs were eliminated by passing the purified antibodies through His6 tag-conjugated sepharose columns (data not shown).
To examine the cross-reactivity of the purified leukocidin IgGs, we immobilized each toxin component on a polyvinylidene difluoride (PVDF) membrane; then, the binding of each IgG was measured by immunoblotting (Fig. 2A) and by quantifying blot intensity (Fig. 2B). Of the S subunit IgGs, LukS-IgG as S subunit IgG showed the broadest cross-reactivity and bound to not only LukS but also HlgA, HlgC, and LukE. Although LukE-IgG also bound all four S subunits, its binding to HlgA and HlgC appeared to be less efficient than LukS-IgG. On the other hand, HlgC-IgGs did not bind LukE, whereas HlgA-IgGs showed the narrowest cross-reactivity and did not recognize LukS or LukE (Fig. 2).
FIG 2.

Cross-reactivity of the purified toxin component antibodies. (A) The recombinant toxin components (each 1 μg) indicated at the top were spotted on the PVDF membrane strips. Each strip was incubated with the toxin component IgG (0.15 μg of each IgG/9 ml of 5% skim milk) shown to the right and then with HRP-conjugated goat anti-rabbit IgG (0.12 μg/9 ml 5% skim milk). The HRP signal was developed with enhanced chemiluminescence (ECL) reagent (Pierce). Red boxes indicate the recognition of cognate antigen, whereas green boxes show the recognition of noncognate antigens. For a negative control, His6-tagged FnbpAN2N3 (the N2N3 domain of S. aureus fibronectin-binding protein A, FnbpA) was used. For a loading control, a strip stained with Ponceau S is shown at the bottom. (B) The obtained blot intensity was measured with the Image Lab program of the ChemiDoc touch imaging system (Bio-Rad, USA). The blot intensity of the cognate antigen and its antibody was set to 1 as a control. Ags, antigens.
Of the F subunit IgGs, HlgB-IgG showed the broadest cross-reactivity and bound not only HlgB but also LukF and LukD (Fig. 2). On the other hand, LukF-IgG and LukD-IgG did not bind HlgB (Fig. 2). None of the IgGs bound FnbpAN2N3, a recombinant protein comprised of a His6 tag and the N2N3 domain of fibronectin-binding protein A, demonstrating the absence of His6-IgG. As expected, LukAB-IgG bound LukAB only, and none of the other IgGs recognized LukAB (Fig. 2).
HlgA-IgG is essential to protect rabbit and human RBCs from staphylococcal PFTs.
Two major targets of S. aureus PFTs are RBCs and phagocytic cells, such as PMNs (3, 4). As reported previously, rRBCs (Fig. 3A) were lysed by Hla and HlgAB (17). To identify IgGs that protect rRBCs from attacks by Hla and HlgAB, we mixed rRBCs with Hla and HlgAB in the presence of HlaH35L-IgG, HlgA-IgG, HlgB-IgG, or their combinations. As shown, when combined, HlgA-IgG and HlaH35L-IgG fully protected rRBCs from lysis at 70 nM (Fig. 3A). On the other hand, HlgB-IgG, which showed the broadest cross-reactivity toward F subunits, failed to confer significant protection. Even in combination with HlaH35L-IgG, HlgB-IgG failed to protect rRBCs.
FIG 3.
Toxin neutralization activity of the purified toxin IgGs in a hemolysis assay. (A) Hemolysis of rabbit RBCs by pore-forming toxins (left) and neutralization of HlgAB and Hla by the cognate toxin component IgGs (right). Toxins and antibodies were indicated to the right of each graph. The concentrations in the x axis are for individual IgGs; when two IgGs were used, both IgGs were added at the concentration indicated. (B) Hemolysis of hRBCs by pore-forming toxins (left) and neutralization of HlgAB and LukED by cognate toxin component antibodies (right). In the hemolysis assay, toxins and RBCs were mixed and incubated at 37°C for 1 h. For the neutralization assay, RBCs were mixed with the toxins indicated and the test antibodies; then, the samples were incubated at 37°C for 1 h.
When human red blood cells (hRBCs) were treated with the recombinant PFTs, they were lysed by HlgAB, LukED, and HlgA/LukD, agreeing with previous studies (17, 18). To examine the protective effect of the toxin component antibodies, we mixed hRBCs with HlgAB and LukED in the presence of individual component IgGs. To our surprise, HlgA-IgGs alone could fully protect hRBCs from toxin-mediated lysis (Fig. 3B).
HlgA-IgG blocks the binding of LukE to hRBCs.
The protection of hRBCs by HlgA-IgG alone was surprising because the immunoblot suggested that HlgA-IgG does not bind to LukE (Fig. 2). To verify that HlgA-IgG can block the binding of LukED under a liquid condition, we carried out a toxin-binding assay with flow cytometry. First, we labeled HlgA and LukE with fluorescein isothiocyanate (FITC), mixed each of the FITC-labeled proteins with hRBCs, and measured the fluorescence of hRBCs as an indicator for the toxin S subunit binding. As shown, both FITC-labeled S subunits bound hRBCs (Fig. 4A). Assuming an equal FITC labeling efficiency, the higher fluorescence intensity of FITC-HlgA-treated hRBCs might indicate that more HlgA molecules bound hRBCs than LukE molecules (Fig. 4A). To compare the efficiency of HlgA-IgG and LukE-IgG on blocking S subunit binding, we added those antibodies to the mix of hRBCs and FITC-HlgA or FITC-LukE. For a negative control, HlgB-IgG was used. Then, the FITC labeling of hRBCs was measured. When added to the mix of hRBCs and FITC-HlgA, HlgA-IgG efficiently blocked HlgA binding to hRBCs, whereas LukE-IgG did not (Fig. 4B). On the other hand, when added to the mix of hRBCs and FITC-LukE, HlgA-IgG blocked LukE binding to hRBCs as efficiently as LukE-IgG (Fig. 4C). Finally, when the antibodies were added to the mix of hRBCs, FITC-HlgA, and FITC-LukE, HlgA-IgG reduced the FITC signal on hRBCs more than LukE-IgG (Fig. 4D). Based on these results, we concluded that, although HlgA-IgG did not bind LukE in the immunoblot assay, it can block the binding of LukE to hRBCs under a liquid condition.
FIG 4.

HlgA-IgG binds to LukE and blocks LukE binding to human RBCs. (A) Binding of FITC-labeled HlgA and LukE to human RBC. (B to D) Comparison of HlgA-IgG and LukE-IgG in their blocking of human RBC binding of FITC-HlgA (B), FITC-LukE (C), and FITC-HlgA/FITC-LukE (D). For a negative control, HlgB-IgG was used (B to D). The FITC intensity of human RBCs was measured by flow cytometry.
The combination of HlgA-IgG, LukS-IgG, and LukAE323AB-IgG protects human PMNs from staphylococcal leukocidins.
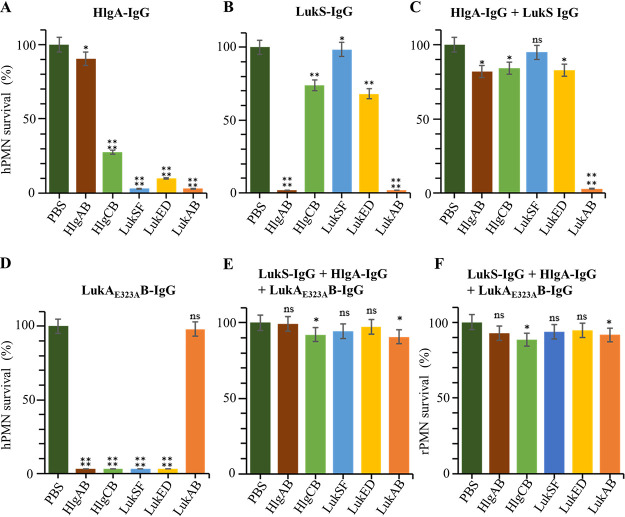
Next, we determined which combination of antitoxin IgGs protects hPMNs from toxin-mediated killing most efficiently. We mixed hPMNs with five individual leukocidins in the presence of each IgG or their combinations; then, the survival of hPMNs was measured (Fig. 5; see also Fig. S3). As expected, LukAE323AB-IgG protected hPMNs only from LukAB, whereas none of the other IgGs protected hPMNs from LukAB (Fig. 5; see also Fig. S3). For individual antibodies, LukE-IgG showed the broadest protection against the four leukocidins (i.e., HlgAB, HlgCB, LukSF, and LukED) (Fig. S3). However, when combined with other S subunit IgGs (e.g., LukS-IgG, HlgA-IgG, or HlgC-IgG), its protective effect was significantly reduced (Fig. S3J, K, and M). HlgA-IgG, which was essential in protecting hRBCs from toxin-mediated killing (Fig. 3), protected hPMNs from HlgAB efficiently; however, it showed only slight protection against HlgCB and LukED (10% to 30%) or almost no protection against LukSF (Fig. 5A). Intriguingly, LukS-IgG showed a protective effect complementary to that of HlgA-IgG. Although it failed to protect hPMNs against HlgAB, it showed reasonably good protection against HlgCB and LukED (60% to 70%) as well as efficient protection against LukSF (Fig. 5B). When combined together, HlgA-IgG and LukS-IgG showed over 80% protection against all leukocidins except for LukAB. This protection was better than any other combination of two antibodies (Fig. S3). When LukAE323AB-IgG was additionally combined, the triple antibodies conferred almost perfect protection for hPMNs against HlgAB, LukSF, and LukED and over 90% protection against HlgCB and LukAB (Fig. 5E). When applied to rabbit PMNs (rPMNs), a similar protective effect was observed (Fig. 5F). Based on these results, we concluded that the triple combination of HlgA-IgG, LukS-IgG, and LukAE323AB-IgG is the best combination for the protection of PMNs from leukocidin-mediated killing.
FIG 5.
Protection of human or rabbit PMNs from staphylococcal leukocidins by various combinations of toxin component antibodies. Human PMNs (A to E) or rabbit PMNs (F) were mixed with each of the five staphylococcal leukocidins in the presence of various combinations of toxin component antibodies indicated above each graph. After 1 h of incubation at 37°C, the viability of PMNs was measured by trypan blue staining and microscopy. Statistical significance of the survival difference was accessed by unpaired Student’s t test. *, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001; ns, not significant. hPMN, human PMN.; rPMN, rabbit PMN.
RBCs are very sensitive to PFT-mediated lysis, but PMNs are not.
Next, we examined the extent of cellular damage afflicted on hRBCs and hPMNs by staphylococcal PFTs. We labeled hRBCs with FITC (Fig. 6A) and, for a control, subjected them to lysis by ammonium chloride or sonication. As shown, upon treatment, a new peak with reduced FITC intensity appeared (Fig. 6A), indicating the breakage of hRBCs into smaller pieces. The FITC intensity of the new peak generated by sonication was lower than that generated by ammonium chloride, suggesting that sonication breaks hRBCs into smaller pieces. As expected, the addition of Hla did not affect the integrity of hRBCs (Hla in Fig. 6A). Intriguingly, the addition of HlgAB and LukED abolished the original peak almost completely and generated a new peak with a FITC intensity similar to that seen following ammonium chloride treatment. These results imply that almost all hRBCs were lysed by the toxins to a size similar to that resulting from ammonium chloride treatment. Finally, when HlgA-IgG was added, a new peak was not observed (Fig. 6A), demonstrating toxin neutralization by the antibody. When the experiment was repeated for rRBCs with HlgAB, Hla, HlgA-IgG, and HlaN35L-IgG, similar results were obtained (Fig. S4A). These results suggest that human and rabbit RBCs are very sensitive to toxin-mediated lysis but can be protected by the right combination of anti-PFT antibodies.
FIG 6.

Lysis of hRBCs and hPMNs by pore-forming toxins and the protection of those human cells by toxin component antibodies. (A) Lysis of hRBCs by HlgAB and LukED and neutralization of those toxins by HlgA-IgG. hRBCs were treated with FITC (+) or PBS (−) and subjected to various treatments indicated above each graph. Then, the FITC intensity of the samples was measured by flow cytometry. Newly generated peaks are indicated by black arrows. (B) Lysis of hPMNs by six pore-forming toxins and neutralization of those toxins by HlgA-IgG, LukS-IgG, and LukAE323AB-IgG. Human PMNs were labeled with FITC (+) and subjected to various treatments shown above the graph. Six PFTs indicate the mix of HlgAB, HlgCB, LukAB, LukED, LukSF, and Hla. Three IgGs indicate the mix of HlgA-IgG, LukS-IgG, and LukAE323AB-IgG.
When we labeled hPMNs with FITC, intriguingly, two different populations were observed; about 20% of hPMNs showed a higher FITC intensity than the rest of the hPMNs (no treatment in Fig. 6B). Since the FITC molecule is reactive to the amine group on the cell surface, the distinct labeling pattern might suggest that about 20% of hPMNs have more surface-exposed amine groups, probably surface proteins, than the rest of hPMNs. Sonication of hPMNs generated a new peak with lower FITC intensity, indicating cell lysis and fragmentation (sonication in Fig. 6B). The addition of Hla, which lacks lytic activity toward hPMNs, did not generate a new peak. However, when either individual PFTs or the mix of six PFTs was added, a new peak with lower FITC intensity appeared (Fig. 6B; see also Fig. S4B), suggesting that PFTs can lyse hPMNs. However, unlike RBCs, a still significant portion of the initial peaks remained, suggesting that not all hPMNs are lysed by PFTs. Considering the fact that almost all hPMNs were dead under this condition (Fig. 5; see also Fig. S3), the remaining initial peak likely represents the hPMN population killed by pore formation without lysis. When HlgA-IgG, LukS-IgG, and LukAE323AB-IgG were added to the hPMN-leukocidin mix, the new peak was greatly reduced (Fig. 6B), demonstrating toxin neutralization by the mix of these antibodies. Based on these results, we concluded that RBCs are very sensitive to toxin-mediated lysis, whereas hPMNs are not, and that both types of cells can be protected by the right combination of antitoxin antibodies.
Although immunization with four PFT antigens enhances the survival of rabbits upon S. aureus infection, it does not prevent bacterial dissemination and colonization.
So far, we identified the optimal combination of antitoxin antibodies to neutralize staphylococcal PFTs. Therefore, next, we examined whether the cognate toxin components can serve as an effective vaccine against staphylococcal infection. Since mice are insensitive to several staphylococcal toxins due to the absence of toxin receptors (19), we carried out the vaccine experiment using rabbits, a suitable animal model for S. aureus infection (20). Because of the sensitivity of rRBCs to Hla (Fig. 3A), the combined vaccine contained not only HlgA, LukS, and LukAE323AB but also Hla toxoid (i.e., HlaH35L). The four antigens were injected into five rabbits four times in a 2- to 4-week interval (Fig. S5). Saline was administered to the control group (5 rabbits). Every week, IgG titers were measured for each antigen by enzyme-linked immunosorbent assay (ELISA). As shown, the combined vaccine induced robust IgG production for all four antigens (Fig. 7A). At 72 days postimmunization, rabbits were challenged with S. aureus USA300 LAC (3.5 × 107 CFU/kg) via the ear vein route (Fig. S5A). By 10 days of the bacterial challenge, four of the five saline-injected rabbits died, whereas all five vaccinated rabbits survived (Fig. 7B), demonstrating the protective effect of the combination vaccine against S. aureus infection. When the bacterial burden was measured for kidneys, it was significantly lower in the vaccinated rabbits than in the control rabbits (P < 0.05, Fig. 7C). However, all kidneys, including kidneys from vaccinated rabbits, contained staphylococcal abscesses (Fig. 7D). We performed another rabbit immunization experiment with a shorter vaccination schedule (three vaccinations in a 2-week interval) and a higher dose of S. aureus infection (7 × 107 CFU/kg) (Fig. S6A). In this experiment, all saline-injected control rabbits (n = 6) died within a day (Fig. S6C). In contrast, of the seven vaccinated rabbits, three died by 6 days postinfection, and four survived until 8 days postinfection (Fig. S6C), confirming the protective effect of the combination vaccine against S. aureus infection. However, the kidneys of the four surviving rabbits contained staphylococcal abscesses (Fig. S6D). Based on these results, we concluded that although the 4 PFT combination vaccine protects rabbits from the lethal effect of staphylococcal infection by neutralizing staphylococcal toxins, it does not prevent the dissemination and abscess formation of S. aureus.
FIG 7.
The protective effect of the combination vaccine in the rabbit model of blood infection by S. aureus USA300 LAC. Rabbits (n = 5) were immunized with the mix of alhydrogel and HlgA, LukS, LukAE323AB, and HlaH35L four times as shown in Fig. S5 in the supplemental material. Then, rabbits were challenged with S. aureus USA300 LAC (3.5 × 107 CFU/kg). For a control, rabbits (n = 5) were injected with the mix of alhydrogel and saline. (A) The vaccine effect on IgG production against each antigen. Sera IgG titers were measured by ELISA at the time indicated. Statistical significance was examined by unpaired Student’s t test (***, P < 0.005; ****, P < 0.001). (B) The vaccine effect on the survival of rabbits. The statistical significance of the survival difference was measured by log rank test (*, P ≤ 0.05). (C) The vaccine effect on the bacterial dissemination and colonization in the kidneys. At day 10 postinfection, all surviving rabbits were euthanized, and the kidneys were harvested and ground. CFUs in the kidneys were measured by serial dilution and spreading on tryptic soy agar (TSA). The statistical significance of the CFU difference was measured by unpaired Student’s t test (**, P ≤ 0.01). (D) The vaccine effect on abscess formation in the kidneys. Staphylococci are indicated with green arrows.
DISCUSSION
To meet the challenges of methicillin-resistant S. aureus (MRSA) infections, it is imperative to develop not only new antibiotics but also vaccines (1). However, despite more than 2 decades of efforts from numerous laboratories, no staphylococcal vaccine has yet been approved. Although initial vaccine development focused on the capsules and surface proteins, it is recognized that surface proteins are not sufficient to induce effective immune responses against S. aureus, and an antitoxin mechanism needs to be a part of the vaccine development strategy (2). In this study, we report that the combination of HlgA, LukS, and LukAE323AB is optimal to protect human RBCs and PMNs from six staphylococcal PFTs. Although Hla is not effective in killing human RBCs and PMNs (Fig. 3B and Fig. 5B), it can disrupt human immune systems and their immune responses. For example, Hla induces vascular injury and programmed cell death of human B cells, T cells, and monocytes (21). It also damages platelets and promotes blood coagulation (22, 23). It is also known to activate the NLRP3 inflammasome in human monocytes (24). Therefore, it is likely that, along with HlgA, LukS, and LukAE323AB, Hla toxoid should also be included in the S. aureus antitoxin vaccine.
It is noteworthy that, of the selected toxin antigens, HlgA and LukS are the S subunits of their cognate toxins HlgAB and LukSF, respectively. When all three F subunit antibodies (i.e., LukF-IgG, HlgB-IgG, and LukD-IgG) were combined with LukAE323AB-IgG, they showed only ∼70% protection for hPMNs against the five leukocidins (Fig. S3T in the supplemental material) compared with over 90% protection by the combination of HlgA-IgG, LukS-IgG, and LukAE323AB-IgG (Fig. 5E). Therefore, compared with F subunits, S subunits might be better antigens to induce neutralizing antibodies. Since the S subunit binds to the toxin receptor and initiates the toxin attack, these results indicate that targeting the earlier step is a more effective way to neutralize bicomponent toxins.
One of our study’s interesting findings is that the cross-reactivity in the immunoblot was not a reliable indicator of the antibody’s toxin neutralization activity. In a BLAST analysis, LukS shows sequence homology to both HlgA (66% identity) and LukE (68% identity). Indeed, in the immunoblot analysis, LukS-IgG bound HlgA and LukE with a similar affinity (Fig. 2). However, in the hPMN cytolysis assay, LukS-IgG neutralized LukED much more efficiently (∼67.6%) than HlgAB (∼3%) (Fig. 5A). It is likely that LukS-IgGs binding to HlgA do not inhibit HlgA binding to its receptor. Intriguingly, HlgA-IgG did not show significant binding to LukS (Fig. 2). These results might indicate that, despite the high amino acid similarity, LukS and HlgA display somewhat different sets of epitopes on the surface. Based on those observations, we concluded that cross-reactivity measured by immunoblotting is not a reliable indicator for the neutralization activity of an antibody.
Functionally, the combination of LukS-IgG, HlgA-IgG, LukAE323AB-IgG, and HlaH35L-IgG is equivalent to the monoclonal antibody ASN100 (14). ASN100 is a combination of two MAbs, ASN-1 and ASN-2. ASN-1 neutralizes five PFTs (i.e., Hla, HlgAB, HlgCB, LukED, and LukSF) (25), whereas ASN-2 neutralizes LukAB (26). In contrast to our active immunization strategy, however, ASN100 is for passive immunization; in the recipients, the monoclonal antibodies will provide a relatively short-lived protective effect against staphylococcal infections by neutralizing staphylococcal cytotoxins. In the rabbit lethal pneumonia model, all rabbits having received 20 mg/kg ASN100 survived, whereas all rabbits having received placebo died within 24 h (10). However, in our rabbit blood infection model with a lethal dosage (i.e., 7 × 107 CFU), only 57% (4/7) of immunized rabbits survived until day 8 postinfection (Fig. S6C). This lower protection might be due to the difference in infection mode (lung versus blood). Staphylococcal toxins may also play a more critical role in pathogenesis during lung infection than they do during blood infection. Nonetheless, the antitoxin strategy does not abolish staphylococcal colonization. All surviving rabbits had abscesses in their kidneys (Fig. 7B and Fig. S6D) and kept losing weight (Fig. S5B and Fig. S6B). In addition, passive immunization with ASN100 did not reduce bacterial burden in the lung (10). Also, although ASN100 reduced the dissemination of S. aureus cells from the lung to other organs, such as the spleen and kidney, it did not abolish staphylococcal colonization of those organs (10). In a recent phase II clinical trial, ASN100 failed to protect patients from ventilator-associated pneumonia (VAP) (ClinicalTrials registration no. NCT02940626). Therefore, although the antitoxin strategy might be effective in a passive immunization setting for certain S. aureus infections, it has limited effectiveness for active immunization and, for a successful vaccine, should be combined with antigens eliciting an immune response to block S. aureus dissemination and colonization.
MATERIALS AND METHODS
Ethics statement.
Human blood was obtained from healthy volunteers. The Institutional Review Board (IRB) of Pusan National University approved this study protocol (PNU IRB/2019_59_BR). Written informed consent was provided by study participants. The animal experiments were performed according to the institutional guidelines and approval (PNU-2018-1892 and PNU-2021-2919) of the Pusan National University Institutional Animal Care and Use Committee (PNU-IACUC).
Bacteria.
S. aureus strain USA300 LAC was grown to mid-logarithmic phase (optical density at 600 nm [OD600] of 0.8 to 1.5) in tryptic soy broth (TSB) at 37°C with shaking as described previously (27). Escherichia coli strains were grown in lysogeny broth (LB) containing kanamycin (10 μg/ml) at 37°C with shaking. For the strains carrying the protein expression plasmid, kanamycin (10 μg/ml) and chloramphenicol (50 μg/ml) were supplemented in LB.
Cloning and expression of PFTs.
The plasmids used in this study are listed in Table S1 in the supplemental material. PFT DNA sequences were PCR amplified with the primers listed in Table S2. The amplified PCR products were cloned in pET28a (Novagen) by Gibson assembly as described previously (28). After confirming the cloned DNA sequence by sequencing, the resulting plasmids were inserted into E. coli BL21(DE3)pLysS.
Purification of the recombinant toxins and toxoids.
His6-tagged recombinant toxin components or toxoids were expressed in E. coli, as described previously (27). The DNA sequences of LukS, LukF, LukE, LukD, HlgA, HlgB, HlgC, LukAB, and Hla were amplified by PCR from USA300 genome sequences using Q5 high fidelity DNA polymerase (Thermo Fisher Scientific). The PCR product was cloned into pET28a vector, resulting in the expression of proteins with an N- or C-terminal His6 tag. For purification of LukAB and LukAE323AB, we inserted a His6 tag in the N- and C-terminal regions of LukA and LukB, respectively. The recombinant proteins were expressed in E. coli BL21 pLysS with 0.2 mM isopropyl-β-d-thiogalactoside (IPTG) and purified with Ni-sepharose 6 fast flow resin (GE Healthcare). In the purification, the following buffers were used: loading buffer (20 mM sodium phosphate containing 0.1% Triton X-100, 150 mM NaCl, and 5 mM imidazole, pH 7.4), washing buffer (20 mM sodium phosphate containing 150 mM NaCl, 5 mM imidazole, pH 7.4), and elution buffer (20 mM sodium phosphate containing 150 mM NaCl, 500 mM imidazole, pH 7.4).
Cloning and expression of toxoids.
Nontoxic HlaH35L, LukAE323A, and LukST244A mutant proteins were generated by PCR-mediated site-directed mutagenesis (29). In the mutagenesis, the CAC codon at position 35 of Hla was mutated to CTC (30). The TTC codon at position 323 of LukA was altered to TCC (16), whereas the TGT codon at position 244 of LukS was changed to TGA (15). The mutant DNA was PCR amplified with the primers containing the mutation (Table S2). The PCR products were purified and inserted into pET28a (Novagen) by Gibson assembly as described previously (27). After confirmation by DNA sequencing, the resulting plasmids were inserted into E. coli BL21(DE3)pLysS.
Antibody generation.
Five hundred microliters of purified toxin or toxoid (1 mg/ml) in phosphate-buffered saline (PBS) was emulsified with 500 μl of complete Freund’s adjuvant (Sigma-Aldrich). The resulting emulsion was injected into a rabbit subcutaneously. One week later, the same amount of the antigen was emulsified with 500 μl of incomplete Freund’s adjuvant (Sigma-Aldrich) and injected subcutaneously into the rabbit. Two weeks later, 500 μl of blood was drawn from the rabbit ear vein, and the generation of antibody was confirmed by Western blotting. When antibody production was confirmed, blood was collected from the rabbit, and the obtained serum was stored at −80°C until use.
Coupling purified toxin and toxoid proteins to CNBr-activated sepharose beads.
The purified recombinant proteins were coupled to CNBr-activated sepharose beads as described previously (27). One gram of CNBr-sepharose beads was activated by washing with coupling buffer (0.1 M NaHCO3, pH 8.5) three times. The activated Sepharose beads were suspended in 4 ml of coupling buffer, and 5 mg of the recombinant protein dissolved with 1 ml of coupling buffer was added. After incubation at room temperature for 2 h, beads were washed three times with coupling buffer. Then, beads were incubated in 4 ml of 1 M ethanolamine (pH 8.0) at room temperature for 4 h. Finally, beads were washed with 0.1 M sodium phosphate (pH 7.4) until no absorbance was observed at 280 nm and were stored at 4°C until use.
Purification of toxin-specific IgGs from rabbit sera.
The rabbit immune sera were subjected to the toxin or toxoid antigen affinity column. The toxin or toxoid antigen-coupled sepharose column was equilibrated with washing buffer (0.1 M sodium phosphate containing 0.1 M NaCl, pH 7.4). Rabbit immune serum (1 ml, about 60 mg of protein) was loaded onto the affinity column, and the column was extensively washed with washing buffer. The bound IgGs were eluted with elution buffer (0.15 M glycine/HCl, pH 2.2) and immediately neutralized to pH 7.5 with neutralizing buffer (1 M Tris-HCl buffer, pH 9.0). The purified IgGs were concentrated with Centricon (Merck Millipore; 10-kDa cutoff) with buffer changing into PBS. The purity of the IgGs was verified by SDS-PAGE under reducing and nonreducing conditions.
Dot immunoblot assay.
The polyvinylidene fluoride (PVDF) membrane strips (Immobilion P, 0.22-μm pore size, Millipore) were activated in methanol for 30 s. The membrane strips were washed with distilled water for 5 min with gentle shaking. The purified recombinant toxin components (1 μg of LukS, LukF, HlgA, HlgB, HlgC, LukE, LukD, and LukAB) were spotted onto the strips. S. aureus FnbpAN2N3 (N2N3 domain of fibronectin-binding protein with His6 tag; 1 μg) was also spotted as a negative control. The PVDF membrane strips were dried at room temperature for 2 h and fixed with methanol for 15 s. The membrane strips were washed one time with distilled water, washed three times with Tris-buffered saline (TBS) (50 mM Tris-HCl, pH 7.5, 150 mM NaCl), and blocked with 5% skim milk for 2 h. After the addition of toxin IgGs (0.15 μg of each IgG/9 ml of 5% skim milk), the membrane strips were incubated at room temperature for 1 h. The membrane strips were washed 3 times with TBST (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.2% Tween 20), and goat anti-rabbit IgG-horseradish peroxidase (HRP) (0.12 μg/9 ml 5% skim milk; Santa Cruz) was added and incubated at room temperature for 1 h. The membrane strips were washed three times with TBST, and antibody binding was detected with a Pico EPD Western blot detection kit (ELPIS-Biotech). The obtained blot intensity was measured with the Image Lab program of the ChemiDoc touch imaging system (Bio-Rad, USA). In the measurement, the blot intensity of the antibody and its cognate antigen combination was set to 1.
Isolation of hPMNs and rPMNs.
The hPMNs were isolated as described previously (31). Briefly, collected human blood (2 ml) was mixed with Polymorphprep (2 ml; Alere Technologies AS) in 5-ml round-bottom tubes at room temperature. The samples were centrifuged (450 × g) at 20°C for 30 min. After removing the supernatant, the lower band containing PMNs was collected, washed 2 times with RPMI (Gibco), and centrifuged (400 × g) at 20°C for 10 min. The collected cells were gently resuspended in 2 ml of RPMI with 0.02% human serum albumin. Equal volumes of the treated cells and trypan blue were mixed and incubated for 3 min before counting on a hemacytometer to determine the percentage of cells stained with trypan blue. The rPMNs were purified as described previously (32). Briefly, three ml of rabbit blood was collected in EDTA-coated tubes (S-Monovette), mixed with an equal volume of ammonium chloride RBC lysis buffer (Sigma-Aldrich), and then incubated for 15 min. After centrifugation at 400 × g for 10 min, the pellet was washed three times and suspended in PBS. Then, rPMNs were counted with a hemacytometer.
Cytotoxicity activity.
Cytotoxicity assays with hPMNs and rPMNs were carried out as described previously with minor modifications (33). Human and rabbit PMNs (2 × 106 cells per well) were seeded in 48-well cell culture plates (SPL), and a mix of S and F subunits (each 65 ng) or LukAB (130 ng) was added and incubated at 37°C and 5% CO2 for 1 h. Then, the numbers of the surviving cells were calculated with a hemacytometer.
Isolation of RBCs.
To prevent coagulation, 1 ml of rabbit blood was collected into hirudin-coated Vacutainer tubes (BD, Becton Drive, Germany) (34). The tube was centrifuged (100 × g) at 4°C for 10 min to collect RBCs. The collected RBCs were washed two times with PBS containing 0.02% bovine serum albumin (BSA) and then collected again by centrifugation (500 × g) at 4°C for 5 min. Finally, the RBCs were diluted with PBS containing 0.02% BSA to 2%.
RBC hemolysis assay.
An RBC hemolysis assay was carried out as described previously with minor modifications (35). The 2% RBCs (100 μl per well) were seeded in a 96-well cell culture plate. As positive controls for RBC hemolysis, 1 μl of 5% Triton X-100 was added into 100 μl of RBCs (0.05% Triton X-100), and NH4Cl lysis solutions (Sigma-Aldrich) were added to a 48-well cell culture plate. PBS (10 μl) was used as a negative control. Then, 1 μg of purified PFTs was added to the plate and incubated with 5% CO2 at 37°C for 1 h. After incubation, the supernatant was collected by centrifugation at 500 × g for 5 min, and absorbance at 405 nm was measured with a spectrophotometer (Eppendorf BioPhotometer).
FITC labeling of cells.
Target cells, 2.5 ml of 2% RBCs (1 × 108 cells/ml), or PMNs (2 × 107 cells/ml) were incubated with 100 μl of FITC stock solution (1 mg/ml) for 15 min at room temperature. After incubation, the labeled cells were washed 2 or 3 times with PBS containing 2% human serum albumin (HSA) or BSA to remove nonreacted FITC. The FITC-labeled cells were collected by centrifugation.
Flow cytometry.
The flow cytometry analysis was carried out with an Accuri C6 Plus flow cytometer (BD Biosciences), as described previously (27, 36).
Rabbit model of immunization and infection.
The injection solution (250 μl) of HlaH35L, LukS, HlgA (25 μg each), and LukAE323AB (50 μg, total 125 μg/125 μl) was mixed with Alhydrogel (125 μl, 10 mg/ml; Invivo Gen, USA) as an adjuvant and then administered intramuscularly into the dorsal lumbar region of the outbred New Zealand White rabbits (2 kg; SamDako, Korea) on days 0, 16, 42, and 65. On day 72, rabbits were injected with S. aureus USA300 LAC (3.5 × 107 CFU/kg in 100 μl of PBS with 0.01% BSA) via the ear vein and were monitored every 12 h for 10 days. Blood was collected every 2 weeks from the rabbits, and the IgG titers against 4 injected antigens were measured by ELISA. The surviving three control rabbits were euthanized before death, whereas all immunized mice were euthanized at day 10 postinfection. Kidneys were removed aseptically and examined for morphology and the presence of abscesses. Then, the kidneys were homogenized, serially diluted, and spread on the blood agar to enumerate the CFU of S. aureus.
In a high-challenge dose experiment, rabbits (6 for the control group and 7 for the vaccinated group) were immunized three times in a 2-week interval (i.e., days 0, 14, and 28) and challenged with a dose of 7 × 107 CFU/kg S. aureus on day 35. The rabbit immunization and bacterial challenge protocol was reviewed and approved by the Pusan National University Institutional Animal Care and Use Committee (protocol number PNU-2018-1892 and PNU-2021-2919).
ELISA for detection of antigen-specific antibodies.
ELISA plates (Falcon, Franklin Lakes, NJ) were coated with 100 μl of each antigen stock solution (5 μg of each antigen dissolved in 5 ml of coating buffer [50 mM sodium bicarbonate, pH 9.4]) and then incubated overnight at 4°C. After removal of the supernatant, each well was washed with 200 μl of wash buffer (PBS with 0.05% Tween 20) 3 times. Each well received 100 μl of blocking buffer (PBS with 0.2% BSA), was incubated at room temperature for 1 h, and was washed with 200 μl of wash buffer 3 times. Threefold serially diluted samples (starting from 1:180 for rabbit sera) were applied to plates and incubated at room temperature for 2 h. HRP-conjugated anti-rabbit lgG antibody (50 μl/each well; Santa Cruz, USA) was added to each well and incubated at room temperature for 2 h. For color development, 50 μl of tetramethylbenzidine solution (TMB; Thermo, USA) was added to each well and incubated for 1 to 3 min. After the addition of the stopping solution (50 μl, 0.5 N HCl), the OD450 nm was measured by an ELISA reader (MULTISKAN GO, Thermo, USA). Endpoint titers of each antigen antibody were expressed as the reciprocal log2 of the last dilution giving a positive color development (OD450 nm of ≥0.3).
Data processing and statistical analysis.
Unless otherwise stated, experiments were carried out at least three times independently, and the results were presented as the mean ± standard deviation (SD). However, if deemed appropriate, a representative result of at least three independent experiments was provided. Statistical significance was measured by unpaired Student’s t test or log rank test with GraphPad Prism (version 8.4.0). Differences were considered significant when the P value was equal to or less than 0.05.
ACKNOWLEDGMENTS
This research was supported by the SGER (2018R1D1A1A02085533) project of the National Research Foundation of Korea and, in part, by “Rediscovery of the Past R&D Result” through the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for Advancement of Technology (KIAT) (grant number P0013891) to B.L.L. This research was supported by the NIH (AI143792) and the Indiana Clinical and Translational Sciences Institute to T.B., who is funded, in part, by grant number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical, and Translational Sciences Award.
Footnotes
Supplemental material is available online only.
Contributor Information
Taeok Bae, Email: tbae@iun.edu.
Bok Luel Lee, Email: brlee@pusan.ac.kr.
Victor J. Torres, New York University School of Medicine
REFERENCES
- 1.Tong SY, Davis JS, Eichenberger E, Holland TL, FowlerVG, Jr.. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LS, Fowler VG, Shukla SK, Rose WE, Proctor RA. 2020. Development of a vaccine against Staphylococcus aureus invasive infections: evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol Rev 44:123–153. 10.1093/femsre/fuz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spaan AN, van Strijp JAG, Torres VJ. 2017. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 15:435–447. 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seilie ES, Bubeck Wardenburg J. 2017. Staphylococcus aureus pore-forming toxins: the interface of pathogen and host complexity. Semin Cell Dev Biol 72:101–116. 10.1016/j.semcdb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko J, Kamio Y. 2004. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem 68:981–1003. 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 6.Spaan AN, Henry T, van Rooijen WJM, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJC, van Kessel KPM, Vandenesch F, Lina G, van Strijp JAG. 2013. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13:584–594. 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Yanai M, Rocha MA, Matolek AZ, Chintalacharuvu A, Taira Y, Chintalacharuvu K, Beenhouwer DO. 2014. Separately or combined, LukG/LukH is functionally unique compared to other staphylococcal bicomponent leukotoxins. PLoS One 9:e89308. 10.1371/journal.pone.0089308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landrum ML, Lalani T, Niknian M, Maguire JD, Hospenthal DR, Fattom A, Taylor K, Fraser J, Wilkins K, Ellis MW, Kessler PD, Fahim RE, Tribble DR. 2017. Safety and immunogenicity of a recombinant Staphylococcus aureus α-toxoid and a recombinant Panton-Valentine leukocidin subunit, in healthy adults. Hum Vaccin Immunother 13:791–801. 10.1080/21645515.2016.1248326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.François B, Mercier E, Gonzalez C, Asehnoune K, Nseir S, Fiancette M, Desachy A, Plantefève G, Meziani F, de Lame PA, Laterre PF, MASTER 1 study group. 2018. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med 44:1787–1796. 10.1007/s00134-018-5229-2. [DOI] [PubMed] [Google Scholar]
- 10.Stulik L, Rouha H, Labrousse D, Visram ZC, Badarau A, Maierhofer B, Groß K, Weber S, Kramarić MD, Glojnarić I, Nagy G, Croisier D, Nagy E. 2019. Preventing lung pathology and mortality in rabbit Staphylococcus aureus pneumonia models with cytotoxin-neutralizing monoclonal IgGs penetrating the epithelial lining fluid. Sci Rep 9:5339. 10.1038/s41598-019-41826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu XQ, Robbie GJ, Wu Y, Esser MT, Jensen K, Schwartz HI, Bellamy T, Hernandez-Illas M, Jafri HS. 2017. Safety, tolerability, and pharmacokinetics of MEDI4893, an investigational, extended-half-life, anti-Staphylococcus aureus alpha-toxin human monoclonal antibody, in healthy adults. Antimicrob Agents Chemother 61:e01020-16. 10.1128/AAC.01020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruzin A, Wu Y, Yu L, Yu XQ, Tabor DE, Mok H, Tkaczyk C, Jensen K, Bellamy T, Roskos L, Esser MT, Jafri HS. 2018. Characterisation of anti-alpha toxin antibody levels and colonisation status after administration of an investigational human monoclonal antibody, MEDI4893, against Staphylococcus aureus alpha toxin. Clin Transl Immunology 7:e1009. 10.1002/cti2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oganesyan V, Peng L, Damschroder MM, Cheng L, Sadowska A, Tkaczyk C, Sellman BR, Wu H, Dall'Acqua WF. 2014. Mechanisms of neutralization of a human anti-α-toxin antibody. J Biol Chem 289:29874–29880. 10.1074/jbc.M114.601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magyarics Z, Leslie F, Bartko J, Rouha H, Luperchio S, Schörgenhofer C, Schwameis M, Derhaschnig U, Lagler H, Stiebellehner L, Firbas C, Weber S, Campanaro E, Jilma B, Nagy E, Stevens C. 2019. Randomized, double-blind, placebo-controlled, single-ascending-dose study of the penetration of a monoclonal antibody combination (ASN100) targeting Staphylococcus aureus cytotoxins in the lung epithelial lining fluid of healthy volunteers. Antimicrob Agents Chemother 63:e00350-19. 10.1128/AAC.00350-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laventie BJ, Guérin F, Mourey L, Tawk MY, Jover E, Maveyraud L, Prévost G. 2014. Residues essential for Panton-Valentine leukocidin S component binding to its cell receptor suggest both plasticity and adaptability in its interaction surface. PLoS One 9:e92094. 10.1371/journal.pone.0092094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuMont AL, Yoong P, Liu X, Day CJ, Chumbler NM, James DB, AlonzoF, III, Bode NJ, Lacy DB, Jennings MP, Torres VJ. 2014. Identification of a crucial residue required for Staphylococcus aureus LukAB cytotoxicity and receptor recognition. Infect Immun 82:1268–1276. 10.1128/IAI.01444-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaan AN, Reyes-Robles T, Badiou C, Cochet S, Boguslawski KM, Yoong P, Day CJ, de Haas CJC, van Kessel KPM, Vandenesch F, Jennings MP, Le Van Kim C, Colin Y, van Strijp JAG, Henry T, Torres VJ. 2015. Staphylococcus aureus targets the Duffy antigen receptor for chemokines (DARC) to lyse erythrocytes. Cell Host Microbe 18:363–370. 10.1016/j.chom.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adhikari RP, Kort T, Shulenin S, Kanipakala T, Ganjbaksh N, Roghmann MC, Holtsberg FW, Aman MJ. 2015. Antibodies to S. aureus LukS-PV attenuated subunit vaccine neutralize a broad spectrum of canonical and non-canonical bicomponent leukotoxin pairs. PLoS One 10:e0137874. 10.1371/journal.pone.0137874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diep BA, Le VTM, Visram ZC, Rouha H, Stulik L, Dip EC, Nagy G, Nagy E. 2016. Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of leukocidins in addition to alpha-hemolysin. Antimicrob Agents Chemother 60:6333–6340. 10.1128/AAC.01213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Malone C, Griffith S, Horswill AR, Torres VJ, Voyich JM. 2012. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One 7:e36532. 10.1371/journal.pone.0036532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manohar M, Maheswaran SK, Frommes SP, Lindorfer RK. 1967. Platelet damaging factor, a fifth activity of staphylococcal alpha-toxin. J Bacteriol 94:224–231. 10.1128/jb.94.1.224-231.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhakdi S, Muhly M, Mannhardt U, Hugo F, Klapettek K, Mueller-Eckhardt C, Roka L. 1988. Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J Exp Med 168:527–542. 10.1084/jem.168.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouha H, Badarau A, Visram ZC, Battles MB, Prinz B, Magyarics Z, Nagy G, Mirkina I, Stulik L, Zerbs M, Jägerhofer M, Maierhofer B, Teubenbacher A, Dolezilkova I, Gross K, Banerjee S, Zauner G, Malafa S, Zmajkovic J, Maier S, Mabry R, Krauland E, Wittrup KD, Gerngross TU, Nagy E. 2015. Five birds, one stone: neutralization of α-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs 7:243–254. 10.4161/19420862.2014.985132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badarau A, Rouha H, Malafa S, Battles MB, Walker L, Nielson N, Dolezilkova I, Teubenbacher A, Banerjee S, Maierhofer B, Weber S, Stulik L, Logan DT, Welin M, Mirkina I, Pleban C, Zauner G, Gross K, Jägerhofer M, Magyarics Z, Nagy E. 2016. Context matters: the importance of dimerization-induced conformation of the LukGH leukocidin of Staphylococcus aureus for the generation of neutralizing antibodies. MAbs 8:1347–1360. 10.1080/19420862.2016.1215791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao X, Kim J, Zhang Q, Jiang T, Ahn DH, Jung Y, Matsushita M, Bae T, Lee BL. 2020. The N2N3 domains of ClfA, FnbpA, and FnbpB in Staphylococcus aureus bind to human complement factor H, and their antibodies enhance the bactericidal capability of human blood. J Biochem 169:543–553. 10.1093/jb/mvaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson DG, Young L, Chuang RY, Venter JC, HutchisonCA, III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 29.Stemmer WP, Morris SK. 1992. Enzymatic inverse PCR: a restriction site independent, single-fragment method for high-efficiency, site-directed mutagenesis. Biotechniques 13:214–220. [PubMed] [Google Scholar]
- 30.Brady RA, Mocca CP, Prabhakara R, Plaut RD, Shirtliff ME, Merkel TJ, Burns DL. 2013. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of Staphylococcus aureus infection. PLoS One 8:e63040. 10.1371/journal.pone.0063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrante A, Thong YH. 1980. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods 36:109–117. 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- 32.Szponder T, Wessely-Szponder J, Smolira A. 2017. Evaluation of platelet-rich plasma and neutrophil antimicrobial extract as two autologous blood-derived agents. Tissue Eng Regen Med 14:287–296. 10.1007/s13770-017-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwizera R, Akampurira A, Kandole TK, Nielsen K, Kambugu A, Meya DB, Boulware DR, Rhein J, on behalf of the ASTRO-CM Study Team. 2017. Evaluation of trypan blue stain in a haemocytometer for rapid detection of cerebrospinal fluid sterility in HIV patients with cryptococcal meningitis. BMC Microbiol 17:182. 10.1186/s12866-017-1093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoong P, Torres VJ. 2015. Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat Commun 6:8125. 10.1038/ncomms9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasquez MT, Lubkin A, Reyes-Robles T, Day CJ, Lacey KA, Jennings MP, Torres VJ. 2020. Identification of a domain critical for Staphylococcus aureus LukED receptor targeting and lysis of erythrocytes. J Biol Chem 295:17241–17250. 10.1074/jbc.RA120.015757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim B, Jiang T, Bae JH, Yun HS, Jang SH, Kim JH, Kim JD, Hur JH, Shibata K, Kurokawa K, Jung Y, Peschel A, Bae T, Lee BL. 2019. In Staphylococcus aureus, the particulate state of the cell envelope is required for the efficient induction of host defense responses. Infect Immun 87:e00674-19. 10.1128/IAI.00674-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00342-21-s0001.pdf, PDF file, 0.09 MB (94.6KB, pdf)
Supplemental material. Download IAI.00342-21-s0002.pdf, PDF file, 0.06 MB (66KB, pdf)
Supplemental material. Download IAI.00342-21-s0003.pdf, PDF file, 0.09 MB (95.3KB, pdf)
Supplemental material. Download IAI.00342-21-s0004.pdf, PDF file, 0.1 MB (124.1KB, pdf)
Supplemental material. Download IAI.00342-21-s0005.pdf, PDF file, 0.1 MB (124.6KB, pdf)
Supplemental material. Download IAI.00342-21-s0006.pdf, PDF file, 0.2 MB (172.6KB, pdf)
Supplemental material. Download IAI.00342-21-s0007.pdf, PDF file, 0.04 MB (38.3KB, pdf)
Supplemental material. Download IAI.00342-21-s0008.pdf, PDF file, 0.04 MB (43KB, pdf)