ABSTRACT
Lyme disease is a multistage inflammatory disease caused by the spirochete Borrelia burgdorferi transmitted through the bite of an infected Ixodes scapularis tick. We previously discovered a B. burgdorferi infectivity gene, bbk13, that facilitates mammalian infection by promoting spirochete population expansion in the skin inoculation site. Initial characterization of bbk13 was carried out using an intradermal needle inoculation model of mouse infection, which does not capture the complex interplay of the pathogen-vector-host triad of natural transmission. Here, we aimed to understand the role of bbk13 in the enzootic cycle of B. burgdorferi. B. burgdorferi spirochetes lacking bbk13 were unable to be acquired by naive larvae fed on needle-inoculated mice. Using a capsule feeding approach to restrict tick feeding activity to a defined skin site, we determined that delivery by tick bite alleviated the population expansion defect in the skin observed after needle inoculation of Δbbk13 B. burgdorferi. Despite overcoming the early barrier in the skin, Δbbk13 B. burgdorferi remained attenuated for distal tissue colonization after tick transmission. Disseminated infection by Δbbk13 B. burgdorferi was improved in needle-inoculated immunocompromised mice. Together, we established that bbk13 is crucial to the maintenance of B. burgdorferi in the enzootic cycle and that bbk13 is necessary beyond early infection in the skin, likely contributing to host immune evasion. Moreover, our data highlight the critical interplay between the pathogen, vector, and host as well as the distinct molecular genetic requirements for B. burgdorferi to survive at the pathogen-vector-host interface and achieve productive disseminated infection.
KEYWORDS: Borrelia burgdorferi, Lyme disease, bbk13, host-pathogen interactions, immune evasion, tick transmission, vector-borne diseases
INTRODUCTION
Lyme disease remains the predominant vector-borne disease in the United States (1). Borrelia burgdorferi, the causative agent of Lyme disease (2, 3), is transmitted to humans via the bite of an infected Ixodes scapularis tick (4). The acute manifestations of Lyme disease involve fever, nausea, and, in some cases, a characteristic bull’s-eye rash (erythema migrans) at the tick bite site. If undiagnosed and untreated, Lyme disease progresses to a late stage as B. burgdorferi disseminates and colonizes various distal tissues such as the joints (Lyme arthritis), the heart (Lyme carditis), and the nervous system (neuroborreliosis) (5).
In nature, the B. burgdorferi reservoir is maintained by the enzootic cycle of transmission and acquisition of B. burgdorferi from various vertebrate hosts as the Ixodes scapularis tick feeds and progresses through its life cycle (6). After hatching, an unfed Ixodes larva will feed on blood from small vertebrate hosts such as rodents, triggering it to molt to the nymph stage. If the host is infected, the larva can acquire B. burgdorferi with the blood meal. B. burgdorferi will then proliferate in the fed larva and persist through the molting phase, which then gives rise to infected unfed nymphs. It is primarily the nymph stage of Ixodes that transmits B. burgdorferi to humans. The risk of contracting Lyme disease rises in areas with high numbers of B. burgdorferi-infected nymphal ticks (7). Nymphs then molt into sexually mature adults, which mate and lay eggs to give rise to the next generation of uninfected Ixodes ticks.
The molecular mechanisms that promote B. burgdorferi infection of its mammalian host are not completely understood, and this has slowed the development of new strategies to curb the rising prevalence of Lyme disease. Genetic manipulation of B. burgdorferi coupled with a mouse model of infection has been central to the discovery of novel B. burgdorferi infectivity genes. Needle inoculation of mouse models of infection allows the precise control of both the inoculum dose and route of inoculation, enabling detailed characterization of infection kinetics. However, needle inoculation is an artificial route of infection that excludes the contribution of the tick vector to B. burgdorferi transmission—both its influence on B. burgdorferi itself and its influence on the host microenvironment at the site of feeding. B. burgdorferi adapts to the conditions present in the unfed tick and undergoes significant transcriptional changes during tick feeding, potentiating its infectivity (8, 9). Moreover, the feeding activity of the ticks themselves, particularly the influence of the tick saliva on the skin feeding site, affects B. burgdorferi infection (10–12). The tick feeding site is unique in that it is the combined interaction of the pathogen, the vector, and the mammalian host, which is likely critical to the adaptation and mechanisms of pathogenesis of B. burgdorferi as well as other tick-borne pathogens.
We recently characterized the role of the novel B. burgdorferi infectivity gene bbk13 in the early phase of B. burgdorferi mammalian infection. Using an intradermal needle inoculation model of infection, we showed that bbk13 is important for spirochete population expansion in the skin, which then drives the downstream steps of disseminated infection, culminating in B. burgdorferi colonization of distal tissues (13). Consequently, the loss of bbk13 leads to reduced colonization of distal tissue sites such as the skin, heart, and joints (13). Furthermore, we have established that BBK13 forms large oligomers in the spirochete membrane and is highly immunogenic (13, 14). In this work, we used Ixodes ticks and mice to study the role of bbk13 in the natural enzootic cycle of B. burgdorferi. We demonstrate that bbk13 is similarly required for productive disseminated infection when delivered by tick bite transmission. Interestingly, B. burgdorferi inoculation by tick feeding bypassed the early requirement for bbk13 to promote B. burgdorferi population expansion in the skin. Despite this, bbk13 remained critical for efficient colonization of distal tissues. In addition, we show that distal tissue colonization by Δbbk13 B. burgdorferi is restored in an immunocompromised mouse host. This work points toward a role of bbk13 in host immune evasion and uncovers a more complex model of bbk13 function in B. burgdorferi mammalian infection, emphasizing the impact of the natural tick vector on the establishment of B. burgdorferi infection as well as the distinct molecular genetic requirements for B. burgdorferi survival during early localized infection at the pathogen-vector-host interface and the establishment of productive disseminated infection of distal host tissues.
RESULTS
Naive larvae fail to acquire Δbbk13 B. burgdorferi from infected mice.
B. burgdorferi’s enzootic cycle depends not only on successful tick transmission but also on its acquisition from the animal host by naive larvae. Needle inoculation of Δbbk13 B. burgdorferi into mice leads to reduced, but not absent, colonization of distal tissues, including the skin (13). This allowed us to ask whether Δbbk13 B. burgdorferi can be acquired from infected mice by feeding Ixodes larvae. We needle inoculated cohorts of C3H/HeN mice with 104 wild-type (WT), Δbbk13, or Δbbk13/bbk13+ B. burgdorferi spirochetes intradermally to generate infected mice for naive tick feeding. Consistent with what we have shown previously (13), Δbbk13 B. burgdorferi spirochetes were found at reduced levels during blood dissemination (Fig. 1A) and in distal tissues (Fig. 1B). More importantly, Δbbk13 B. burgdorferi was still present in the skin, as indicated by reisolation from the ear (Fig. 1B, top row). B. burgdorferi colonization of tissues was assessed via semiquantitative tissue reisolation assay (see Materials and Methods). Briefly, reisolation cultures were inspected daily for the presence of spirochetes by dark-field microscopy. A numerical score was assigned to each culture corresponding to the observed density of spirochetes (0 for no spirochetes and 1 to 3 depending on the number of spirochetes).
FIG 1.
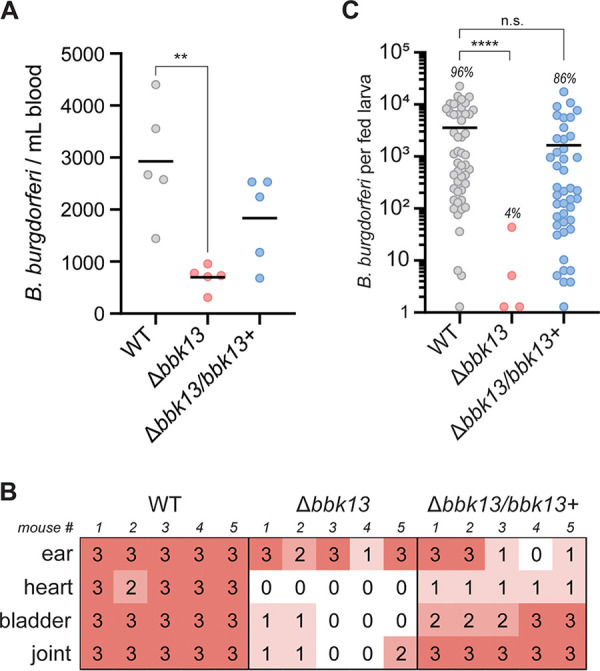
Naive larval ticks fail to acquire Δbbk13 B. burgdorferi during feeding on infected mice. Groups of six C3H/HeN mice were needle inoculated intradermally with 104 WT, Δbbk13, or Δbbk13/bbk13+ B. burgdorferi spirochetes. (A) At 6 days postinoculation, disseminating B. burgdorferi spirochetes were enumerated by plating blood in solid BSK-agarose medium and enumerating CFU. Each dot represents an individual mouse. The mean value is indicated by a black horizontal line. The Kruskal-Wallis test with Dunn’s multiple comparisons was used to determine statistical significance (**, P ≤ 0.01). (B) At 4 weeks postinoculation, the indicated tissues were assessed for B. burgdorferi by a reisolation assay. Semiquantitative scoring of reisolation cultures was performed (“0” indicates no spirochetes, and “1” to “3” indicate increasing spirochete densities). (C) Naive tick larvae fed to repletion on C3H/HeN mice 3 weeks after needle inoculation with 104 WT, Δbbk13, or Δbbk13/bbk13+ B. burgdorferi spirochetes. Individual fed larvae were homogenized and plated in solid BSK-agarose medium, and B. burgdorferi loads were determined by enumerating CFU. Each data point represents an individual tick. The tick infection rate for each B. burgdorferi clone is indicated above the corresponding scatterplot. The mean is indicated by a horizontal black line. The Kruskal-Wallis test with Dunn’s multiple comparisons was used to determine statistical significance (****, P < 0.0001; n.s., not significant).
At 3 weeks postinoculation, naive Ixodes larvae were fed to repletion on the three groups of B. burgdorferi-infected mice. Individual fed larvae were assessed for B. burgdorferi acquisition via homogenization and plating in solid Barbour-Stoenner-Kelly (BSK)-agarose medium to quantify the number of B. burgdorferi spirochetes per tick. The rate of acquisition of wild-type B. burgdorferi after larval tick feeding was 96% (48/50 larvae), with an average of ∼5 × 103 B. burgdorferi spirochetes per tick (Fig. 1C). In stark contrast, only 4% of the Ixodes larvae (4/100 larvae) that fed on Δbbk13 B. burgdorferi-infected mice acquired spirochetes and with B. burgdorferi loads at dramatically reduced levels (Fig. 1C). The rate of acquisition of Δbbk13/bbk13+ B. burgdorferi from infected mice was 86% (43/50 larvae), with the average number of B. burgdorferi spirochetes per tick being comparable to that of the wild-type B. burgdorferi group (Fig. 1C). These data indicated that there is a significant deficiency in the ability of Ixodes larvae to acquire spirochetes from Δbbk13 B. burgdorferi-infected mice.
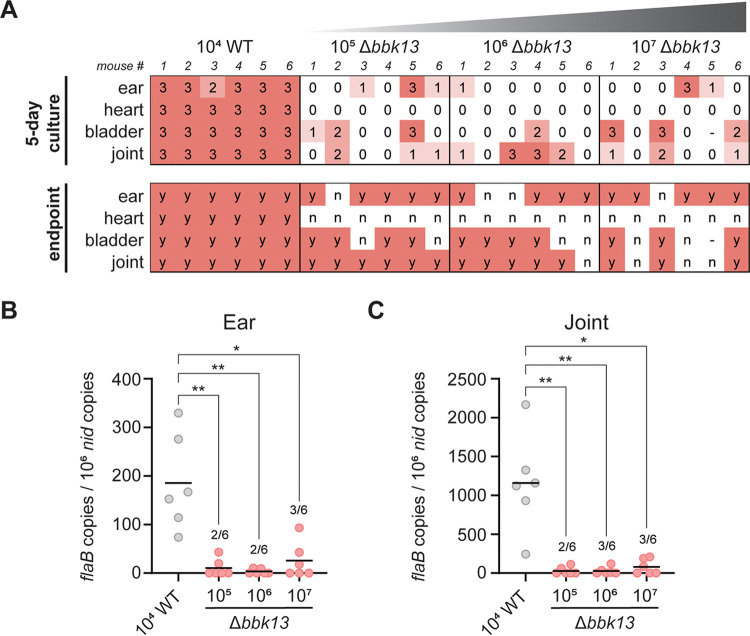
Increasing the inoculum dose does not rescue low Δbbk13 B. burgdorferi numbers in distal tissues.
For Ixodes ticks to acquire B. burgdorferi through feeding, the spirochetes must be present in sufficient numbers at the skin feeding site. Although both wild-type and Δbbk13 B. burgdorferi spirochetes were present at a skin site (Fig. 1B, ear), Δbbk13 B. burgdorferi spirochetes were found at reduced numbers compared to wild-type B. burgdorferi during late infection (13). Reduced Δbbk13 B. burgdorferi numbers in the skin likely negatively influence acquisition via tick feeding, raising the question of whether low Δbbk13 B. burgdorferi acquisition was due to reduced spirochete numbers and/or a molecular function of BBK13. To address this, we attempted to increase the Δbbk13 B. burgdorferi load in the skin by increasing the inoculum dose. We needle inoculated 105, 106, or 107 Δbbk13 B. burgdorferi spirochetes intradermally into C3H/HeN mice. At 3 weeks postinoculation, distal tissues were collected and assessed for B. burgdorferi colonization via the semiquantitative tissue reisolation assay (13). After 5 days of incubation, nearly all of the reisolation cultures from mice inoculated with 104 wild-type B. burgdorferi spirochetes demonstrated a score of “3” (23/24 cultures) (Fig. 2A). At the same time point, reisolation cultures from mice inoculated with increasing doses of Δbbk13 B. burgdorferi were mostly negative for spirochetes (105 dose, 9/24 positive; 106 dose, 6/24 positive; 107 dose, 8/24 positive), and the positive cultures demonstrated varying spirochete densities (scores ranging from 1 to 3) (Fig. 2A). Thus, no observable increase in distal tissue colonization was detected upon increasing the inoculum dose of Δbbk13 B. burgdorferi. These trends were maintained at the 2-week endpoint of the reisolation assay (Fig. 2A). Direct measurement of B. burgdorferi loads in the distal tissues by quantitative PCR (qPCR) supported the findings in the tissue reisolation assay. Despite the increased inoculum doses, the mean Δbbk13 B. burgdorferi loads in the ears and joints remained consistently lower than those of wild-type B. burgdorferi at a lower inoculum (Fig. 2B and C). Moreover, Δbbk13 B. burgdorferi loads were above the level of detection in only 2 or 3 tissues out of 6 mice per inoculum group, further emphasizing the reduced efficiency of distal tissue colonization of Δbbk13 B. burgdorferi.
FIG 2.
Increasing the inoculum dose does not alleviate the infectivity defect of Δbbk13 B. burgdorferi. Groups of six C3H/HeN mice were intradermally inoculated with 104 WT B. burgdorferi or increasing doses (105, 106, and 107) of Δbbk13 B. burgdorferi spirochetes. (A) At 3 weeks postinoculation, tissues were assessed for B. burgdorferi by a reisolation assay. Semiquantitative scoring of reisolation cultures was performed following 5 days (0 indicates no spirochetes, and 1 to 3 indicate increasing spirochete densities) and 14 days (endpoint, scored for the presence [“y”] or absence [“n”] of spirochetes) of culture incubation. (B and C) Total DNA was extracted from the ears (B) and joints (C). The B. burgdorferi load was measured by quantifying B. burgdorferi flaB copies normalized to 106 mouse nid copies using quantitative PCR. In cases where not all samples per group had detectable B. burgdorferi DNA, the numbers of positive samples out of the total mice per group are indicated in the scatterplot. Data points below the level of detection are not shown. The mean is indicated by a horizontal black line. The Kruskal-Wallis test with Dunn’s multiple comparisons was used to determine statistical significance. (*, P < 0.05; **, P < 0.01).
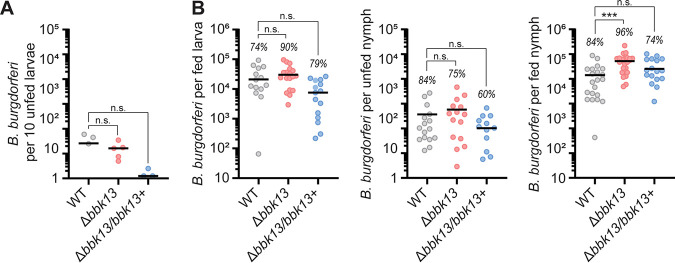
bbk13 is dispensable for B. burgdorferi maintenance in Ixodes ticks.
To further understand the role of bbk13 in the enzootic cycle of B. burgdorferi, we investigated the survival of Δbbk13 B. burgdorferi in ticks after the blood meal and through the molt. Given the inefficiency of Δbbk13 B. burgdorferi to be acquired by feeding ticks, we artificially infected unfed Ixodes larvae with wild-type, Δbbk13, or Δbbk13/bbk13+ B. burgdorferi by immersion (15). After recovery from immersion, positive infection was confirmed and quantified by plating homogenates from groups of 10 larvae per infecting B. burgdorferi clone. No significant difference was detected between the numbers of B. burgdorferi spirochetes per 10 larvae across the three B. burgdorferi clones (Fig. 3A). The infected larvae were fed to repletion on naive C3H/HeN mice. Approximately 7 days after feeding, 20 fed larvae per infecting B. burgdorferi clone were individually homogenized and plated in solid BSK-agarose medium to measure spirochete loads. Robust multilog expansion was detected for all B. burgdorferi clones (Fig. 3B, left), with no statistical difference between the average numbers of Δbbk13 or Δbbk13/bbk13+ B. burgdorferi spirochetes and those of wild-type B. burgdorferi (Fig. 3B, left). A portion of the fed larvae were allowed to molt to unfed nymphs, from which 20 nymphs per B. burgdorferi clone were then individually assayed for B. burgdorferi loads by homogenization and plating for CFU. B. burgdorferi loads were comparable across all three groups and displayed the expected decline in number following the molt (Fig. 3B, middle) (16). The nymphs were then allowed to feed to repletion on mice, and the number of B. burgdorferi spirochetes per fed nymph was determined. No bbk13-dependent decrease in the B. burgdorferi load per fed nymph was detected; rather, the number of Δbbk13 B. burgdorferi spirochetes per fed nymph was found to be significantly increased compared to that of wild-type B. burgdorferi (Fig. 3B, right). Although not statistically significant, the average number of Δbbk13/bbk13+ B. burgdorferi spirochetes in all tick stages except for fed nymphs tended to be lower than those of wild-type and Δbbk13 B. burgdorferi (Fig. 3), perhaps due to dysregulated expression of bbk13 from the shuttle vector (13). In all, the loss of bbk13 did not result in a defect in the replication and survival of B. burgdorferi in ticks.
FIG 3.
bbk13 is dispensable for replication and survival in ticks. Naive larvae were artificially infected by immersion with wild-type (WT), Δbbk13, or Δbbk13/bbk13+ B. burgdorferi. B. burgdorferi loads in ticks at various stages of development were measured by plating tick homogenates in solid BSK-agarose medium and enumerating CFU. (A) Unfed larvae were assessed for B. burgdorferi loads following artificial infection. Each data point represents a group of 10 larvae. (B) B. burgdorferi loads in fed larvae, unfed nymphs, and fed nymphs were determined. Each data point represents an individual tick. The tick infection rate for each B. burgdorferi clone is indicated above the corresponding scatterplot. The mean is indicated by a horizontal black line. The Kruskal-Wallis test with Dunn’s multiple comparisons was used to determine statistical significance (***, P < 0.001; n.s., not significant).
bbk13 is critical for B. burgdorferi infection of mice by Ixodes nymph transmission.
Our previous study used needle inoculation to demonstrate the importance of bbk13 during mouse infection (13). In nature, B. burgdorferi is transmitted via the feeding activity of Ixodes ticks. Moreover, tick feeding influences both B. burgdorferi and the host to promote infection (6, 17). Unfed nymphs carrying equivalent loads of wild-type, Δbbk13, or Δbbk13/bbk13+ B. burgdorferi (Fig. 3B, middle) were used to test the requirement for bbk13 during natural B. burgdorferi infection of mice by tick feeding. Three weeks after free feeding of infected nymphs on mice, distal tissues were collected and tested for B. burgdorferi colonization via the semiquantitative tissue reisolation assay. After 5 days of incubation, a majority (17/20) of the cultures containing tissues from mice fed on by wild-type B. burgdorferi-infected nymphs had a reisolation score of 3 (Fig. 4A, 5-day culture). In contrast, nearly all (19/20) reisolation cultures from mice fed on by Δbbk13 B. burgdorferi-infected nymphs had no spirochetes upon microscopic inspection and were given a score of 0 (Fig. 4A, 5-day culture). Moreover, at the 2-week endpoint, only 4 out of 20 Δbbk13 B. burgdorferi reisolation cultures were positive for spirochetes, with three of the five mice having no detectable spirochetes in any distal tissue examined (Fig. 4A, endpoint). In contrast, all reisolation cultures from the wild-type and Δbbk13/bbk13+ B. burgdorferi-infected mouse tissues were positive for spirochetes at the 2-week endpoint (Fig. 4A, endpoint). Consistent with the observed deficit in tissue reisolation, Δbbk13 B. burgdorferi was below the level of detection by qPCR in the hearts (0/5 mice) and joints (0/5 mice) (Fig. 4B) of mice after tick feeding. Spirochete numbers were measurable in two out of five ear tissues from Δbbk13 B. burgdorferi-infected mice but were reduced compared to those in wild-type and Δbbk13/bbk13+ B. burgdorferi-infected mice (Fig. 4B). Moreover, in agreement with reduced disseminating spirochetes and reduced overall loads in distal tissues, mice fed on by Δbbk13 B. burgdorferi-infected ticks exhibited a serological response with a reduced signal intensity yet a banding pattern similar to that of wild-type B. burgdorferi- or Δbbk13/bbk13+ B. burgdorferi-infected mice (Fig. 4C), as demonstrated previously for mice infected with spirochetes lacking bbk13 (13). The attenuated infection phenotype of Δbbk13 B. burgdorferi by nymph transmission was recapitulated by free-feeding artificially infected larvae (Fig. 4D), together supporting the importance of bbk13 for B. burgdorferi infection by tick bite transmission.
FIG 4.
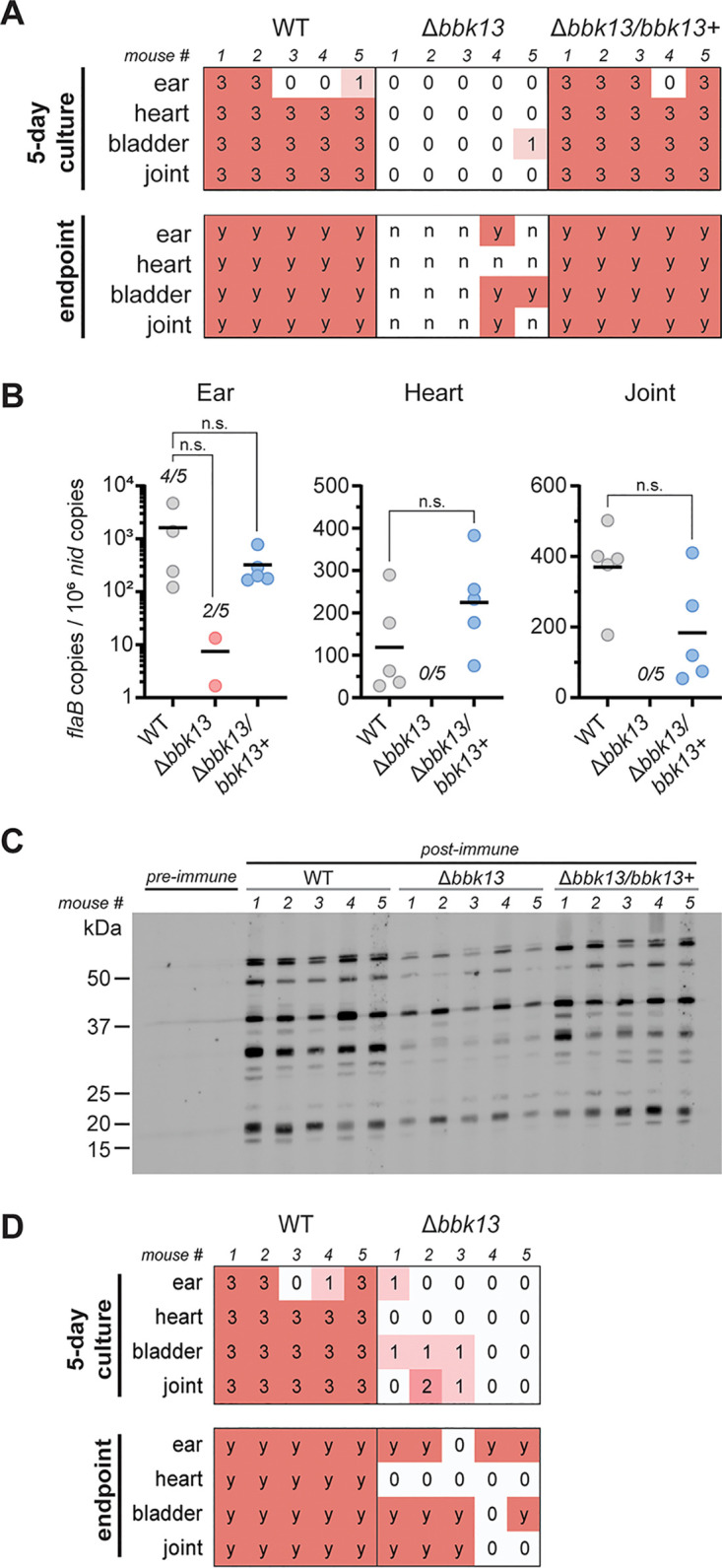
bbk13 is critical for B. burgdorferi infection of mice by tick transmission. Unfed nymphs infected with wild-type (WT), Δbbk13, or Δbbk13/bbk13+ B. burgdorferi were fed to repletion on groups of five C3H/HeN mice. Mice were assessed for B. burgdorferi infection 3 weeks after tick feeding by a reisolation assay, quantitative PCR, and serology. (A) Distal tissues were collected for spirochete reisolation. Semiquantitative scoring of reisolation cultures was performed following 5 days (0 indicates no spirochetes, and 1 to 3 indicate increasing spirochete densities) and 14 days (endpoint, scored for the presence [y] or absence [n] of spirochetes) of culture incubation. (B) Total DNA was extracted from ear, heart, and joint tissues. B. burgdorferi loads in each tissue were measured by quantifying B. burgdorferi flaB copies normalized to 106 mouse nid copies using quantitative PCR. The mean for each group is represented by a horizontal line. In cases where not all samples per group had detectable levels of B. burgdorferi DNA, the numbers of positive samples out of 5 mice per group are indicated. The Kruskal-Wallis test with Dunn’s multiple comparisons was used to determine statistical significance (n.s., not significant). (C) Preimmune and postimmune sera were assessed by immunoblotting for the presence of anti-B. burgdorferi antibodies. Post-immune serum from individual mice was used to blot total B. burgdorferi lysates. Preimmune sera were pooled across each of the three groups. Molecular weight standards are shown in kilodaltons. (D) Unfed Ixodes larvae were artificially infected with WT or Δbbk13 B. burgdorferi by immersion. Artificially infected larvae were allowed to feed freely to repletion on groups of five C3H/HeN mice. Three weeks after larva application, tissues were assessed for B. burgdorferi by a reisolation assay. Semiquantitative scoring of reisolation cultures was performed following 5 days (0 indicates no spirochetes, and 1 to 3 indicate increasing spirochete densities) and 14 days (endpoint, scored for the presence [y] or absence [n] of spirochetes) of culture incubation.
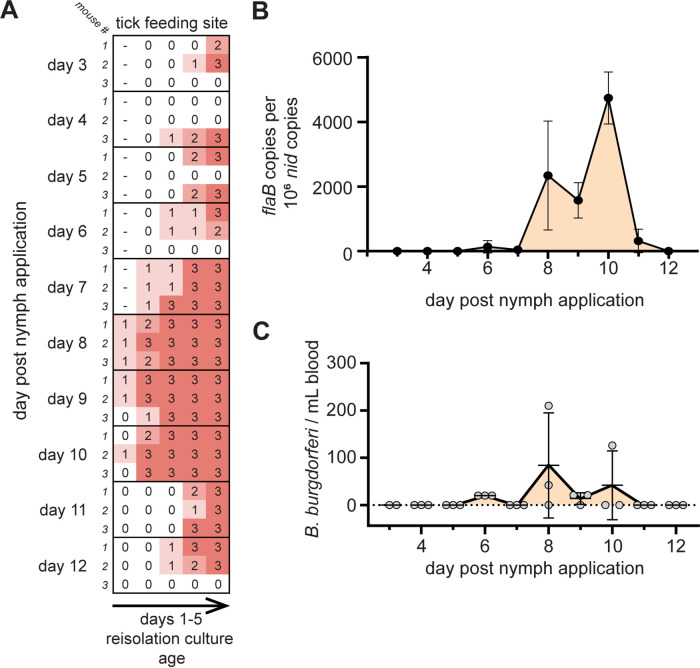
B. burgdorferi population expansion occurs at the tick feeding site.
We previously showed that bbk13 is important early in B. burgdorferi mammalian infection to promote spirochete expansion in the skin within days after intradermal needle inoculation (13). The deficiency of Δbbk13 B. burgdorferi in distal tissue colonization was observed by both needle inoculation (Fig. 1A and B) (13) and natural tick transmission (Fig. 4). We were interested in determining whether wild-type B. burgdorferi undergoes a similar population expansion in the skin after tick bite transmission and if bbk13 contributes to this process. To achieve this, we confined wild-type B. burgdorferi-infected nymphs to capsules affixed to the dorsal skin of mice and determined the kinetics of the B. burgdorferi population in the skin feeding site. The feeding site was harvested daily from cohorts of mice starting at day 3 out to day 12 after nymph application. The spirochete load in the skin was assessed using the semiquantitative reisolation assay as well as quantitative PCR. Feeding sites collected on days 3 to 6 after nymph application showed inconsistent spirochete reisolation, with one or two of the three mice in the cohort showing an absence of spirochetes and peak scores being achieved only at the end of the scoring period in some cultures (Fig. 5A). However, from days 7 to 10 after nymph application, spirochetes were detected in all cultures as soon as day 1 of incubation and reached a peak score of 3 as early as day 2 of incubation (Fig. 5A). Reisolation from skin on days 11 to 12 after nymph application resembled that at the early time points, with spirochetes being detected later in the scoring period (Fig. 5A). Quantification of B. burgdorferi loads from the skin feeding site by quantitative PCR corroborated the findings from the reisolation assay. There were few or no measurable B. burgdorferi spirochetes in the skin feeding site from days 3 to 7 after nymph application, followed by a dramatic increase in the B. burgdorferi load during days 8 to 10 after nymph application (Fig. 5B). A precipitous drop in B. burgdorferi loads was detected on days 11 to 12 (Fig. 5B). Blood was also collected from mice throughout the duration of the nymph capsule feeding experiment and assessed for the presence of disseminating spirochetes in circulation. Plating of whole blood in solid BSK-agarose medium and CFU enumeration revealed peak numbers of B. burgdorferi in the blood on days 8 to 10 after nymph application (Fig. 5C). Taken together, these results show that B. burgdorferi undergoes population expansion in the skin after tick transmission, and the peak of this expansion occurs on days 8 to 10 after nymph application.
FIG 5.
B. burgdorferi spirochetes delivered by tick bite undergo population expansion in mouse skin. Unfed nymphs infected with wild-type (WT) B. burgdorferi were placed in capsules adhered to the dorsal skin of naive C3H/HeN mice. Cohorts of three mice each were assessed for B. burgdorferi infection at the indicated days after nymph application. (A) The tick feeding site was assessed for B. burgdorferi by a tissue reisolation assay. Semiquantitative scoring of reisolation cultures was performed daily over 5 days of culture incubation (0 indicates no spirochetes, and 1 to 3 indicate increasing spirochete densities). (B) Total DNA was extracted from the skin feeding site, and B. burgdorferi loads were measured by quantifying B. burgdorferi flaB copies normalized to 106 mouse nid copies using quantitative PCR. The mean B. burgdorferi load (black dots) for each cohort was plotted over time. Error bars represent standard deviations. (C) Peripheral blood from each mouse was plated in solid BSK-agarose medium, and circulating B. burgdorferi spirochetes were enumerated by counting CFU. Gray dots represent individual mice. Error bars represent standard deviations.
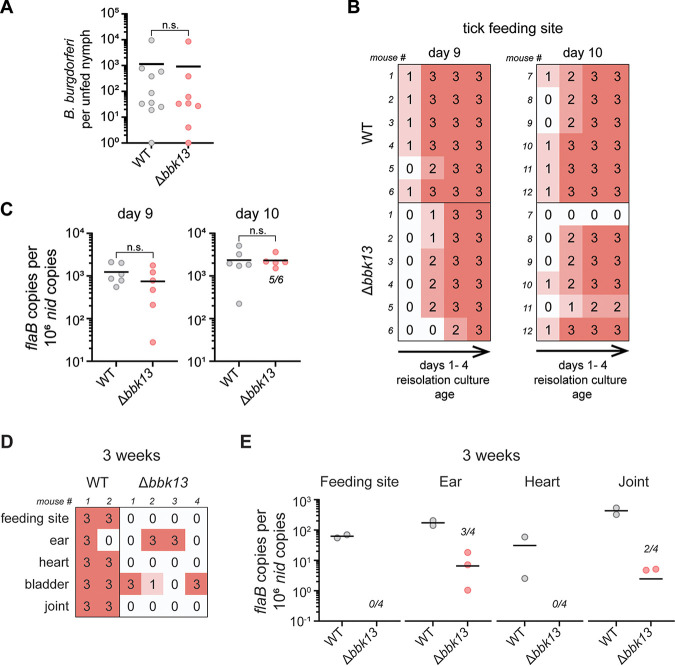
By tick bite transmission, bbk13 is dispensable for B. burgdorferi early population expansion but critical for disseminated infection.
Having established the kinetics of wild-type B. burgdorferi population expansion in the skin following tick transmission, we used the nymph capsule feeding model to assess the contribution of bbk13 to this process. Unfed nymphs infected with comparable numbers of either wild-type B. burgdorferi or Δbbk13 B. burgdorferi spirochetes (Fig. 6A) were placed in feeding capsules on the dorsal skin of C3H/HeN mice to feed to repletion. The skin feeding site was collected on days 9 and 10 after nymph application, which corresponded to the peak of spirochete expansion of tick-transmitted wild-type B. burgdorferi (Fig. 5B). A reisolation assay of the skin site fed on by wild-type B. burgdorferi-infected nymphs collected on both days 9 and 10 demonstrated the detection of spirochetes within 1 day of culture incubation (Fig. 6B). Cultures containing the skin site fed on by Δbbk13 B. burgdorferi-infected nymphs showed little reisolation delay compared to wild-type B. burgdorferi, with spirochetes being detected on day 2 of incubation (Fig. 6B). Surprisingly, no difference in B. burgdorferi loads measured by quantitative PCR was detected between wild-type and Δbbk13 B. burgdorferi-infected skin sites collected on either day (Fig. 6C). These results are in contrast to previous findings that, when delivered by intradermal needle inoculation, Δbbk13 B. burgdorferi failed to undergo efficient population expansion in the skin inoculation site compared to wild-type B. burgdorferi, thus negatively impacting distal tissue colonization at later time points of infection (13). Yet our data indicated that Δbbk13 B. burgdorferi is highly attenuated for disseminated mouse infection by free-feeding tick transmission (Fig. 4). Therefore, we examined the disseminated infection outcomes of Δbbk13 B. burgdorferi delivered by nymph capsule feeding. Three weeks after nymph application in feeding capsules, the feeding site and multiple distal tissues were dissected and assessed for spirochete colonization. Interestingly, despite the lack of B. burgdorferi load differences between wild-type and Δbbk13 B. burgdorferi at the feeding site early in infection, Δbbk13 B. burgdorferi demonstrated reduced numbers in all tissues examined at this later time point. Nearly all reisolation cultures containing tissues from mice fed on by wild-type B. burgdorferi-infected nymphs were positive for spirochetes, while only 5 out of 20 cultures were positive for spirochetes from mice fed on by Δbbk13 B. burgdorferi-infected nymphs (Fig. 6D). Furthermore, quantitative PCR analysis of spirochete loads consistently showed reduced or undetectable Δbbk13 B. burgdorferi spirochetes in tissues (Fig. 6E), indicating the critical requirement for bbk13 for productive disseminated infection.
FIG 6.
When delivered by tick transmission, bbk13 is not required for B. burgdorferi population expansion in the skin but is critical for disseminated infection. (A to C) Unfed nymphs infected with wild-type (WT) or Δbbk13 B. burgdorferi were placed in capsules adhered to the dorsal skin of groups of six naive C3H/HeN mice to feed. The tick feeding site was collected at days 9 and 10 after nymph application for analysis. (A) Prior to feeding, subsets of individual unfed B. burgdorferi-infected nymphs were homogenized and plated in solid BSK-agarose medium to assess spirochete loads by enumerating CFU. (B) The tick feeding site was assessed for B. burgdorferi by a tissue reisolation assay. Semiquantitative scoring of reisolation cultures was performed daily over 4 days of culture incubation (0 indicates no spirochetes, and 1 to 3 indicate increasing spirochete densities). (C) Total DNA was extracted from the tick feeding site. The B. burgdorferi load was measured by quantifying B. burgdorferi flaB copies normalized to 106 mouse nid copies using quantitative PCR. In cases where not all samples per group had detectable B. burgdorferi DNA, the numbers of positive samples out of 6 mice per group are indicated in the scatterplot. Data points below the level of detection are not shown. The mean is indicated by a horizontal black line. The Mann-Whitney test was used to determine statistical significance (n.s., not significant). (D and E) Unfed nymphs infected with WT or Δbbk13 B. burgdorferi were placed in capsules adhered to the dorsal skin of groups of 2 to 4 naive C3H/HeN mice to feed. Three weeks after nymph application, the tick feeding site and various distal tissues were collected for analysis. (D) Tissues were assessed for B. burgdorferi by a tissue reisolation assay. Semiquantitative scoring of reisolation cultures was performed on day 6 of culture incubation (0 indicates no spirochetes, and 1 to 3 indicate increasing spirochete densities). (E) Total DNA was extracted from the indicated tissues. B. burgdorferi loads were measured by quantifying B. burgdorferi flaB copies normalized to 106 mouse nid copies using quantitative PCR. In cases where not all samples per group have detectable B. burgdorferi DNA, the numbers of positive samples out of the total mice per group are indicated in the scatterplot. Data points below the level of detection are not shown. The mean is indicated by a horizontal black line.
Δbbk13 B. burgdorferi infectivity is restored in immunocompromised mice.
The above-described results show that delivery by tick bite locally rescued the defect of Δbbk13 B. burgdorferi in spirochete expansion in the skin during early infection. It is known that the feeding activity of Ixodes ticks leads to immunomodulation at the bite site, promoting B. burgdorferi survival (9, 17), which therefore prompted the question as to whether bbk13 is involved in immune evasion. To test this, we determined whether Δbbk13 B. burgdorferi mammalian infectivity was restored if the host immune response was reduced overall. We delivered 104 wild-type, Δbbk13, or Δbbk13/bbk13+ B. burgdorferi spirochetes by intradermal needle inoculation into highly immunocompromised Nod-scid IL2Rγnull (NSG) mice and the congenic control strain, Nod/ShiLtJ. At day 6 postinoculation, blood was collected from mice and plated in solid BSK-agarose medium to enumerate circulating B. burgdorferi spirochetes. No B. burgdorferi clone-specific differences were observed between the number of circulating B. burgdorferi spirochetes in immunocompromised NSG mice and that in the control mouse strain (Fig. 7A). At 3 weeks postinoculation, infection of the inoculation site and distal tissues was assessed by a semiquantitative reisolation assay. As expected, Δbbk13 B. burgdorferi spirochetes were highly attenuated for infection of the control mice, with only 6 out of 30 cultures having a score of 1 or 2 following 5 days of incubation. In contrast, 29 out of 30 and 24 out of 30 reisolation cultures from wild-type B. burgdorferi- and Δbbk13/bbk13+ B. burgdorferi-infected NOD mice, respectively, received a score of 1 to 3 at the same time point (Fig. 7B, top). Strikingly, in immunocompromised NSG mice, Δbbk13 B. burgdorferi demonstrated robust infection. Twenty-one out of 30 reisolation cultures from Δbbk13 B. burgdorferi-inoculated NSG mice received a score of 3 following 5 days of incubation, congruent with those of wild-type B. burgdorferi (25/30 cultures) and Δbbk13/bbk13+ B. burgdorferi (22/30 cultures) (Fig. 7B, bottom). These data indicated that without host immune pressure, the infection defect of Δbbk13 B. burgdorferi was overcome, resulting in productive disseminated infection of distal tissues and suggesting a role for BBK13 in immune evasion.
FIG 7.
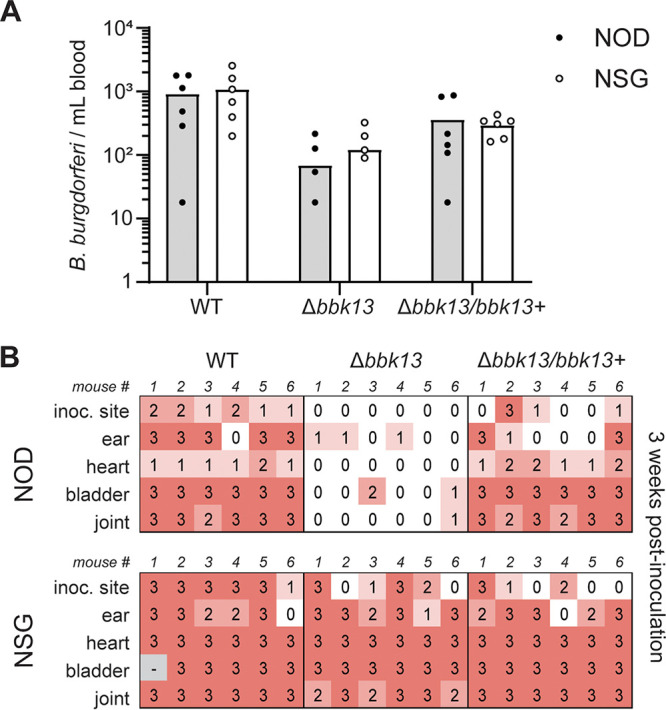
Infectivity of Δbbk13 B. burgdorferi is restored in immunocompromised mice. Groups of six control NOD/ShiLtJ (NOD) or immunocompromised Nod-scid IL2Rγnull (NSG) mice were intradermally inoculated with 104 wild-type (WT), Δbbk13, or Δbbk13/bbk13+ B. burgdorferi spirochetes. (A) At 6 days postinoculation, blood was collected and assessed for disseminating spirochetes by plating in solid BSK-agarose medium and enumerating CFU. (B) At 3 weeks postinoculation, tissues were assessed for B. burgdorferi by a reisolation assay. Semiquantitative scoring of reisolation cultures was performed on day 5 of culture incubation (0 indicates no spirochetes, and 1 to 3 indicate increasing spirochete densities).
DISCUSSION
The maintenance of B. burgdorferi in the natural reservoir depends on its ability to colonize a vertebrate host, to position itself to be acquired during tick feeding, and to subsequently be transmitted to a new vertebrate host. Although B. burgdorferi colonizes several mammalian tissues that contribute to the pathogenesis of Lyme disease in humans, colonization of the skin of reservoir hosts is most pertinent to the upkeep of the enzootic cycle. Intravital imaging of the skin of infected mice revealed that B. burgdorferi undergoes directed migration toward a feeding tick (18). Furthermore, B. burgdorferi has shown positive chemotactic responses to components of the tick salivary gland and that these factors are important during spirochete acquisition by feeding ticks (19, 20). This evidence supports the ability of B. burgdorferi to sense the presence of the feeding tick and to position itself to best be taken up by a feeding tick. However, these cues are likely restricted to the immediate vicinity of an active tick feeding site. Therefore, efficient B. burgdorferi acquisition likely depends on achieving the appropriate density of B. burgdorferi spirochetes in the skin such that enough B. burgdorferi spirochetes will encounter the cues from a feeding tick. B. burgdorferi spirochetes must then be present in the feeding site at a sufficient number for successful tick acquisition and continuation of the enzootic cycle.
We and others have previously described the population expansion of B. burgdorferi at the site of needle inoculation (13, 21–24). We further showed that this population expansion is promoted by gene bbk13, and the inability to expand in numbers at the skin inoculation site leads to attenuated dissemination and distal tissue colonization (13). We now show that naive larvae fail to acquire Δbbk13 B. burgdorferi from needle-inoculated mice. Our data suggest that the number of Δbbk13 B. burgdorferi spirochetes colonizing the skin is likely below the threshold required for successful tick acquisition but do not rule out the possibility of a specific function of bbk13 as a driver of the acquisition defect. Matching the wild-type and Δbbk13 B. burgdorferi numbers in the skin would allow any bbk13-specific requirement to be tested during tick acquisition. However, increasing the needle inoculum dose of the mutant did not result in increased spirochete loads in the skin.
Using the semiquantitative tissue reisolation assay as a measure of tissue burden and highly immunocompromised NSG mice, we discovered that when host immune pressures are relieved, Δbbk13 B. burgdorferi is able to colonize distal tissues similarly to wild-type B. burgdorferi. Thus, it appears that bbk13 is important for circumventing the host immune response, either directly or indirectly, to promote B. burgdorferi survival in the mammalian host. The restoration of Δbbk13 B. burgdorferi loads in the tissues of NSG mice to apparent wild-type levels suggests that this model could be used to test for a bbk13-specific requirement for tick acquisition. However, there are a number of limitations to this model in its ability to address this question. The semiquantitative nature of the tissue reisolation assay does not provide sufficient resolution at a score of 3 (>15 spirochetes in the field of view) to precisely distinguish quantitative differences in tissue loads between wild-type and Δbbk13 B. burgdorferi infections. Furthermore, assessment of spirochete loads in distal tissues of NSG mice using quantitative PCR enumeration of B. burgdorferi genome copies was unreliable. Tissues that had no reisolation of live spirochetes recorded high spirochete loads by qPCR (data not shown). This observation indicates that B. burgdorferi DNA is retained in the tissues despite the absence of viable spirochetes. The NSG mice and the congenic control strain used in our studies are of the Nod/ShiLtJ background, commonly called NOD (nonobese diabetic). NOD mice have been shown to have poor clearance of apoptotic cells and the generation of autoantibodies against DNA (25), which indicates poor clearance of DNA. Moreover, hyperglycemic mice are suspected to suffer from deficiencies in clearing B. burgdorferi debris and DNA from tissues (26). This is an important consideration for mouse infection studies since quantitative PCR measurement of B. burgdorferi genome copies in distal tissues, the current gold standard for assessing B. burgdorferi tissue loads, assumes a correlation between the amounts of B. burgdorferi genomic DNA and viable spirochetes. The caveats of precise quantitation of B. burgdorferi in the tissues of NSG mice have the potential to confound the interpretation of tick acquisition, or the lack thereof, of Δbbk13 B. burgdorferi. Therefore, NSG mice were deemed not to be the appropriate model to test this question. In addition, while the removal of immune pressures clearly allowed Δbbk13 B. burgdorferi to achieve disseminated infection, given the technical limitations of these mice, we cannot rule out the potential that BBK13 also contributes to a broader mechanism of dissemination not overcome by the immunodeficiency of the NSG background. Nonetheless, the broadly immunocompromised background of NSG mice is a useful tool to eliminate a large swath of the host immune response across both innate and adaptive arms. Future studies will focus on the identification of the specific aspect(s) of the host immune response that BBK13 contributes to providing protection against.
Consistent with previous findings for B. burgdorferi lacking the entire linear plasmid 36 (lp36) (27), on which the bbk13 gene resides (28), no bbk13-dependent defect in B. burgdorferi replication or survival was detected across the tick life stages. Tick bite transmission of B. burgdorferi induces significant changes in both the spirochete and the mammalian host to promote infection (8, 9), which are not recapitulated by in vitro-grown B. burgdorferi delivered by needle inoculation. Therefore, we sought to assess the contribution of the bbk13 gene to mouse infection by tick bite transmission. B. burgdorferi spirochetes lacking bbk13 were highly attenuated for disseminated infection 3 weeks following free feeding of infected ticks on mice. Our previous findings indicated a role for bbk13 in B. burgdorferi population expansion in the skin site of infection and the importance of this population expansion for the establishment of productive disseminated infection (13). To investigate the contribution of bbk13 to this model of infection in the context of tick bite transmission, we first established the early kinetics of wild-type B. burgdorferi in the skin site of tick feeding using capsule-confined nymphs. Similar to the spirochete numbers in the skin delivered by needle inoculation, tick-delivered B. burgdorferi underwent population expansion in the feeding site, with peak numbers of spirochetes being detected on days 8 to 10 after nymph application. Given that B. burgdorferi transmission can occur 24 to 48 h after the start of tick feeding and that tick feeding is not synchronized, the observed kinetics of spirochete population expansion in the skin was comparable to that determined by needle inoculation (13). Using the nymph capsule feeding approach, analysis of the numbers of Δbbk13 B. burgdorferi spirochetes in the tick feeding site on days 9 and 10 after nymph application revealed the striking result that the loss of bbk13 had no impact on spirochete population expansion in the skin. It is important to note that peak numbers of wild-type B. burgdorferi spirochetes in the blood were also detected on days 8 to 10 after nymph application. As the skin is a highly vascularized tissue and the mice were not perfused prior to harvest of the tick feeding site, it remains a possibility that circulating B. burgdorferi spirochetes in the blood may have contributed to the numbers of wild-type and Δbbk13 B. burgdorferi spirochetes detected in the skin at these time points. Despite the amelioration of the early infection defect of Δbbk13 B. burgdorferi when delivered by tick feeding, assessment of mice fed on by capsule-confined nymphs 3 weeks after nymph application revealed that the bbk13 gene remained critical for disseminated infection. Together, these data suggest that one or more of the biological changes that the spirochetes and/or the host undergoes during tick feeding allowed Δbbk13 B. burgdorferi to overcome the barrier to population expansion in the skin. However, the relief of this early phenotype was not sufficient to overcome the need for bbk13 during disseminated infection. These findings emphasize that B. burgdorferi population expansion in the skin and dissemination to distal tissues are distinct events of mammalian infection that are uniquely influenced by the feeding tick and likely require different sets of B. burgdorferi genes. Furthermore, this work underscores the significance of the vector as a driver of vector-borne pathogen adaptation and pathogenesis.
Our studies have provided greater insight into the role of bbk13 in the biology of B. burgdorferi, yet the molecular function of BBK13 remains unknown. Recently, we established that BBK13 forms large oligomeric complexes in the spirochete membrane (14). Current work is focused on investigating how BBK13 oligomerization contributes to the mechanisms by which B. burgdorferi surmounts immune barriers in the mammalian host in order to establish a disseminated infection. In sum, this work cements the importance of bbk13 in promoting B. burgdorferi mammalian infectivity and highlights the influence of the tick vector on the pattern of infection, particularly at the skin—the interface between host, vector, and pathogen.
MATERIALS AND METHODS
B. burgdorferi clones and growth conditions.
The Borrelia (Borreliella) burgdorferi clones used in this study were derived from the low-passage-number infectious clone B31 A3-68 Δbbe02, referred to here as the wild type, which lacks plasmids cp9 and lp56 as well as the bbe02 gene on lp25 (29). The Δbbk13 mutant clone and the Δbbk13/bbk13+ complemented strain have been described previously (13). B. burgdorferi cultures were grown at 35°C in liquid Barbour-Stoenner-Kelly II (BSKII) medium containing gelatin and 6% rabbit serum. Alternatively, B. burgdorferi spirochetes were plated in solid BSK-agarose medium and incubated at 35°C under 2.5% CO2. The following antibiotics were used, as needed: kanamycin (200 μg/ml), streptomycin (50 μg/ml), and gentamicin (40 μg/ml).
Ethics statement.
The University of Central Florida is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care. Protocols for all animal experiments were prepared according to the guidelines of the National Institutes of Health and were reviewed and approved by the University of Central Florida Institutional Animal Care and Use Committee.
Needle inoculation of mice.
B. burgdorferi cultures were grown from frozen glycerol stocks in BSKII medium with the appropriate antibiotics to stationary phase (1 × 108 spirochetes/ml). B. burgdorferi cultures were kept at stationary phase for ∼24 h prior to mouse inoculation. Culture density was determined using a Petroff-Hauser chamber under dark-field microscopy. B. burgdorferi cultures were diluted in BSKII medium to the desired inoculum dose. Groups of C3H/HeN, Nod-scid IL2Rγnull (NSG), or NOD/ShiLtJ (NOD) mice were inoculated intradermally into the shaved dorsal skin with wild-type, Δbbk13, or Δbbk13/bbk13+ B. burgdorferi at a dose of 104, 105, 106, or 107 spirochetes. All inoculum cultures were analyzed for plasmid content by PCR and plated in solid BSK-agarose medium to verify the presence of virulence plasmids lp25, lp28-1, and lp36 in individual colonies (27). All inoculum cultures had the expected plasmid profile, and 80% to 100% of the individual clones examined contain all three virulence plasmids. Seven days or 3 to 4 weeks after tick feeding, mice were assayed for B. burgdorferi infection by a semiquantitative tissue reisolation assay (as described below) (13), quantitative PCR loads in tissues (13, 27), and/or serology (30).
B. burgdorferi acquisition by naive Ixodes larvae.
Three weeks after inoculation with wild-type, Δbbk13, or Δbbk13/bbk13+ B. burgdorferi, mice were fed upon by groups of ∼150 naive Ixodes scapularis larval ticks (CDC, BEI Resources, or Oklahoma State University). Replete larvae were collected and individually assessed for B. burgdorferi infection, as described below.
Artificial infection of Ixodes larvae and free-feeding tick transmission.
Approximately 4-month-old naive Ixodes scapularis larval ticks (CDC, BEI Resources, or Oklahoma State University) were dehydrated by exposure to saturated ammonium sulfate for 24 h. Log-phase-grown B. burgdorferi clones were diluted to 2 × 107 cells/ml in BSKII medium. Five hundred microliters of the spirochetes was incubated with dehydrated ticks at 35°C for ∼1.5 h and washed twice with phosphate-buffered saline (PBS) (15). The inoculum cultures were verified to contain the expected endogenous plasmids (27, 31). Infectious plasmids lp25, lp28-1, and lp36 were present in 80 to 100% of individuals of each inoculum culture. Three days after artificial infection, cohorts of ∼150 larvae were fed to repletion on groups of 5 naive C3H/HeN mice (Envigo) per B. burgdorferi clone. A subset of fed larvae was allowed to molt into nymphs, and subsequently, cohorts of 25 nymphs were fed on groups of 5 naive C3H/HeN mice (Envigo) per B. burgdorferi clone. Three weeks after tick feeding, mice were assayed for B. burgdorferi infection by a semiquantitative tissue reisolation assay (as described below) (13), quantitative PCR loads in tissues (13, 27), and/or serology (30).
Capsule feeding tick transmission.
The top portion of a 2-ml screw-cap tube with a perforated cap (capsules) was glued onto the shaved dorsal skin of groups of 2 to 6 C3H/HeN mice using veterinary tag cement (Nasco) and allowed to completely adhere overnight. Groups of 15 B. burgdorferi-infected unfed nymphs were added to each capsule and allowed to feed. Seven days after nymph application, replete nymphs were collected. Seven days or 3 weeks after nymph application, mice were euthanized, and the skin feeding site and distal tissues were collected for analysis. Infection was determined by a semiquantitative tissue reisolation assay (as described below) (13), quantitative PCR loads in tissues (13, 27), and/or serology (30).
Semiquantitative tissue reisolation scoring method.
Semiquantitative tissue reisolation scoring was performed as described previously (13). Briefly, tissues were dissected at the indicated time points after inoculation and placed into BSKII medium containing an antibiotic cocktail of rifampin (50 μg/ml), amphotericin B (2.5 μg/ml), and phosphomycin (20 μg/ml) (RPA cocktail). B. burgdorferi is naturally resistant to these antibiotics. Reisolation cultures were incubated at 35°C. Cultures were visually inspected daily for the presence of spirochetes using dark-field microscopy. While viewing at a ×200 magnification, a numerical score is assigned, as follows: 0 for the absence of spirochetes, 1 for a low spirochete density (<5 spirochetes in the field of view), 2 for a medium spirochete density (5 to 15 spirochetes in the field of view), and 3 for a high spirochete density (>15 spirochetes in the field of view). Finally, the presence or absence of spirochetes (denoted by “y” [yes] or “n” [no], respectively) was scored at the endpoint of the assay, after 14 days of incubation.
Quantification of B. burgdorferi loads in Ixodes ticks.
The B. burgdorferi densities in ticks were assessed before and 7 to 14 days after feeding to repletion on mice. Ticks were surface sterilized using sequential washes with 3% hydrogen peroxide, 70% ethanol, and sterile water. Groups of 10 unfed larvae, individual fed larvae, or individual nymphs (fed or unfed) were crushed and homogenized in BSKII medium using a plastic pestle in microcentrifuge tubes, first by hand and then by motorized adapter. Homogenates were serially diluted and plated in solid BSK-agarose medium supplemented with the RPA cocktail. CFU were enumerated, and the B. burgdorferi load per tick was calculated (30, 32). Alternatively, total DNA was isolated from groups of 40 unfed larvae using a NucleoSpin tissue kit (Clontech Laboratories) according to the manufacturer’s specifications, and qPCR was performed as previously described (30).
Quantification of B. burgdorferi density in the blood.
Peripheral blood was collected from mice on day 6 after needle inoculation with B. burgdorferi or days 3 to 12 after application of B. burgdorferi-infected ticks. Whole blood was serially diluted in BSKII medium, plated in solid BSK-agarose medium plus the RPA cocktail, and incubated at 35°C under 2.5% CO2. CFU were enumerated, and the spirochete density in circulating blood was calculated (13).
Graphs, figures, and statistical tests.
GraphPad Prism v.9 was used to generate graphs and perform statistical tests. Adobe Illustrator was used for figure assembly and minor editing of graphic elements.
ACKNOWLEDGMENTS
We thank the three expert reviewers for their thoughtful comments. We thank Travis Jewett and members of the Jewett labs for insightful discussions. We thank the UCF NAF animal care staff. The following reagent was provided by the Centers for Disease Control and Prevention for distribution by BEI Resources, NIAID, NIH: Ixodes scapularis larvae (live) (catalog number NR-44115).
We have no conflicts of interest to declare.
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI099094 to M.W.J.) and the Deborah and Mark Blackman-Global Lyme Alliance postdoctoral fellowship (G.F.A.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Mollie W. Jewett, Email: Mollie.Jewett@ucf.edu.
Jeroen P. J. Saeij, UC Davis School of Veterinary Medicine
REFERENCES
- 1.Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. 2017. Surveillance for Lyme disease—United States, 2008-2015. MMWR Surveill Summ 66:1–12. 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319. 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N Engl J Med 308:733–740. 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 4.Burgdorfer W, Keirans JE. 1983. Ticks and Lyme disease in the United States. Ann Intern Med 99:121. 10.7326/0003-4819-99-1-121. [DOI] [PubMed] [Google Scholar]
- 5.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc’h G, Melton F, Hickling GJ, Tsao JI, Bunikis J, Barbour AG, Kitron U, Piesman J, Fish D. 2012. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg 86:320–327. 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helble JD, McCarthy JE, Hu LT. 2021. Interactions between Borrelia burgdorferi and its hosts across the enzootic cycle. Parasite Immunol 43:e12816. 10.1111/pim.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovius JW, van Dam AP, Fikrig E. 2007. Tick-host-pathogen interactions in Lyme borreliosis. Trends Parasitol 23:434–438. 10.1016/j.pt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Bernard Q, Grillon A, Lenormand C, Ehret-Sabatier L, Boulanger N. 2020. Skin interface, a key player for Borrelia multiplication and persistence in Lyme borreliosis. Trends Parasitol 36:304–314. 10.1016/j.pt.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Boulanger N, Wikel S. 2021. Induced transient immune tolerance in ticks and vertebrate host: a keystone of tick-borne diseases? Front Immunol 12:625993. 10.3389/fimmu.2021.625993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikel S. 2013. Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol 4:337. 10.3389/fmicb.2013.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aranjuez GF, Kuhn HW, Adams PP, Jewett MW. 2019. Borrelia burgdorferi bbk13 is critical for spirochete population expansion in the skin during early infection. Infect Immun 87:e00887-18. 10.1128/IAI.00887-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn HW, Aranjuez GF, Jewett MW. 2021. The Borrelia burgdorferi infection critical BBK13 protein forms large oligomers in the spirochete membrane. Biochem Biophys Res Commun 537:1–6. 10.1016/j.bbrc.2020.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Policastro PF, Schwan TG. 2003. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J Med Entomol 40:364–370. 10.1603/0022-2585-40.3.364. [DOI] [PubMed] [Google Scholar]
- 16.Piesman J, Oliver JR, Sinsky RJ. 1990. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am J Trop Med Hyg 42:352–357. 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- 17.Kurokawa C, Lynn GE, Pedra JHF, Pal U, Narasimhan S, Fikrig E. 2020. Interactions between Borrelia burgdorferi and ticks. Nat Rev Microbiol 18:587–600. 10.1038/s41579-020-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bockenstedt LK, Gonzalez D, Mao J, Li M, Belperron AA, Haberman A. 2014. What ticks do under your skin: two-photon intravital imaging of Ixodes scapularis feeding in the presence of the Lyme disease spirochete. Yale J Biol Med 87:3–13. https://www.ncbi.nlm.nih.gov/pubmed/24600332. [PMC free article] [PubMed] [Google Scholar]
- 19.Murfin KE, Kleinbard R, Aydin M, Salazar SA, Fikrig E. 2019. Borrelia burgdorferi chemotaxis toward tick protein Salp12 contributes to acquisition. Ticks Tick Borne Dis 10:1124–1134. 10.1016/j.ttbdis.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narasimhan S, Sukumaran B, Bozdogan U, Thomas V, Liang X, DePonte K, Marcantonio N, Koski RA, Anderson JF, Kantor F, Fikrig E. 2007. A tick antioxidant facilitates the Lyme disease agent’s successful migration from the mammalian host to the arthropod vector. Cell Host Microbe 2:7–18. 10.1016/j.chom.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernard Q, Wang Z, Di Nardo A, Boulanger N. 2017. Interaction of primary mast cells with Borrelia burgdorferi (sensu stricto): role in transmission and dissemination in C57BL/6 mice. Parasit Vectors 10:313. 10.1186/s13071-017-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodzic E, Feng S, Freet KJ, Borjesson DL, Barthold SW. 2002. Borrelia burgdorferi population kinetics and selected gene expression at the host-vector interface. Infect Immun 70:3382–3388. 10.1128/IAI.70.7.3382-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horka H, Cerna-Kyckova K, Skallova A, Kopecky J. 2009. Tick saliva affects both proliferation and distribution of Borrelia burgdorferi spirochetes in mouse organs and increases transmission of spirochetes to ticks. Int J Med Microbiol 299:373–380. 10.1016/j.ijmm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Kern A, Collin E, Barthel C, Michel C, Jaulhac B, Boulanger N. 2011. Tick saliva represses innate immunity and cutaneous inflammation in a murine model of Lyme disease. Vector Borne Zoonotic Dis 11:1343–1350. 10.1089/vbz.2010.0197. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien BA, Geng X, Orteu CH, Huang Y, Ghoreishi M, Zhang Y, Bush JA, Li G, Finegood DT, Dutz JP. 2006. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun 26:104–115. 10.1016/j.jaut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Javid A, Zlotnikov N, Pětrošová H, Tang TT, Zhang Y, Bansal AK, Ebady R, Parikh M, Ahmed M, Sun C, Newbigging S, Kim YR, Santana Sosa M, Glogauer M, Moriarty TJ. 2016. Hyperglycemia impairs neutrophil-mediated bacterial clearance in mice infected with the Lyme disease pathogen. PLoS One 11:e0158019. 10.1371/journal.pone.0158019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jewett MW, Lawrence K, Bestor AC, Tilly K, Grimm D, Shaw P, VanRaden M, Gherardini F, Rosa PA. 2007. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol Microbiol 64:1358–1374. 10.1111/j.1365-2958.2007.05746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, Freeman S, Radune D, Weidman JF, Dimitrov GI, Khouri HM, Sosa JE, Halpin RA, Dunn JJ, Fraser CM. 2012. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7:e33280. 10.1371/journal.pone.0033280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rego RO, Bestor A, Rosa PA. 2011. Defining the plasmid-borne restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. J Bacteriol 193:1161–1171. 10.1128/JB.01176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jewett MW, Lawrence KA, Bestor A, Byram R, Gherardini F, Rosa PA. 2009. GuaA and GuaB are essential for Borrelia burgdorferi survival in the tick-mouse infection cycle. J Bacteriol 191:6231–6241. 10.1128/JB.00450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elias AF, Schmutzhard J, Stewart PE, Schwan TG, Rosa P. 2002. Population dynamics of a heterogeneous Borrelia burgdorferi B31 strain in an experimental mouse-tick infectious cycle. Wien Klin Wochenschr 114:557–561. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12422601. [PubMed] [Google Scholar]
- 32.Adams PP, Flores Avile C, Jewett MW. 2017. A dual luciferase reporter system for B. burgdorferi measures transcriptional activity during tick-pathogen interactions. Front Cell Infect Microbiol 7:225. 10.3389/fcimb.2017.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]