Abstract
Background and Aims:
Excessive bleeding is a major concern in functional endoscopic sinus surgery (FESS) under general anaesthesia; this can be decreased by various hypotensive agents. This study was conducted to compare the hypotensive effectiveness and haemodynamic stability of dexmedetomidine and clonidine in patients undergoing elective FESS.
Methods:
In this prospective double-blinded interventional study, 70 adult patients of either sex, 20–50 years of age, posted for elective FESS were randomly assigned to two groups. Group A received a loading dose of intravenous (IV) dexmedetomidine 1 μg/kg, followed by infusion of 1 μg/kg/h, and group B received a loading dose of IV clonidine 2 μg/kg, followed by 1 μg/kg/h infusion. Surgical field quality, emergence time, sedation score, visual analogue score, recovery profile and haemodynamic parameters were recorded. Statistical analysis was done by Student's unpaired t-test to evaluate the significance of normally distributed variables, whereas Mann–Whitney test and Chi-square test were used for ordinal data and categorical variables and proportions, respectively.
Results:
In both the groups, target mean arterial pressure (MAP) of 65–70 mmHg and improved surgical field quality were achieved. MAP and heart rate (HR) were statistically significantly lower in the dexmedetomidine group with a longer duration of post-operative analgesia (P = 0.001). None of the groups showed any statistically significant adverse effects.
Conclusions:
Both dexmedetomidine and clonidine can be used for controlled hypotension to improve surgical field quality in FESS. Dexmedetomidine provides more haemodynamic stability and an additional benefit of post-operative analgesia and conscious sedation.
Keywords: Clonidine, controlled, dexmedetomidine, hypotension
INTRODUCTION
Functional endoscopic sinus surgery (FESS) is an important therapeutic technique for nasal sinus pathologies. Diminished surgical field visibility due to bleeding at the operative site is a major issue in FESS.[1] Controlled hypotension has been used during FESS to reduce blood loss and to improve the visibility of the surgical field by decreasing the mean arterial pressure (MAP).[2] In hypotensive anaesthesia, the patient's baseline MAP is reduced by 30% or kept at 65–70 mmHg. Side effects of induced hypotension are ischaemia to vital organs, cerebral hypoperfusion, acute kidney injury and acidosis, and all must be taken care of. Several agents such as nitroglycerine, higher doses of inhaled anaesthetics, vasodilators like sodium nitroprusside, and beta-blockers have been used either alone or in combination for achieving controlled hypotension; however, an ideal agent for this purpose has not been asserted. The agents having characteristics such as faster onset, rapid elimination without toxic metabolites, easy administration, short context-sensitive half-life and dose-dependent predictable effects should be considered ideal for controlled hypotension.[3] Dexmedetomidine has been approved by the United States Food and Drug Administration and is a more selective α-2 adrenoceptor agonist (α2:α1 = 1620:1) than clonidine (α2:α1 = 220:1). It has been approved for use as a sedative–analgesic and/or total anaesthetic in adult and paediatric patients. It acts through central α-2A and imidazoline type 1 receptors. The activation of these central receptors results in a decrease in norepinephrine release, which leads to a decrease in blood pressure and heart rate.[4] Clonidine is also a selective α-2 adrenergic agonist with some α-1 agonist properties and acts by decreasing the sympathetic nervous system output from the central nervous system. Various studies have found that preoperative administration of clonidine decreases mucosal bleeding in FESS, which improves surgical field visibility and reduces the duration of surgery.[5,6,7]
Dexmedetomidine and clonidine both have been used for blunting of haemodynamic response to laryngoscopy and tracheal intubation.[8,9] We designed this study to evaluate and compare the efficacy of dexmedetomidine and clonidine for producing controlled hypotension during FESS. The primary objective of the study was to assess and compare the hypotensive effectiveness and haemodynamic stability of dexmedetomidine and clonidine in FESS by comparing the haemodynamic parameters from the baseline at different time intervals within each group (intragroup) and between both the groups (intergroup). The secondary objectives were to assess and compare the quality of the intraoperative surgical field, emergence time, sedation score, visual analogue scale (VAS) score and time to first rescue analgesic demand in the post-operative period and to compare the proportion of cases with side effects.
METHODS
This hospital-based prospective randomised double-blind interventional study was conducted in our institute from January 2020 to December 2020 after obtaining permission from the institutional ethics committee and after registering in the Clinical Trials Registry-India. All patients who gave written informed consent were included in the study. Randomisation was done by simple randomisation technique via chit-in-box method. Concealment of randomisation was performed through the sealed envelope method. Double blinding was done in such a manner that the anaesthesiologist who administered anaesthesia was different from the anaesthesiologist who recorded study variables. A routine pre-anaesthetic check-up was done a day before surgery. We included patients of either sex, American Society of Anesthesiologists (ASA) physical status I–II, aged 20–50 years, weighing 45–65 kg and scheduled for elective FESS of 60–70 min duration. Patients having a history of hypertension, coronary artery disease, heart blocks, autonomic neuropathy, renal dysfunction, hepatic dysfunction, cerebral insufficiency, rhinorrhoea, coagulation abnormalities, recurrent sinus surgery and allergy to study drugs were excluded from the study. Seventy patients were randomly divided into two groups of 35 each [Figure 1]. Group A received dexmedetomidine 1 μg/kg in 10 ml of saline over 10 min followed by 1 μg/kg/h infusion. Group B received clonidine 2 μg/kg in 10 ml of saline over 10 min followed by 1 μg/kg/h infusion. The patient's fasting status and informed written consent were confirmed. After taking the patient into the operating room, all standard monitors were attached and an 18-G intravenous catheter was inserted. All patients were premedicated with inj. midazolam 0.02 mg/kg, inj. glycopyrrolate 4 μg/kg and inj. fentanyl 2 μg/kg intravenously and pre-oxygenated with 100% oxygen. Anaesthesia was induced with inj. thiopentone sodium 5 mg/kg and inj. succinylcholine 1.5 mg/kg and maintained on oxygen-nitrous oxide (40:60), isoflurane (0.4%–1%) and intermittent boluses of atracurium. Loading dose of study drug was given 10 min before induction of general anaesthesia (GA), and its maintenance dose infusion was started soon after induction of anaesthesia and continued intraoperatively until 5 min before the completion of surgery or stopped on the occurrence of hypotension below our target, whichever was earlier. Intraoperative haemodynamic parameters such as heart rate (HR), systolic blood pressure, diastolic blood pressure, mean arterial pressure (MAP) and oxygen saturation (SpO2) were recorded at baseline, after the loading dose, after induction, 1 min after intubation, 5 min after intubation and thereafter every 10 min until shifting of the patient to the recovery area. The surgical site was observed for the severity of bleeding and the need for frequent suctioning by using the average category scale proposed by Fromme and Boezaart.[10,11] [score 0 = no bleeding; score 1 = slight bleeding, no suctioning of blood required; score 2 = slight bleeding, occasional suctioning required, surgical field not threatened; score 3 = slight bleeding, frequent suctioning required, bleeding threatens surgical field a few seconds after suction is removed; score 4 = moderate bleeding, frequent suctioning required, bleeding threatens surgical field directly after suction is removed; score 5 = severe bleeding, constant suctioning required, bleeding appears faster than can be removed by suction, surgical field severely threatened and surgery suspended]. Blood loss estimation was done by measuring suction canister volume minus irrigation fluid used in surgery. The number of small gauges soaked in blood was also counted and estimated accordingly. Patients were reversed with inj. neostigmine 0.05 mg/kg and inj. glycopyrrolate 0.01 mg/kg intravenously. Extubation was done when the patient was responding to verbal commands. Emergence time was defined as the time interval between discontinuation of anaesthetics to the response of eye opening to verbal commands. Post-operatively, patients were kept in the recovery room, monitored for 30 min and later shifted to the post-operative wards. Post-operative haemodynamic parameters, emergence time and sedation score were recorded every 30 min. Sedation was assessed by using Ramsay Sedation Score.[12] Post-operative pain was assessed by VAS score every 15 min until the patient reached a VAS score of 3. Time to first rescue analgesia was noted and patients were allowed to receive intravenous diclofenac 75 mg as rescue analgesia. This was the endpoint of our study. Post-operative complications such as nausea, vomiting, shivering, dryness of mouth, hypotension and bradycardia were also recorded. Hypotension was defined as MAP <65 mmHg and was treated by stopping hypotensive agent and giving fluid bolus and inj. mephentermine 6 mg bolus as per need. Bradycardia was defined as HR <50/min and treated with intravenous atropine 0.6 mg if not resolved by stopping study drug infusion. Inj. ondansetron 0.1 mg/kg was given to treat post-operative nausea/vomiting.
Figure 1.
CONSORT flow diagram
The sample size was calculated to be 32 subjects for each of the two groups at an alpha error of 0.05 (95% confidence) and power of 80% expecting a minimum detectable difference of 4.96 ± 6.9 mmHg in mean arterial pressure in both groups from the baseline, at 15 min after intubation as per a study done by Suggala et al.[13] Further, the sample size was rounded off to 35 subjects in each group. Statistical analysis was done by using the Statistical Package for the Social Sciences software version 21 (SPSS Inc., Chicago, IL, USA). We used the Student's unpaired t-test to evaluate the significance of normally distributed variables, whereas Mann–Whitney test was used for comparison of ordinal data. Categorical variables/proportion of cases like the occurrence of complications were analysed using Chi-square test. P < 0.05 was considered statistically significant.
RESULTS
Demographic data were statistically comparable in both groups [Table 1]. We observed that both HR and MAP were significantly decreased (P = 0.001) at all observation time points after giving a loading dose of study drugs in comparison to baseline in both the groups. The reduction in HR and MAP was more in group A as compared to group B, and the difference was statistically significant (P = 0.001) [Tables 2 and 3]. The average category score for surgical field visibility ranged between 1 and 3 in group A and between 2 and 3 in group B. The difference in average category scale was statistically insignificant and surgical field visibility was comparable in both groups. Mean estimated blood loss was statistically comparable in both groups A and B (128.14 ± 6.54 ml vs 129.71 ± 6.85 ml) (P = 0.329). The mean emergence time was statistically significantly longer in group A (7.36 ± 0.60 min) in comparison to group B (6.42 ± 0.74 min) (P = 0.001). Mean sedation scores were statistically significantly higher in group A as compared to group B at all the time intervals post-operatively (P = 0.001) [Figure 2]. Although the sedation that occurred in group A was more than that in group B, the depth of sedation was such that it could be termed as conscious sedation (appeared to be asleep but readily arousable). The mean VAS score in group A was lower at different time intervals in comparison to group B [Figure 3]. Time to first rescue analgesia was significantly longer in group A (110.43 ± 12.27 min) as compared to group B (84.29 ± 10.08 min) (P = 0.001). Post-operative complications were statistically comparable between the two groups. The main side effect in group A was dry mouth (4/35 = 11.42% vs 1/35 = 2.85% in group B) while nausea and vomiting occurred more in group B (4/35 = 11.42% vs 1/35 = 2.85% in group A). Hypotension and bradycardia occurred in 3 (8.57%) patients in group A and in 2 (5.71%) patients in group B but reverted spontaneously after stopping infusion of study drug and giving fluids. None of the patients had severe adverse effects.
Table 1.
Demographic profile of both groups
| Variable | Group A (n=35) | Group B (n=35) | P |
|---|---|---|---|
| Age (Years), Mean+SD | 27.23+7.75 | 27.49+8.68 | 0.896 |
| Gender- Male/Female (No.) | 20/15 | 18/17 | 0.810 |
| Weight (kg), Mean+SD | 55.06+6.22 | 53.77+4.53 | 0.326 |
| ASA Physical Status- I/II | 28/7 | 30/5 | 0.751 |
| Duration Of Surgery (min), Mean+SD | 66.71+2.67 | 67.20+2.31 | 0.419 |
SD: Standard Deviation, ASA: American Society of Anesthesiologists
Table 2.
Mean heart rate (beats/min)
| Time point | P | ||||
|---|---|---|---|---|---|
|
| |||||
| Group A (n=35) | Intragroup | Group B (n=35) | Intragroup | Intergroup | |
| Baseline | 93.69±8.15 | - | 92.69±7.52 | - | 0.592 |
| After loading dose of study drug | 80.29±6.83 | <0.001* | 81.89±8.20 | <0.001* | 0.378 |
| After induction | 76.77±5.93 | <0.001* | 80.17±7.58 | <0.001* | 0.040* |
| 1 min after intubation | 74.80±4.98 | <0.001* | 79.83±7.89 | <0.001* | 0.002* |
| 5 min after intubation | 69.77±5.95 | <0.001* | 78.71±7.26 | P<0.001* | <0.001* |
| 10 min | 66.54±5.53 | <0.001* | 76.54±6.83 | <0.001* | <0.001* |
| 20 min | 65.94±4.90 | <0.001* | 73.69±7.18 | <0.001* | <0.001* |
| 30 min | 65.20±4.68 | <0.001* | 72.94±6.67 | <0.001* | <0.001* |
| 40 min | 65.31±4.38 | <0.001* | 73.17±7.06 | <0.001* | <0.001* |
| 50 min | 64.26±4.30 | <0.001* | 73.06±6.97 | <0.001* | <0.001* |
| 60 min | 66.40±4.24 | <0.001* | 76.49±6.36 | <0.001* | <0.001* |
| 70 min | 72.52±2.46 | <0.001* | 79.68±5.56 | <0.001* | <0.001* |
Original, Student’s unpaired t-test. *P is significant, SD: Standard Deviation
Table 3.
Mean arterial pressure (mmHg)
| Time point | P | ||||
|---|---|---|---|---|---|
|
| |||||
| Group A (n=35) | Intra group | Group B (n=35) | Intra group | Inter group | |
| Baseline | 96.70±3.45 | 97.31±2.85 | 0.420 | ||
| After loading of study drug | 85.95±3.29 | <0.001* | 86.39±4.14 | <0.001* | 0.628 |
| After induction | 80.13±3.60 | <0.001* | 85.99±6.57 | <0.001* | <0.001* |
| 1 min after intubation | 78.97±2.48 | <0.001* | 84.21±4.74 | <0.001* | <0.001* |
| 5 min after intubation | 72.78±5.44 | <0.001* | 79.73±6.03 | <0.001* | <0.001* |
| 10 min | 70.21±4.27 | <0.001* | 78.05±5.92 | <0.001* | <0.001* |
| 20 min | 70.59±3.51 | <0.001* | 77.91±5.24 | <0.001* | <0.001* |
| 30 min | 69.41±2.98 | <0.001* | 76.58±5.91 | <0.001* | <0.001* |
| 40 min | 69.36±2.31 | <0.001* | 75.57±6.04 | <0.001* | <0.001* |
| 50 min | 69.03±2.28 | <0.001* | 76.05±5.50 | <0.001* | <0.001* |
| 60 min | 70.30±2.55 | <0.001* | 77.81±4.63 | <0.001* | <0.001* |
| 70 min | 70.27±2.38 | <0.001* | 77.48±4.14 | <0.001* | <0.001* |
Original, Student’s unpaired t-test, *P is significant, SD: Standard Deviation NS: Non- significant
Figure 2.
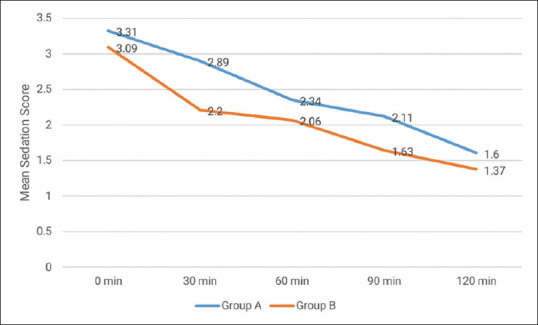
Mean sedation score (post-operative)
Figure 3.
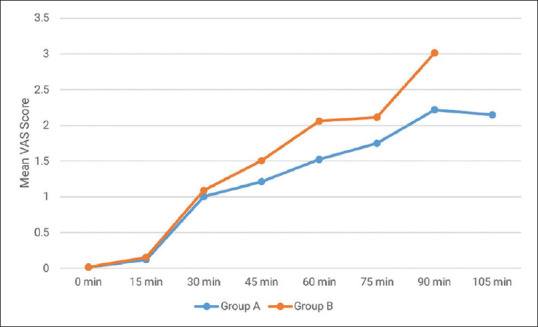
Mean VAS score (post-operative)
DISCUSSION
Dexmedetomidine causes a reduction in blood pressure, slowing of HR, sedation and analgesia. The fall in blood pressure is mainly due to inhibition of central sympathetic outflow and due to stimulation of presynaptic α-2 adrenoceptors decreasing norepinephrine release.[14] Dexmedetomidine has a very minimal respiratory depressant effect with potent sedative and analgesic effects compared with opioids and other sedatives. The important problem involved in FESS is bleeding from the sinuses. Controlled hypotension has a definitive role in FESS as it reduces bleeding during surgery and improves visibility of the surgical field, which can decrease the duration of surgery and anaesthesia. There are several studies comparing dexmedetomidine with other agents for FESS but very few directly comparing it with clonidine. Moreover, we used continuous infusion of dexmedetomidine or clonidine while previous studies used bolus doses. In the present study, we compared these two drugs in terms of haemodynamic parameters, mean average category scale, mean emergence time, mean sedation score, time to first rescue analgesic demand and adverse effects. We found that though induced hypotension was achieved with both the drugs, dexmedetomidine produced more stable haemodynamics with lower readings of MAP and HR along with more prolonged post-operative analgesia and conscious sedation in comparison to clonidine. Our results are similar to the study done by Suggala et al.[13], who compared dexmedetomidine and clonidine for controlled hypotension during FESS and concluded that dexmedetomidine provided more effective controlled hypotension and analgesia and thus allowed less nasal bleeding as well as comparable surgical field visibility. They also noted that the time to first rescue analgesic request was significantly prolonged in the dexmedetomidine group along with higher sedation scores as compared to clonidine.
In another study, Chhabra A, et al.[15] compared dexmedetomidine and magnesium sulphate for induced hypotension during FESS and found that haemodynamics were superior in the dexmedetomidine group. They also observed good post-operative analgesia and sedation. It produces analgesic effects by acting at α-2 receptors within the locus coeruleus and spinal cord. Dexmedetomidine also has the unique property of providing conscious sedation.[16]
We found that both the drugs have improved and comparable surgical field quality. These results were in line with those of the study done by Escamilla et al.[17] who, when comparing the efficacy of clonidine and dexmedetomidine to improve the quality of the surgical field by hypotensive anaesthesia in FESS, found no significant differences between clonidine and dexmedetomidine in the quality of surgical field. Soliman R, et al.[18] compared dexmedetomidine and magnesium sulphate and found that better operating condition was provided by dexmedetomidine but with more hypotension and bradycardia like our study. Kim et al.,[19] in a meta-analysis of randomised controlled trials comparing the perioperative administration of hypotensive agents, found dexmedetomidine to be a superior agent. They also concluded that systemic use of dexmedetomidine reduces intraoperative bleeding and operating time, provides relatively stable haemodynamics by alleviating stress response and reduces the fentanyl requirement significantly. Moshiri et al.[20] compared dexmedetomidine with propofol and found that the desired surgical field is made possible by reducing HR rather than vasoconstriction. In our study, the HR was comparatively lower and less fluctuating in the dexmedetomidine group, which is in favour of more stable haemodynamics and blunting of response to sympathomimetic stimuli by dexmedetomidine.
The limitation of our study is that we did not use a control group because it would have been unethical not to try to control bleeding in FESS where surgical field visibility may be compromised due to bleeding. Invasive monitoring of blood pressure can also be done in hypotensive anaesthesia, but a recent retrospective study done by Lee et al.[21] concluded that it does not aid in achieving lower target blood pressures.
CONCLUSIONS
Dexmedetomidine provides better haemodynamic stability in comparison to clonidine. Both the drugs achieve ideal operative field visibility and decrease in blood loss; however, dexmedetomidine provides an additional benefit of prolonged analgesia and conscious sedation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sieśkiewicz A, Reszeć J, Piszczatowski B, Olszewska E, Klimiuk PA, Chyczewski L, et al. Intraoperative bleeding during endoscopic sinus surgery and microvascular density of the nasal mucosa. Adv Med Sci. 2014;59:132–5. doi: 10.1016/j.advms.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Khalifa OS, Awad OG. A comparative study of dexmedetomidine, magnesium sulphate, or glyceryl trinitrate in deliberate hypotension during functional endoscopic sinus surgery. Ain-Shams J Anaesthesiol. 2015;8:320–6. [Google Scholar]
- 3.Degoute CS. Controlled hypotension: A guide to drug choice. Drugs. 2007;67:1053–76. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Jiwanmall M, Joselyn AS, Kandasamy S. Intravenous clonidine as a part of balanced anaesthesia for controlled hypotension in functional endoscopic sinus surgery: A randomised controled trial. Indian J Anaesth. 2017;61:418–23. doi: 10.4103/ija.IJA_58_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramchandani S, Lakra AM, Shah PJ, Lalwani J, Sahare KK. Effect of intravenous clonidine premedication for the bloodless surgical field in patients undergoing middle ear or nasal surgery: A comparison of three different doses. Anesth Essays Res. 2015;9:397–400. doi: 10.4103/0259-1162.161821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajwa SJ, Kaur J, Kulshrestha A, Haldar R, Sethi R, Singh A. Nitroglycerine, esmolol and dexmedetomidine for induced hypotension during functional endoscopic sinus surgery: A comparative evaluation. J Anaesthesiol Clin Pharmacol. 2016;32:192–7. doi: 10.4103/0970-9185.173325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar NR, Jonnavithula N, Padhy S, Sanapala V, Naik VV. Evaluation of nebulised dexmedetomidine in blunting haemodynamic response to intubation: A prospective randomised study. Indian J Anaesth. 2020;64:874–9. doi: 10.4103/ija.IJA_235_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary S, Sharma S, Kumari I, Kalluraya S, Meena K, Dave T. Comparative evaluation of oral melatonin and oral clonidine for the attenuation of haemodynamic response to laryngoscopy and tracheal intubation–A prospective randomised double blind study. Indian J Anaesth. 2020;64:696–703. doi: 10.4103/ija.IJA_76_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fromme GA, MacKenzie RA, Gould AB, Jr, Lund BA, Offord KP. Controlled hypotension for orthognathic surgery. Anesth Analg. 1986;65:683–6. [PubMed] [Google Scholar]
- 11.Boezaart AP, van der Merwe J, Coetzee A. Comparison of sodium nitroprusside- and esmolol-induced controlled hypotension for functional endoscopic sinus surgery. Can J Anaesth. 1995;42:373–6. doi: 10.1007/BF03015479. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suggala KK, Kishan Rao B, Nagrale MH. Comparison of dexmedetomidine, with clonidine-based anaesthesia for controlled hypotension in functional endoscopic sinus surgery. J Evid Based Med Healthc. 2020;7:782–6. [Google Scholar]
- 14.Giovannitti JA, Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth Prog. 2015;62:31–9. doi: 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra A, Saini P, Sharma K, Chaudhary N, Singh A, Gupta S. Controlled hypotension for FESS: A randomised double-blinded comparison of magnesium sulphate and dexmedetomidine. Indian J Anaesth. 2020;64:24–30. doi: 10.4103/ija.IJA_417_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guven DG, Demiraran Y, Sezen G, Kepek O, Iskender A. Evaluation of outcomes in patients given dexmedetomidine in functional endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2011;120:586–92. doi: 10.1177/000348941112000906. [DOI] [PubMed] [Google Scholar]
- 17.Escamilla Y, Cardesín A, Samara L, López S, Izquierdo A, Fradera M, et al. Randomized clinical trial to compare the efficacy to improve the quality of surgical field of hypotensive anesthesia with clonidine or dexmedetomidine during functional endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2019;276:3095–104. doi: 10.1007/s00405-019-05575-6. [DOI] [PubMed] [Google Scholar]
- 18.Soliman R, Fouad E. The effects of dexmedetomidine and magnesium sulphate in adult patients undergoing endoscopic transnasal transsphenoidal resection of pituitary adenoma: A double-blind randomised study. Indian J Anaesth. 2017;61:410–7. doi: 10.4103/ija.IJA_581_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Lee J, Kim SW, Hwang SH. The efficacy of hypotensive agents on intraoperative bleeding and recovery following general anesthesia for nasal surgery: A network meta-analysis. Clin Exp Otorhinolaryngol. 2021;14:200–9. doi: 10.21053/ceo.2020.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moshiri E, Modir H, Yazdi B, Susanabadi A, Salehjafari N. Comparison of the effects of propofol and dexmedetomidine on controlled hypotension and bleeding during endoscopic sinus surgery. Ann Trop Med Public Health. 2017;10:721–5. [Google Scholar]
- 21.Lee YL, Thangavelautham S, Harikrishnan S, Karthekeyan R, Kothandan H. Is hypotensive anaesthesia guided by invasive intraarterial monitoring required for orthognathic surgery.– A retrospective review of anaesthetic practice and intraoperative blood loss in orthognathic surgery in a tertiary hospital? Indian J Anaesth. 2021;65:525–32. doi: 10.4103/ija.IJA_201_21. [DOI] [PMC free article] [PubMed] [Google Scholar]


