Abstract
Background and Aims:
Baska Mask, a newly designed third-generation supraglottic device, has a sump where the pharyngeal secretions can collect and be suctioned out continuously. We aimed to study the effectiveness of Baska Mask in preventing airway contamination during nasal surgeries. Our primary objective was to assess airway soiling using fibreoptic bronchoscopy. Total airway manipulation time, haemodynamic parameters during device insertion and post-operative oro-pharyngeal morbidities were the secondary objectives.
Methods:
Eighty-four participants undergoing nasal surgeries were randomised to either have their airway maintained with Baska Mask (Group-BM) or Endotracheal tube (Group-TT). Fibreoptic bronchoscopy was performed at the end of the surgery and the airway was inspected for signs of contamination. Total airway manipulation time, haemodynamic parameters during device insertion and post-operative oro-pharyngeal morbidities were also assessed. Unpaired Student's t test was used for parametric data and Chi-square test for nonparametric data. One-way analysis of variance (ANOVA) was used for the intra-group analysis of haemodynamic data.
Results:
Tracheal contamination was not observed in any patient in either group. Time taken for device insertion (Group TT: 24.24 ± 6.86 s vs. Group BM: 24.22 ± 7.3 s; P = 0.97) was similar in both the groups. The total airway manipulation time was 2 min longer in Group-TT (P = 0.000) due to additional time taken for insertion of throat pack. Haemodynamic parameters during device insertion were stable and post-operative oro-pharyngeal morbidities were fewer with Baska Mask when compared to Tracheal tube.
Conclusions:
Baska Mask is non-inferior to tracheal tube in preventing tracheal contamination in patients undergoing nasal surgeries.
Keywords: Bronchoscopy, Laryngeal Masks, nasal surgical procedures
INTRODUCTION
Tracheal intubation with throat packing is the standard of practice during nasal surgeries as these surgeries carry the inherent risk of blood trickling down the nasopharynx and contaminating the lower airway. The throat pack also prevents blood from entering the stomach and provoking post-operative nausea and vomiting. Supraglottic devices such as Laryngeal mask airways (LMAs), are associated with minimal cardiovascular responses during insertion and fewer post-operative oropharyngeal morbidities.[1,2] LMAs have been tested as an alternative to tracheal tubes in nasal surgeries but are not routinely used for such surgeries as the seal is not definitive and pooling of trickled blood can occur proximal to the LMA cuff.[3,4] Baska Mask (BM), a newly designed third-generation supraglottic device is provided with a sump where the pharyngeal secretions can collect and be suctioned out continuously, thereby increasing the safety of the device in nasal surgeries.
The efficiency of this device during positive pressure ventilation has been shown.[5,6] However its effectiveness in protecting the lower airway from contamination during nasal surgeries has not yet been investigated. We hypothesised that the BM is non-inferior to the Tracheal tube in preventing tracheal contamination during nasal surgeries and aimed to study the effectiveness of BM for nasal surgeries. The primary objective was to assess airway soiling using fibreoptic bronchoscopy. The secondary objectives were to compare the total airway manipulation time, haemodynamic parameters during device insertion and post-operative oro-pharyngeal morbidities between the two groups.
METHODS
This prospective, randomised controlled trial was approved by the institutional human ethics committee, and registered with the Clinical Trial Registry of India (CTRI/2019/06/019724). The study was done from July 2019 to December 2019 and followed the principles laid down in the declaration of Helsinki. Patients scheduled for elective nasal surgeries during this period formed the study population. From the study population, patients between 18 and 60 years of age, American Society of Anesthesiologists physical status I and II who gave written informed consent were included. Patients with an anatomically difficult airway, reactive airway disease, body mass index >25 kg/m2 were excluded.
Eighty-six patients were enroled. Block randomisation was performed using the 'Permuted block' feature of the 'Statistics and Sample Size' app, version 1.0 developed by Truc TT, with a pre-defined block size of six. The randomisation sequence was generated by a resident not involved in the study and handed over to the investigators in sealed opaque sequentially numbered envelopes containing the allocated group, BM (Group-BM) or Tracheal tube (Group-TT). The patients were blinded to the allocated group.
All patients were fasted for 6 h and received standardised premedication. In the operation theatre, an 18-G intravenous access was secured, routine monitoring (GE B40 monitor; GE Healthcare, Wisconsin) was initiated and baseline haemodynamic parameters were noted. Patients were placed in a sniffing position and pre-oxygenated for 3 min. A standardised anaesthesia induction-maintenance protocol including fentanyl, propofol, vecuronium, nitrous-oxide and desflurane was used.
In Group-TT, direct laryngoscopy was performed, the trachea was intubated using an appropriate sized tracheal tube and the cuff inflated to 30 cmH2O with cuff pressure monitor. In Group-BM, the Baska Mask (PROACT Medical Systems, Frenchs Forest NSW, Australia) was selected based on the weight of the patient [Size 3: 30-50 kg, size 4: 50-70 kg, size 5: 70–100 kg]. All intubations and BM insertions were done by the investigators, who had prior experience with intubation and BM insertions. Correct placement of the device was confirmed by observation of adequate chest rise and the appearance of a square-shaped end-tidal carbon dioxide waveform. If the first attempt at insertion failed, one more attempt was allowed. Failure of insertion in the second attempt or an audible leak during ventilation was recorded as a failure of BM insertion. Conventional tracheal intubation was performed and the patients were excluded.
Removal of face mask to the appearance of square-shaped capnography waveform was taken as the time for device insertion. Patients were ventilated with a tidal volume of 8 ml/kg and a respiratory rate of 12/min. Subsequently, in Group-TT, direct laryngoscopy was performed, the throat pack was inserted and the endotracheal tube was shifted to the left side of the mouth as required for nasal surgeries. Removal of the facemask to the readiness for handing over of the patient to the surgeon was taken as total airway manipulation time.
After securing the airway, fibreoptic bronchoscopy was performed and trachea was inspected up to the carina to rule out contamination due to trauma during device insertion or intubation. Any airway soiling was attributed to trauma during device insertion and the patients were excluded from the study. In Group-BM, the alignment of the device to the larynx was also noted by placing the tip of the scope at the rim of the airway tube (visualised as a crescent) and graded using the Brimacombe scale (Grade-1: 75%–100% Anterior-Posterior rimaglottidis distance (APRD); Grade-2: 50%–75% APRD; Grade-3: 25-50% APRD; Grade-4: 0-25% APRD; Grade-5: only epiglottis visible; Grade-6: No vocal cords/epiglottis visible).[7] The leak fraction (LF) during controlled ventilation and laryngeal seal pressure (LSP) were also determined in the same sniffing position. Subsequently, continuous suction (pressure -300 mmHg) was attached to the suction elbow, whereas the other drain tube of the BM was left open to the atmosphere. Haemodynamic parameters were noted at baseline, before intubation, every minute for the first 6 min after airway device insertion. Patients were monitored intra-operatively for signs of airway contamination such as gurgling sounds during ventilation, increase in airway pressure, desaturation or increase in heart rate. In the event of an accidental dislodgement of the BM, the plan was to remove the device and intubate conventionally with a tracheal tube after thorough suctioning of the nasopharynx and insertion of a tight nasal pack.
Once the surgery was completed, tracheal contamination distal to the device was reassessed with fiberoptic bronchoscopy. In Group-TT, laryngoscopy was done, the upper airway was cleared of blood and the throat pack was removed. In Group-BM, a 10 French nasogastric tube was passed through the drain tube and the stomach was cleared of any collections. Neuromuscular blockade was reversed with 2.5 mg of neostigmine and 0.5 mg of glycopyrrolate and patients were extubated awake. Any incidence of laryngospasm, desaturation and other complications following extubation were noted. In Group-BM, check laryngoscopy was performed following the removal of the device to note for presence of blood or clots in the pharynx. All patients were assessed for sore-throat, hoarseness of voice, cough, odynophagia and post-operative nausea and vomiting by one of the authors not involved in the management of the case, in the immediate post-operative period and followed up daily till symptoms settled. The trial ended after the recruitment of the sample size.
We calculated the sample size using the 'Sealed Envelope Ltd. power calculator for binary outcome non-inferiority trial' software. Webster et al. have observed tracheal tube to prevent airway contamination in 94% of patients undergoing nasal surgery.[4] We assumed that BM will be equally successful in preventing tracheal contamination and the non-inferiority limit was taken as 14. With a power of 80% and type 1 error of 5%, the sample size was estimated as 72. To compensate for the drop-outs, 84 patients were recruited.
Statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS) version 16.0 (International Business Machines) software. Unpaired Student's t test was used for parametric data and Chi-square test for nonparametric data. One-way analysis of variance (ANOVA) was used for the intra-group analysis of haemodynamic data.
RESULTS
Eighty-six patients were assessed for eligibility. Two patients with anatomically difficult airway were excluded. Eighty-four patients completed the study [Figure 1]. The two study groups were comparable for age, weight, sex, Mallampati class (MPC), ASA physical status, type of surgeries and duration of surgeries [Table 1].
Figure 1.
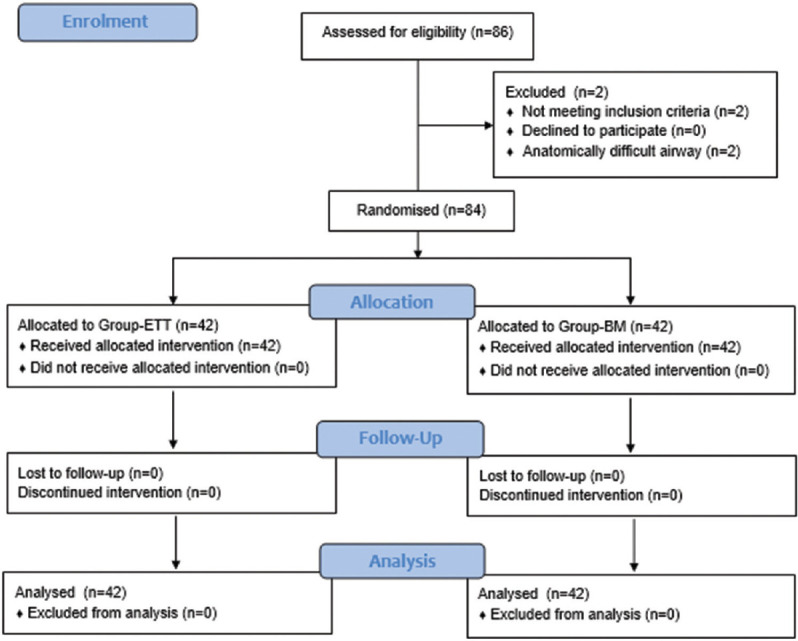
CONSORT flow diagram
Table 1.
Demographic data and duration of surgery
| Group-ETT (n=42) | Group-BM (n=42) | |
|---|---|---|
| Age (years)* | 33.69±10.23 | 30.10±8.53 |
| Sex (male:female) | 30:12 | 32:10 |
| BMI* | 23.95±1.9 | 23.55±2.0 |
| MPC (1:2) | 20:22 | 23:19 |
| ASA (1:2) | 27:15 | 29:13 |
| Surgery | ||
| FESS | 22 | 18 |
| Septoplasty | 18 | 22 |
| FESS + septoplasty | 2 | 2 |
| Duration of surgery (min)* | 128.35±31.72 | 126.07±22.88 |
BMI=body mass index, MPC=Mallampati airway classification, ASA=American Society of Anesthesiologists Physical Status Classification, FESS=functional endoscopic sinus surgery. *Denotes mean± standard deviation (SD)
All intubations and BM insertions were done in the first attempt itself. Fibreoptic bronchoscopy performed after securing the airway as well as at the end of the surgery did not show tracheal contamination in any patient in either group. No pooling of blood or blood clots was seen in the pharynx during check laryngoscopy following removal of the BM. The anterior surface of the BM cuff was not blood-stained in any patient.
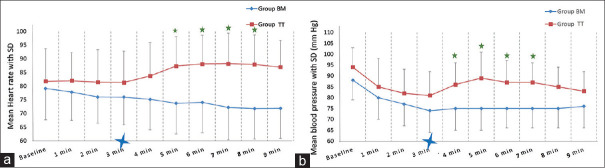
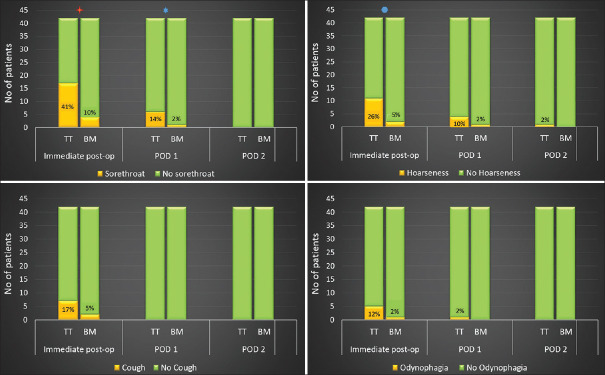
Time taken for device insertion (Group-TT: 24.24 ± 6.86 s vs. Group-BM: 24.22 ± 7.3 s; P = 0.97) was similar in both the groups [Figure 2]. The total airway manipulation time (Group-TT: 144.15 ± 40 s vs. Group-BM: 24.22 ± 7.3 s; P = 0.000) was longer in Group-TT than the Group-BM. In Group-TT, there was an increase in heart rate and blood pressure post-intubation, whereas no such increase was seen in Group-BM [Figure 3]. During surgery, none of the patients in either group developed haemodynamic deviations that needed active management. Post-operative sore throat, odynophagia, hoarseness of voice and cough were higher in Group-TT than in Group-BM, on postoperative day (POD)-0 and POD-1, which settled by POD-2 [Figure 4]. Accidental dislodgement of the device, laryngospasm, desaturation and PONV did not occur in any patient in either group.
Figure 2.
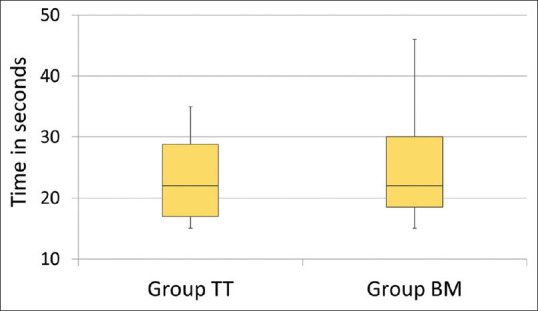
Time taken for intubation and Baska Mask insertion
Figure 3.
Comparison of haemodynamic changes following airway manipulation in the two groups. (a) Mean heart rate changes. (b) Mean blood pressure changes.  On x-axis indicates the time at which device was inserted.
On x-axis indicates the time at which device was inserted.  Denotes P < 0.05 compared to the time of insertion.
Denotes P < 0.05 compared to the time of insertion.
Figure 4.
Incidence of post-operative oropharyngeal morbidities in the two groups. Sore throat was higher in Group-TT in the immediate post-operative period ( P = 0.01) and POD-1 (
P = 0.01) and POD-1 ( P = 0.048). The incidence of hoarseness of voice was higher in Group-TT in the immediate post-operative period (
P = 0.048). The incidence of hoarseness of voice was higher in Group-TT in the immediate post-operative period ( P = 0.01)
P = 0.01)
The median LF with BM was 0.00% [range 0.0 to 0.04%] and the median LSP was 36 [range 20 to 40] cmH2O. Fibreoptic grading of the glottic view through the device showed a Grade-1 view in 69% of patients and Grade-2 view in 31% of patients.
DISCUSSION
Our study shows that BM provides adequate sealing of the larynx preventing contamination of the lower airway during nasal surgeries. The proposed function of the sump was adequate as seen by continuous suctioning of blood and clot through the suction elbow [Figure 5]. Check laryngoscopy after device removal also showed no clots or pooled blood in the pharynx.
Figure 5.
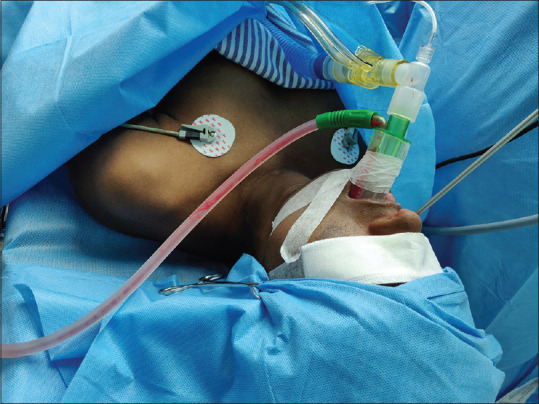
Functioning of sump suctioning
When using LMAs, investigators have either directly inspected the lower airway using a flexible fiberoptic bronchoscope as direct evidence or looked at the pattern of staining of the supraglottic airway device cuff after its removal as indirect evidence of airway contamination.[8,9,10] We preferred to examine the lower airway with fibreoptic bronchoscope to identify this. In our study, fibreoptic bronchoscopy was done twice. The first bronchoscopy was done soon after the placement of either device to eliminate patients who had airway contamination due to trauma during device insertion. Hence, the presence of blood in the lower airway at the end of surgery was entirely attributed to the inadequate sealing of the glottis.
A majority of studies using LMA during nasal surgeries have concluded it as an effective alternative to ETT in nasal surgeries.[8,10,11,12,13] John et al. noticed no major leak of dye past the LMA cuff or staining of the larynx even after injecting 10 mL of methylene blue into the pharynx following LMA insertion.[13] Other investigators have however reported instances of tracheal contamination with LMA. 19.5% and 3% incidence of lower airway contamination with LMA was reported by Kaplan et al. and Webster et al. respectively.[4,10]
Blood staining the anterior surface of the LMA cuff provides only a crude indication of airway contamination and it does not always relate to tracheal contamination. Williams et al. observed laryngeal contamination only in 2.5% of the 13.5% patients who had staining of the anterior surface of the reinforced LMA cuff.[12] Ahmed et al. report blood staining the interior of the RLMA cuff in 2% of the 200 patients studied.[8] However, none of them manifested any signs suggestive of airway contamination. Al-Mazrou et al. report soiling of the anterior aspect of the LMA cuff in 60% of patients, without any staining of the subglottis or trachea on fibreoptic examination.[9] We did not find blood staining the anterior surface of the BM or tracheal contamination on fibreoptic examination in any of our patients.
Post-extubation laryngospasm is another concern during nasal surgeries. The incidence of laryngospasm decreased from 19% to 6% merely by changing the plane of anaesthesia during extubation from deep to awake.[4] Similarly pooling of blood proximal to the LMA cuff can trigger a laryngospasm post LMA removal. It is therefore necessary to inspect the pharynx for clots or blood after removal of the LMA in nasal surgeries. Zhou et al. have addressed this issue by attaching two 12-Fr Tri-Flo suction catheters to the distal barrel of the flexible LMA on the posterior surface.[14] With this modification, they were able to prevent post-extubation complications even in patients with significant intra-operative bleeding. The BM supraglottic airway, has an inbuilt provision for performing the same function.[15]
Several anaesthesia-related factors can contribute to post-operative sore throat amongst which endotracheal intubation and pharyngeal packing are important.[16,17,18,19,20,21] The incidence according to Griffith is as high as 70%.[16] He also stresses the importance of direct questioning in eliciting this in about 40% of his study population. A majority of patients in our study too reported sore throat only on questioning. The advantage of the non-inflatable membranous cuff of BM in limiting the pressure exerted on the pharyngeal mucosa could also be seen in the fewer incidences of oro-pharyngeal morbidities. Our incidence of sore throat with ETT was similar to that reported by Rieger et al. and Higgins et al.[2,17] However, with BM, sore throat was only 9.5%, which is much less when compared to 23.5% reported by Rieger et al. and 17.5% reported by Higgins et al. with the use of LMA.[2,17] Reiger et al. also observed a 28% incidence of dysphonia and 23.8% odynophagia with LMA, which was contrary to our observations with BM.[17] Although a high incidence of PONV is reported following nasal surgeries, we did not encounter this in any patient in either group.[22,23] As with other LMAs, haemodynamic stability during device insertion was better with BM when compared to ETT.
BM insertion was easy and quick in a majority of patients. Manipulation of the insertion tab was not required in any of our study patients. As we only included patients with a normal airway, the same may not apply in difficult airway scenarios. Adequate sealing is important for PPV with supraglottic airway devices.[24] BM provided a good laryngeal seal and the maximum allowable sealing pressure of 40 cmH2O was achieved in 26% of patients. Similar sealing pressures with BM have been described earlier.[5,25,26,27] Total airway manipulation took 2 min longer in Group ETT due to the additional time taken for throat pack insertion and fixation of the ETT to the left angle of the mouth. Although statistically significant, a 2 min longer time taken in handling the airway for throat pack cannot be considered clinically relevant.
As the study involved an airway device, the person performing the fibreoptic assessment for airway contamination could not be blinded to the study group. The Baska-FESS has an angled connector, specifically designed to prevent hindrance during manipulation of the endoscope during nasal surgeries.[28] We have used the BM with a straight connector to facilitate the insertion of the fibreoptic bronchoscope. The surgeons were comfortable with the device during septoplasty but complained of difficulty in manipulating the endoscope during sinus surgeries. Our anaesthetic technique included N2O which may be considered a confounding factor in the development of postoperative sore throat in Group-TT.
CONCLUSION
BM is non-inferior to the tracheal tube in preventing tracheal contamination in patients undergoing nasal surgeries with the added advantage of haemodynamic stability and less post-operative oro-pharyngeal morbidities.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tanaka A, Isono S, Ishikawa T, Sato J, Nishino T. Laryngeal resistance before and after minor surgery.Endotracheal tube versus Laryngeal Mask AirwayTM. Anesthesiology. 2003;99:252–8. doi: 10.1097/00000542-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Higgins PP, Chung F, Mezei G. Postoperative sore throat after ambulatory surgery. Br J Anaesth. 2002;88:582–4. doi: 10.1093/bja/88.4.582. [DOI] [PubMed] [Google Scholar]
- 3.Francois A, Rosenbaum M, Adams W, Schantz O, Stankiewicz J. The use of supraglottic airway vs tracheal tube for endoscopic nasal sinus surgery. Trends Anaesth Crit Care. 2020;33:17–22. [Google Scholar]
- 4.Webster AC, Morley-Forster PK, Janzen V, Watson J, Dain SL, Taves D, et al. Anesthesia for intranasal surgery: A comparison between tracheal intubation and the flexible reinforced Laryngeal Mask Airway. Anesth Analg. 1999;88:421–5. doi: 10.1097/00000539-199902000-00037. [DOI] [PubMed] [Google Scholar]
- 5.Jayalekshmi S, Paul C, Thomas M. Efficacy of Baska mask and Laryngeal mask airway supreme during positive pressure ventilation – A comparative study. J Anaesthesiol Clin Pharmacol. 2020;36:31–6. doi: 10.4103/joacp.JOACP_17_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SR, Lee TY, Kim SW, Park SY, Chung C. Comparison of clinical performance of i-gel® and Baska Mask® during laparoscopic cholecystectomy. Korean J Anesthesiol. 2019;72:576–82. doi: 10.4097/kja.19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zundert AV, Brimacombe J, Kamphuis R, Haanschoten M. The anatomical position of three extraglottic airway devices in patients with clear airways. Anaesthesia. 2006;61:891–5. doi: 10.1111/j.1365-2044.2006.04745.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed MZ, Vohra A. The reinforced laryngeal mask airway (RLMA) protects the airway in patients undergoing nasal surgery — An observational study of 200 patients. Can J Anesth. 2002;49:863–6. doi: 10.1007/BF03017421. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mazrou KA, Abdullah KM, ElGammal MS, Ansari RA, Turkistani A, Abdelmeiguid ME. Laryngeal mask airway vs.uncuffed endotracheal tube for nasal and paranasal sinus surgery: Paediatric airway protection. Eur J Anaesthesiol. 2010;27:16–9. doi: 10.1097/EJA.0b013e32832c5f09. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan A, Crosby GJ, Bhattacharyya N. Airway protection and the laryngeal mask airway in sinus and nasal surgery. Laryngoscope. 2004;114:652–5. doi: 10.1097/00005537-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Daum REO, O'Reilly BJ. The laryngeal mask airway in ENT surgery. J Laryngol Otol. 1992;106:28–30. doi: 10.1017/s0022215100118511. [DOI] [PubMed] [Google Scholar]
- 12.Williams PJ, Thompsett C, Bailey PM. Comparison of the reinforced laryngeal mask airway and tracheal intubation for nasal surgery. Anaesthesia. 1995;50:987–9. doi: 10.1111/j.1365-2044.1995.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 13.John RE, Hill S, Hughes TJ. Airway protection by the laryngeal mask: A barrier to dye placed in the pharynx. Anaesthesia. 1991;46:366–7. doi: 10.1111/j.1365-2044.1991.tb09545.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou GX, Rosenblatt W, Zhou SE, Dai F, Heerdt PM. Flexible laryngeal mask with pharyngeal suction for nasal surgery. Trends Anaesth Crit Care. 2019;26-27:42–7. [Google Scholar]
- 15.Gatt S, Zundert T. The Baska Mask® -A new concept in Self-sealing membrane cuff extraglottic airway devices, using a sump and two gastric drains: A critical evaluation. J Obstet Anaesth Crit Care. 2012;2:23–30. [Google Scholar]
- 16.Griffiths DP, Lindop MJ, Samuels SI, Roberts GD. Pharyngeal packs and the incidence of post-operative sore throat. Anaesthesia. 1973;28:320–30. doi: 10.1111/j.1365-2044.1973.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 17.Rieger A, Brunne B, Hass I, Brummer G, Spies C, Striebel W, et al. Laryngo-pharyngeal complaints following laryngeal mask airway and endotracheal intubation. J Clin Anesth. 1997;9:42–7. doi: 10.1016/S0952-8180(96)00209-7. [DOI] [PubMed] [Google Scholar]
- 18.Fine J, Kaltman S, Bianco M. Prevention of sore throat after nasotracheal intubation. J Oral Maxillofac Surg. 1988;46:946–7. doi: 10.1016/0278-2391(88)90331-x. [DOI] [PubMed] [Google Scholar]
- 19.Marais J, Prescott RJ. Throat pain and pharyngeal packing: A controlled randomized double-blind comparison between gauze and tampons. Clin Otolaryngol Allied Sci. 1993;18:426–9. doi: 10.1111/j.1365-2273.1993.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 20.Tay JYY, Tan WKS, Chen FG, Koh KF, Ho V. Postoperative sore throat after routine oral surgery: Influence of the presence of a pharyngeal pack. Br J Oral Maxillofac Surg. 2002;40:60–3. doi: 10.1054/bjom.2001.0753. [DOI] [PubMed] [Google Scholar]
- 21.Biro P, Seifert B, Pasch T. Complaints of sore throat after tracheal intubation: A prospective evaluation. Eur J Anaesthesiol. 2005;22:307–11. doi: 10.1017/s0265021505000529. [DOI] [PubMed] [Google Scholar]
- 22.Korkut AY, Erkalp K, Erden V, Teker AM, Demirel A, Gedikli O, et al. Effect of pharyngeal packing during nasal surgery on postoperative nausea and vomiting. Otolaryngol Head Neck Surg. 2010;143:831–6. doi: 10.1016/j.otohns.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Naderian M, Jangholi E, Tavakol TK. Postoperative sore throat, nausea and vomiting in routine nasal surgery: Influence of pharyngeal packing. Galen Med J. 2012;1:24–8. [Google Scholar]
- 24.Solanki SL, Johnson JE, Samantaray A. Supraglottic airway devices: Placement and pharyngeal seal matters! Indian J Anaesth. 2020;64:649–52. doi: 10.4103/ija.IJA_938_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahajan SR, Mahajan M, Chaudhary UK, Kumar S. Evaluation of Baska mask performance in laparoscopic cholecystectomy. IOSR J Dent Med Sci. 2017;4:74–8. [Google Scholar]
- 26.Sidhu GK, Jindal S, Mahajan R, Bhagat S. Influence of head and neck positions on oropharyngeal seal pressure with Baska Mask® versus I-gelTM; A randomised clinical study. Indian J Anaesth. 2020;64:675–80. doi: 10.4103/ija.IJA_185_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López AM, Muñoz-Rojas G, Fontanals M, San Jose I, Hermoso A, Valero R. Clinical evaluation of the Baska Mask® laryngeal mask in adult patients in ambulatory surgery. Rev Esp Anestesiol Reanim. 2015;62:551–6. doi: 10.1016/j.redar.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Foo LL, Shariffuddin II, Chaw SH, Lee PK, Lee CE, Chen YS, et al. Randomized comparison of the Baska FESS mask and the LMA Supreme in different head and neck positions. Expert Rev Med Dev. 2018;15:597–603. doi: 10.1080/17434440.2018.1506329. [DOI] [PubMed] [Google Scholar]