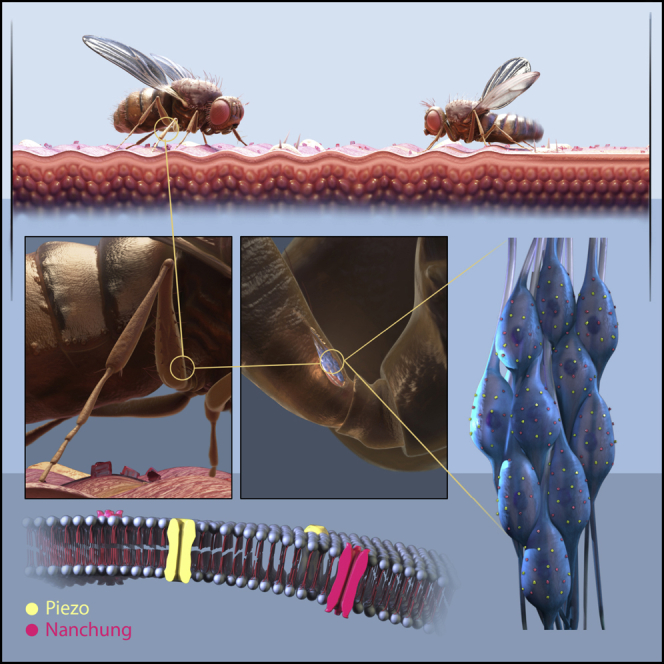
Summary
Substrate-borne vibratory signals are thought to be one of the most ancient and taxonomically widespread communication signals among animal species, including Drosophila flies.1, 2, 3, 4, 5, 6, 7, 8, 9 During courtship, the male Drosophila abdomen tremulates (as defined in Busnel et al.10) to generate vibrations in the courting substrate.8,9 These vibrations coincide with nearby females becoming immobile, a behavior that facilitates mounting and copulation.8,11, 12, 13 It was unknown how the Drosophila female detects these substrate-borne vibratory signals. Here, we confirm that the immobility response of the female to the tremulations is not dependent on any air-borne cue. We show that substrate-borne communication is used by wild Drosophila and that the vibrations propagate through those natural substrates (e.g., fruits) where flies feed and court. We examine transmission of the signals through a variety of substrates and describe how each of these substrates modifies the vibratory signal during propagation and affects the female response. Moreover, we identify the main sensory structures and neurons that receive the vibrations in the female legs, as well as the mechanically gated ion channels Nanchung and Piezo (but not Trpγ) that mediate sensitivity to the vibrations. Together, our results show that Drosophila flies, like many other arthropods, use substrate-borne communication as a natural means of communication, strengthening the idea that this mode of signal transfer is heavily used and reliable in the wild.3,4,7 Our findings also reveal the cellular and molecular mechanisms underlying the vibration-sensing modality necessary for this communication.
Keywords: biotremology, Drosophila, courtship, tremulation, substrate-borne vibrations, mechanosensation, femoral chordotonal organ, nanchung, piezo, trpγ, female immobility
Graphical abstract
Highlights
-
•
Courting male abdominal tremulations signal via substrate-borne, not air-borne, vibrations
-
•
Substrate-borne vibrations propagate through natural food substrates
-
•
Neurons of the leg fCHO mediate female immobility response to these vibrations
-
•
Mechanically gated ion channels Nan and Piezo mediate response in these neurons
McKelvey et al. find that courting male D. melanogaster abdominal tremulations signal via substrate-borne, not air-borne, vibrations. These vibrations propagate through natural food substrates, promoting female immobility. Neurons and their mechanically gated ion channels within the leg fCHO mediate female response to the vibrations.
Results and discussion
Drosophila melanogaster wild flies exhibit substrate-borne communication signals similar to laboratory fly stocks
Substrate-borne vibratory signals during courtship have been reported in D. melanogaster laboratory stocks,8,9,11 but not in wild D. melanogaster. Single wild males courted single wild females vigorously, including wing fluttering and abdominal tremulations (Video S1), and the wild females responded similarly to Oregon-R laboratory stocks (Figures 1A–1C and S1A–S1C).8,9,11 The durations of the interpulse intervals (IPIs) are often used by animals for signal recognition;1,4,7,14, 15, 16, 17, 18, 19 we used laser vibrometry to measure and compare the IPI of the substrate-borne vibrations produced by wild and laboratory male’s abdominal tremulations and found them to be similar to one another (Figure S1D). Therefore, all our later experiments were performed on laboratory Oregon-R flies.
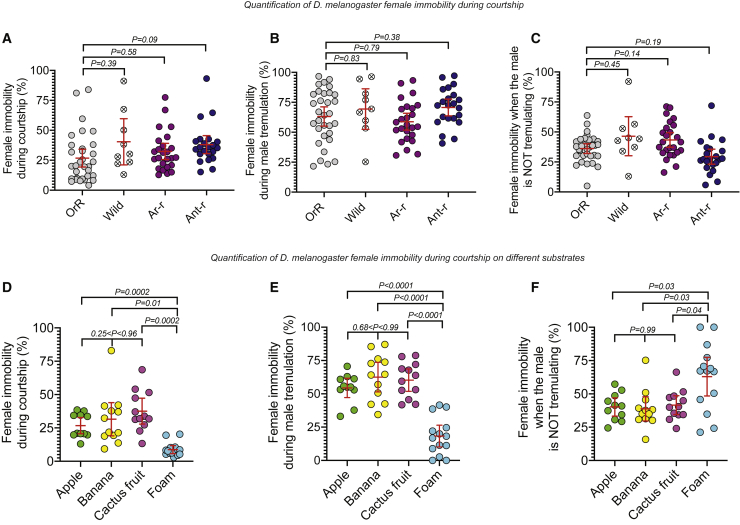
Figure 1.
Quantification of D. melanogaster female immobility during courtship
(A–C) Data for intact OregonR (OrR) pairs, wild flies, and aristae-removed or antennae-removed OrR females paired with intact OrR males (Ar-r pairs and Ant-r pairs, respectively) filmed in plastic chambers. Ethograms constructed from analysis of video clips of 32, 9, 25, and 22 pairs, respectively.
(A) The total percentage of time females were immobile during courtship is similar for all pairs.
(B) The percentage of time where females were immobile while the male abdomen was tremulating is similar for all pairs. Note that, in all cases, the male was tremulating for a similar duration during courtship (Figure S1C).
(C) The percentage of time where females were immobile while the male abdomen was not tremulating is similar in all pairs.
(D–F) Data for OrR pairs on different substrates, including apple, banana, cactus fruit, or foam. Ethograms are constructed from analysis of 11, 12, 12, and 14 pairs, respectively.
(D) The total percentage of time females were immobile during courtship is significantly lower on foam (9% ± 1%) compared to the other substrates (27% ± 3%, 31% ± 6%, and 38% ± 4%, respectively), where it is similar.
(E) The percentage of time where females were immobile while the male tremulated was similar on apple (54% ± 3%), banana (62% ± 5%), and cactus (60% ± 4%) but significantly lower on foam (18% ± 4%).
(F) The percentage of time where females were stationary while the male was not tremulating his abdomen is similar on the natural substrates (40% ± 3%, 39% ± 4%, and 41% ± 3%, respectively) but significantly higher on foam (63% ± 7%). There is no significant difference between OrR female immobility in plastic chambers and OrR female immobility on apple, banana, and cactus fruit (p > 0.99, p = 0.86, and p = 0.19, respectively), suggesting that the presence of an edible substrate does not modify female’s immobility and response to the vibrations during courtship.
The male chases the female while fluttering his wing. There is one very short bout of tremulation associated with wing fluttering, the female stops briefly. Finally, a long bout of male tremulation (first, male tremulation is associated with wing fluttering, and then tremulation is performed on its own) coincides with female immobility; this is soon followed by copulation.3
The signals produced by male abdominal tremulations, and received by the female to promote her immobility, are not air borne
During courtship, a number of cues convey information about the pairs’ identity and fitness,20, 21, 22, 23, 24, 25 particularly the reproductive, nutritional, and receptive status of the female.21,23 Chemical, visual, and air-borne signals modify the behavior of the male and the responsiveness of the female.21, 22, 23, 24, 25, 26, 27, 28, 29, 30 D. melanogaster courtship relies on a near-field air-borne signal, the “love song,” which is produced by the male’s wing fluttering.21, 22, 23, 24, 25, 26, 27, 28 We surgically removed the whole antennae or only the aristae (essential for air-borne sound reception)26 from females, paired them with normal males, studied their courtship, and compared it with the courtship of intact Oregon-R couples (Figures 1A–1C, S1C, and S1E). In all three treatments, female immobility strongly coincided with bouts of male tremulation (Figure 1B), and female immobility was low when the male did not tremulate (Figures 1B and 1C; Table S1). These data are consistent with the hypotheses that female immobility is not regulated by air-borne signals and that females do not detect males’ tremulations via air-borne signals.
Drosophila vibrations’ propagation varies through natural substrates, but the females’ responses are similar
The fidelity of transmission of the substrate-borne signals and the response to those signals may depend heavily on the physical properties of the courting substrates.31, 32, 33, 34, 35, 36, 37, 38, 39 So far, laser vibrometry has been used to study Drosophila substrate-borne vibrations on reflective materials.8,9,11,40 Drosophila typically meet and court on soggy and rotten fruits.41, 42, 43, 44 By recording vibrations on apple, banana, and prickly pear cactus fruits, we found that these natural substrates propagate fly vibrations (Table S2; Audio S1). Signal amplitude was highest on cactus fruits (Figure 2A; Audio S1). Our laser vibrometer did not record substrate vibrations on stone and wood, even though these substrates are used for signaling by spiders and termites,35,45, 46, 47, 48 nor on insulating foam material (Table S2).39,49
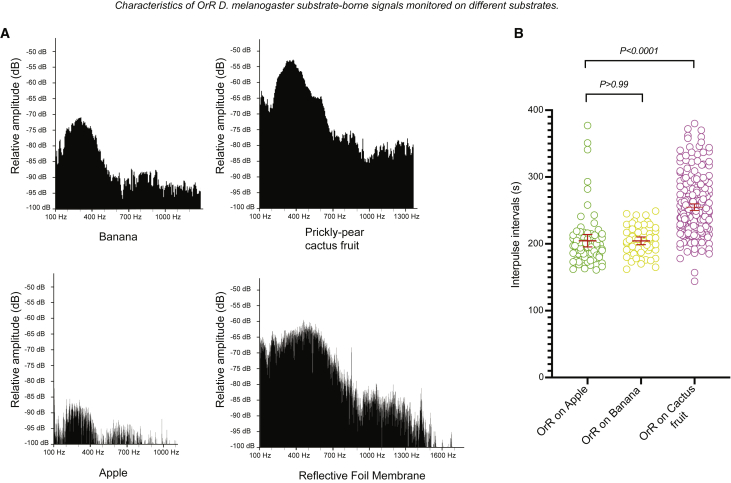
Figure 2.
Characteristics of D. melanogaster substrate-borne vibrations monitored on different substrates during courtship
(A) Pattern of frequencies and amplitude of 1–3 vibratory pulses generated by tremulations of OrR males recorded on banana, apple, prickly-pear cactus fruit, or on the artificial foil membrane during courtship with OrR females.
(B) Interpulse intervals (IPIs) of the substrate-borne vibrations of OrR males on apple (n = 74 pulses recorded, 2 flies), banana (n = 55 pulses recorded, 2 flies), and cactus fruit (n = 251 pulses recorded, 4 flies). The mean IPIs recorded on apple and banana were similar, but they were significantly different from the mean IPI recorded on the cactus fruits.
See also Table S2.
We next investigated the physical parameters of the vibrations. We analyzed the IPIs of male tremulations transmitted through the various substrates. The IPI of the vibrations generated on cactus fruits (258 ± 5 ms) were significantly different from those on banana (204 ± 6 ms) and apple (207 ± 9 ms; Figure 2B). These results suggest that properties of the substrates, such as viscosity, may affect the male’s ability to raise his abdomen to tremulate, and the properties of the cactus fruits may cause the males to tremulate in a unique fashion. The repetition rate was near 3.9 Hz (for cactus fruits) and around 4.9 Hz (for banana and apple); these values are similar to those measured when we modified other parameters in the environment, such as the ambient temperature.11 Large differences in the pulse repetition rate of vibratory signals have previously been reported for different Drosophila species,8,9,40 some of which share food substrates. For example, the prickly pear cactus is a natural breeding and courting ground for several species of Drosophila.41,43,50, 51, 52 These large differences may have a role in intraspecific courtship communication to avoid interspecific breeding, as in other vibratory insects (see, for example, Hrabar et al.53 and Miklas et al.54).
Substrate-borne signals are also characterized by the frequency spectra and the dominant frequencies of vibratory pulses, which may vary on different substrates.3,32,37,55, 56, 57 Both of these measures were complex (Figure 2A): on the reflective foil membrane, the spectrum of a vibratory unit showed a broad peak of high amplitude at frequencies between 200 Hz and 800 Hz, with a peak around 500 Hz. Another broad peak of lower amplitude was visible at frequencies between 1,000 Hz and 1,300 Hz. On the cactus fruit, values showed high amplitude from 200 Hz to 600 Hz, with a peak at about 400 Hz. On banana, we observed a narrow frequency peak around 300 Hz, and the amplitude of the signal was much lower than that observed on cactus fruits. The signal obtained on apple was similar but of even lower amplitude. On apple and banana, frequencies above 1,000 Hz did not display another peak (Figure 2A). These results suggest that Drosophila natural courting substrates modify the spectral properties of the signals and act as filters, attenuating the high frequencies.6,32,58, 59, 60 Herbaceous plants have similar properties, also acting as low pass filters.33,61, 62, 63
We studied the behavior of courting flies on these substrates. Females remained immobile for a similar percentage of time during courtship on all fruits but moved more on foam (Figure 1D). There was a strong correlation between male tremulation and female immobility on all the fruits, but not on foam (Figures 1E and 1F; Table S1). These results show that substrate-borne signals are effective through a variety of fruit substrates, regardless of the different frequency and IPI patterns (Figure 2), arguing that these signal variations fit within the sensitivity range of the receiving sensory structures. On foam, a striking impairment of female responsiveness (Figures 1D–1F) suggests a lack of propagation of male substrate-borne signals.39 This impairment could possibly result from the taste and odor of the foam, which could alter the female’s multisensory integration of male cues through her gustatory and olfactory receptor neurons,23 although we did not observe such a lack of responsiveness in plastic chambers where vibratory signals can propagate8,39 nor with antennaeless females (this report). The total time the male spent tremulating was significantly higher on cactus than on foam (Figure S1F), suggesting that the male may increase tremulation when on a conducive substrate, perhaps in reaction to responses from the female.64, 65, 66
The signals produced by abdominal tremulations are received by females via specific leg chordotonal neurons
Next, we asked which organs and neurons might act as vibration receptors in flies. In other arthropods, chordotonal organs within the legs detect substrate-borne vibrations, particularly the subgenual organ, which is absent in Drosophila.7,48,67, 68, 69, 70, 71, 72, 73 Flies do, however, possess a chordotonal organ in the femur (the fCHO) and also in the tibia (the tCHO). Their location and anatomy make them candidates for the reception of substrate-borne vibrations during Drosophila courtship;74, 75, 76 indeed, the calcium responses of a subset of fCHO “club” neurons showed that these neurons respond to artificial vibratory stimuli in addition to bidirectional movements of the tibia (the latter relates to a role in proprioception and locomotion).75 Also, fCHO club neurons project toward the thoracic ganglions, where central interneurons 10Bα respond to vibrations and mediate female immobility.77 To investigate whether neurons in the female legs respond to the vibrations, we reviewed Gal4 lines78 that expressed within distinct subsets of the CHO neurons in the leg, and we tested them by driving the expression of a neuronal inhibitor (UAS-TNTE; during metamorphosis and adulthood)79, 80, 81 in females, in courtship assays. One of these lines was 86D09-Gal4. We used membrane-bound GFP in combination with 86D09-Gal4 to observe expression in the periphery (Figure 3A) and in the brain and ventral nerve cord (VNC) (Figure 3B). We identified ∼10 neurons in the fCHO and 3 neurons in the tCHO (Figures 3A and 3C). 86D09-Gal4 neuron projections in the gnathal ganglion of the brain resemble those described for fCHO neuron axonal projections (Figure 3B),82 and the central projections of these neurons in the VNC resemble closely those of the fCHO club neurons and of the tibial chordotonal neurons (Figure 3B).75,82,83 86D09-Gal4 femoral expression appeared to be included within that of the large R46H11-Gal4 club line78 and to lie beside that of the smaller R64C04-Gal4 club neuron line (Figure S2A).75 The neuronal inhibitor driven by 86D09-Gal4 was associated with a striking reduction in female immobility (Figure S2B) during male tremulation (Figure 4A; a decrease of 40% from the 86D09-Gal4 control line and of 31% from the UAS-TNTE control line; Table S1). There was no clear effect when the male was not tremulating (Figure 4B) and locomotion of 86D09-Gal4 > UAS-TNTE females was normal (Figure S2C). Male tremulation was reduced, suggesting that, as on foam, the male may reduce tremulation as a response to low female immobility (Figure S2D). Females expressing the neuronal activator (UAS-TRPA1)84 driven by 86D09-Gal4 became more immobile when the male was not tremulating (Figure 4D). Their immobility during tremulation remained at the normal high level (Figure 4C), and no locomotor defects were observed (Figure S2C). Another line, R73D10-Gal4, drives expression in a different subset of 20 fCHO neurons, the “claw” neurons.75 Expression of the neuronal inhibitor (UAS-TNTE) under the control of R73D10-Gal4 had no effect on mean female immobility during courtship (Figures 4B and S2B) nor on the high level of female immobility during male tremulations (Figure 4A). These results argue that some or all of the ∼thirteen 86D09-Gal4-expressing neurons are club neurons that act specifically in, and are necessary for, the female’s response to the male tremulations. A lack of locomotor defects suggests that 86D09-Gal4-expressing neurons have no role in locomotion as in other insects where neurons in the leg CHOs are specialized to detect vibrations or proprioception.70,72,85, 86, 87, 88, 89 It is not known whether Drosophila males also detect substrate-borne vibrations and whether they may use these neurons to do so and to regulate their own tremulation. Our study used behavioral proxies to infer the function of CHO neurons, but further calcium imaging and electrophysiology experiments on single neurons in males and females, ideally during pair courtship, could help understand the thirteen 86D09-Gal4-expressing neurons further.
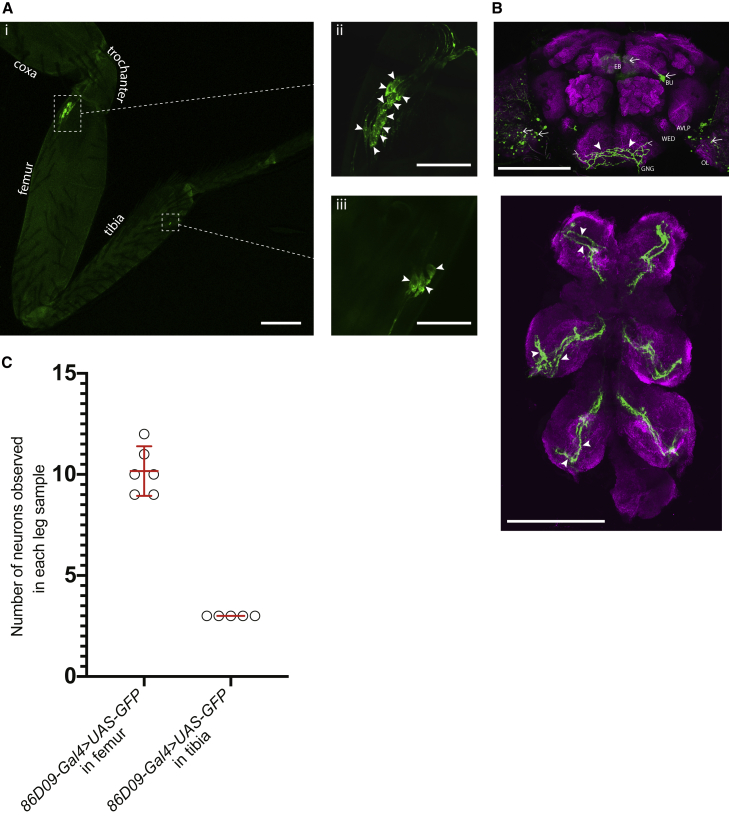
Figure 3.
Pattern of expression of the 86D09-Gal4 line in the leg and in the central nervous system
(A) Confocal image of the front leg of a 86D09-Gal4>UAS-mCD8GFP female; (i) expression (bright green) in the fCHO and the tCHO, scale bar, 100 μm; (ii) expression in 10 cell bodies of the fCHO (bright green; arrowheads) and in their associated neurites that bundle to project upward toward the trochanter and the central nervous system (CNS) and downward toward the cuticle,74 scale bar, 40 μm; (iii) expression in the cell bodies (arrowheads) of the 3 tCHO neurons, as well as in their axons bundling to project toward the leg nerve and the CNS. Scale bar, 40 μm. Light green is autofluorescence from the cuticle.
(B) Confocal image showing axon terminals (green) of 86D09-Gal4>UAS-mCD8GFP neurons in a female brain (top) and ventral nerve cord (VNC) (bottom) labeled by the neuropil marker NC82 (magenta). Top: the processes targeting the anterior side of the brain in the gnathal ganglion (GNG) (arrowheads) resemble descriptions that a few fCHO neurons target directly this region of the brain and pursue anterior-dorsally along the lateral side of the gnathal ganglion (thin arrowheads). These processes are not seen continuing deeper in the brain toward the wedge neuropil (WED) and the ventral-most part of the anterior ventrolateral protocerebrum (AVLP),82 probably due to the fact that only a small subset of neurons is stained in comparison to Tsubouchi et al.;82 GFP is also seen in neuron cell bodies in the optic lobes (OLs) (arrows; they resemble retinal neurons), in a pair of bilateral neurons of the posterior brain in the bulb region (BU) that resemble PBG1-8.b-EBw.s-D/VGA.b ring neurons that respond to visual stimuli,90, 91, 92 and weakly in the ellipsoid body (EB) where ring neurons project (arrow) and which is a central brain region for visual processing.90 Female’s vision is not necessary for her immobility response to the tremulations,8 but we cannot fully exclude that these regions known for visual processing may also be involved in vibratory signal processing. Bottom: two thin sets of axon projection bundles (arrowheads in first ganglion) enter each thoracic ganglion and bundle together toward the midline of the VNC. The bundles present the club shape typical of club neurons of the fCHO (arrowheads in second and third ganglia).75,82,83 Scale bars, 100 μm.
(C) The number of cell bodies labeled with GFP was counted in the femoral and tibial chordotonal organs of females carrying the constructs 86D09-Gal4>UAS-mCD8GFP (5/6 legs among the first pair of legs).
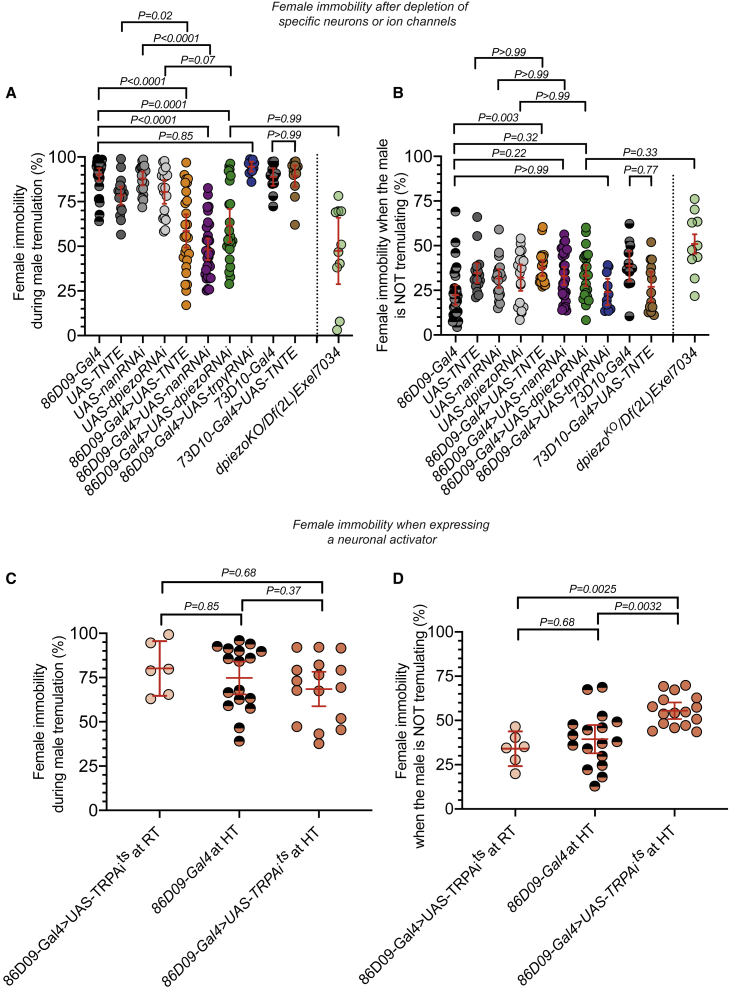
Figure 4.
Quantification of experimental and control female immobility during tremulation and during the rest of the courtship in pairs with OrR males
Data for pairs including control females carrying either 86D09-Gal4 or 73D10-Gal4 (each Gal4 targets different subsets of neurons in the legs) or either the UAS-TNTE, UAS-TRPA1 or one of the UAS-RNAi lines and for pairs including experimental females carrying a combination of both a Gal4 and an upstream activating sequence (UAS) and pairs including a dpiezoKO/Df(2L)Exel7034 female. In (A) and (B), ethograms were constructed from analysis of 30, 18, 19, 18, 26, 30, 23, 10, 12, 13, and 10 pairs (in the order illustrated on the graphs).
(A) The percentage of time where females were immobile while the male was tremulating is significantly lower only for pairs that included a female 86D09-Gal4>UAS-TNTE, 86D09-Gal4>UAS-nanRNAi, or 86D09-Gal4 > UAS-dpiezoRNAi. dpiezoKO/Df(2L)Exel7034 females behave similarly to 86D09-Gal4>UAS-dpiezoRNAi females.
(B) Same pairs as (A). The percentage of time where females were immobile while the male was not tremulating is 40% lower for 86D09-Gal4 control females compared with 86D09-Gal4>UAS-TNTE females, but the immobility of the UAS-TNTE control line is only 7.5% lower than that of 86D09-Gal4>UAS-TNTE females; all other pairs behaved similarly to all their associated controls. The immobility of dpiezoKO/Df(2L)Exel7034 females is similar to that of 86D09-Gal4 > UAS-dpiezoRNAi females.
(C) The percentage of time where females were immobile while the male tremulated is shown for pairs filmed at high temperature (HT) (31°C), including control females carrying only the 86D09-Gal4 construct (n = 17) or 86D09Gal4 > UAS-TRPA1 females (n = 16). Data are also shown at room temperature (RT) (23°C) for pairs including females 86D09-Gal4>UAS-TRPA1 (n = 6). Female immobility during male tremulation is similarly high in all 3 types of pairs. This is also the case for total female immobility and male tremulation during courtship (Figures S2E and S2F).
(D) The percentage of time where females were immobile while the male abdomen was not tremulating is shown for the same pairs as (C). 86D09-Gal4>UAS-TRPA1 female immobility at high temperature is significantly higher than controls at RT and HT.
See also Figures S2 and S3, Table S1, and Video S2.
Nanchung and Piezo, but not Trpγ, mediate female vibration sensing in 86D09-Gal4-expressing neurons
Finally, we asked which mechanotransducer ion channels, most likely mechanically gated cation channels,93, 94, 95, 96 might mediate vibration sensing in the 86D09-Gal4-expressing neurons. We tested three genes for cation channels: nanchung (nan), dpiezo, and transient receptor potential cation channel γ (trpγ), which are expressed in the fCHO;97, 98, 99 Nanchung is involved in mechanosensory transduction;97,100,101 dPiezo is mechanically activated;102,103 and Trpγ is required for photomechanosensation, proprioception, and proper gait.99,104 The roles of these cation channels in leg CHO during fly courtship had not been previously investigated. We verified nan and dpiezo expression in the leg CHOs using reporter lines and found that they are expressed exclusively in the fCHO, not in the tCHO (Figures S3A and S3B). In previous reports, immunohistochemistry on the central nervous system showed Nan-Gal4>UAS-mGFP projections in the gnathal ganglion of the brain, as well as club-shaped projections in the thoracic ganglia of the VNC.101,105 dPiezo-Gal4>UAS-mGFP also displayed expression in the gnathal ganglion of the brain and in club-shaped projections of the thoracic ganglia (Figure S3C; see also Extended Data in Ramdya et al.98). These patterns of expression are reminiscent of those observed centrally for fCHO club neurons.75,82 We knocked down each of these channels in females using UAS-RNAi lines under control of 86D09-Gal4 and observed courtship (Figures 4A, 4B, S2B, and S2D). 86D09-Gal4>UAS-nanRNAi and 86D09-Gal4>UAS-dpiezoRNAi females showed a dramatic decrease in total immobility and in immobility coinciding with male tremulations (Figures 4A and S2B). In the UAS-nanRNAi control line, female immobility during tremulation was 64% higher than female immobility when the male was not tremulating. But in 86D09-Gal4>UAS-nanRNAi females, it was only 16% higher, suggesting that female movement becomes less dependent on the tremulations (Figures 4A and 4B; Table S1; Video S2). This was also associated with significantly reduced levels of copulation success (Figure S2G). Females 86D09-Gal4>UAS-dpiezoRNAi displayed levels of immobility during tremulation that were 36% lower than those of UAS-dpiezoRNAi controls (Figure 4A). In addition, dpiezoKO/Df(2L)Exel7034 females, in which dpiezo expression was abolished, showed reduced levels of immobility during courtship, and female immobility became independent of the tremulations (Figures 4A, 4B, and S2B; Table S1). 86D09-Gal4>UAS- trpγRNAi females were unaffected (Figures 4A, 4B, S2B, and S2D). In all three RNAi experiments, climbing and walking trajectories were normal and there were no locomotor defects (Figures S2C, S2D, and S2H).
The first video clip shows a control female 86D09-Gal4 paired with an OrR male. Male tremulation coincides with female immobility. The second video clip shows a female 86D09-Gal4>UAS-nanRNAi paired with an OrR male. Male tremulations are not associated with female immobility, and on the contrary the female walks away.4
Together, these results suggest that Nan and dPiezo function in 86D09-Gal4-expressing neurons to mediate the female’s immobility response to tremulations. In flies, Nan, Inactive (Iav), and NompC have been found to work together in the antennal CHO for hearing.97,100,106 Nan functions with the TRPA channel Waterwitch and with the TRPV channel Iav during hygrosensing.101 Our findings in the leg CHOs provide an important entry point to investigate the mechanotransducer complex that detects substrate-borne vibrations; the other actors involved in this mechanotransduction remain to be determined, as well as whether Nan and dPiezo act in the same mechanosensory pathway for the reception of the vibrations. Piezo proteins account for most of the gentle touch sensitivity of vertebrates, including vibrations applied to the skin, and they are expressed in the dorsal root ganglion innervating vibration-sensitive cells, such as the Merkel cells and the Meissner’s corpuscles.107, 108, 109, 110 Our results and those of other Drosophila studies102,103,111, 112, 113, 114, 115 suggest that the roles of Piezo in mechanotransduction (here, for the gentle touch modality of vibration sensation) are diverse and conserved from adult flies to vertebrates.116
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat FITC-conjugated anti-GFP | AbCam | Cat# ab6662; RRID: AB_305635 |
| mouse anti-NC82 | Hybridoma bank | Cat# nc82; RRID: AB_2314866 |
| anti-mouse Cy5 | JacksonImmunoResearch | Cat# 715-175-151; RRID: AB_2340820 |
| Experimental models: organisms/strains | ||
| Wild Drosophila melanogaster | Collected from Wild (see Experimental model and subject details) | N/A |
| Drosophila melanogaster Oregon-R | Gift from the Lawrence laboratory, Cambridge | N/A |
| Drosophila melanogaster dPiezo-Gal4 | BDSC 58771 (on chr. II) | FBti0164865 |
| BDSC 59266 (on chr. III) | FBti0166812 | |
| Drosophila melanogaster Nanchung-Gal4 | BDSC 24903 | FBti0101148 |
| Drosophila melanogaster R86D09-Gal4 | BDSC 40459 | FBti0139154 |
| Drosophila melanogaster R73D10-Gal4 | BDSC 39819 | FBti0138087 |
| Drosophila melanogaster R64C04-Gal4 | BDSC 39296 | FBti0137440 |
| Drosophila melanogaster R46H11-Gal4 | BDSC 50284 | FBti0136107 |
| Drosophila melanogaster UAS nanchungRNAi | BDSC 53312 | FBti0157920 |
| Drosophila melanogaster UAS-trpγRNAi | BDSC 53313 | FBti0157921 |
| Drosophila melanogaster UAS-TNTE | BDSC 28837 | FBti0038528 |
| Drosophila melanogaster UAS-TRPA1 | BDSC 26264 | FBti0114502 |
| Drosophila melanogaster UAS-piezoRNAi | NIG-FLY 8486R-1 | FBal0267721 |
| Drosophila melanogaster UAS-mCD8-GFP | BDSC 5137 | FBti0012685 |
| Drosophila melanogaster PiezoKO | BDSC 58770 | FBti0147345 |
| Drosophila melanogaster Df(2L)Exel7034 | BDSC 7807 | FBab0037912 |
| Software and algorithms | ||
| Excel Macro to build ethograms from annotated movies | Described in Fabre et al.8 | Movie-to-ethogram.xlsm available at: https://github.com/CarolineFabre/Excel-Macro-for-ethograms- |
| R algorithm to analyze overlapping courtship behaviors | Described in Fabre et al.8 | script_behavior_R available at: https://github.com/CarolineFabre/Script_behaviour.R |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Caroline C. Fabre (c.c.g.fabre.03@cantab.net)
Materials availability
This study did not generate new unique reagents.
Data and code availability
Excel macros, R code and datasets used to analyze the overlapping behaviors of pairs of flies during courtship are available in the public repository at the following links:
The Excel Macro used to build ethograms from annotated movies is available at: https://github.com/CarolineFabre/Excel-Macro-for-ethograms-.
The R algorithm used to analyze overlapping courtship behaviors script_behavior_R is available at: https://github.com/CarolineFabre/Script_behaviour.R.
Raw data can be found at: https://github.com/CarolineFabre/Raw-data-from-McKelvey-et-al-...Fabre.
Experimental model and subject details
For experiments requiring females without aristae (“aristae-removed females”) or without antennnae (“antennae-removed females”), aristae or antennae were cut at their base using microscissors upon eclosion, and the flies were kept in tubes for 4 days to allow for recovery and maturation.
For laser vibrometry experiments, wings were removed at collection so as to reduce noise in the recordings.
Before courting, Oregon R individual males or small groups of five to ten virgin females of the appropriate genotype were kept in vials with fresh laboratory food.
Wild Drosophila melanogaster
Wild Drosophila flies and pupae were collected in San Michele all’Adige (Trentino, Italy) during the International Symposium on Biotremology by means of local fruit baits. After collection, wild Drosophila were kept on a mix of fruits from the region, and reared under 12:12 hr light:dark cycle, at 23°C and with 65% humidity. Subsequently, Drosophila melanogaster flies were identified morphogically under light CO2 anesthesia117 and kept together to reproduce and give rise to progeny.
Oregon R, dpiezo mutant flies, all Gal4 and UAS lines during maintenance in the laboratory fly stocks, as well as R64C04-Gal4, R46H11-Gal4, 86D09-Gal4, UASmCD8GFP, R64C04-Gal4 > UASmCD8GFP, 86D09Gal4 > UASmCD8GFP and R46H11-Gal4 > UASmCD8GFP flies used for fluorescent expression
Flies were reared under 12:12 hr light:dark cycle, at 23°C and with 65% humidity. Virgin female/male progeny were collected upon eclosion from the pupal case and, if used in courtship assays, tested at 4 days-old. dpiezo null mutant females were obtained by generating females carrying the dpiezo knockout (KO) allele and the deficiency Df(2L)Exel7034 (where the entire dpiezo genomic region is deleted) on each of the homologous chromosomes 2.103
86D09-Gal4, 73D10-Gal4, UAS-dpiezoRNAi, UAS-TNTE, UAS-nanchungRNAi, UAS-trpγRNAi, UAS-TRPA1 and their combined crosses, used for behavioral assays
To restrict expression of the UAS to metamorphosis and adulthood we kept all flies at 18°C during embryonic and larval development before moving them to room temperature (23°C). Control flies were subjected to the same conditions as experimental flies preceding pair assays.
UAS-TNTE (Tetanus toxin) specifically cleaves neuronal Synaptobrevin, which is essential for synaptic vesicle release and neurotransmitter release, and its expression leads to impairment of neuronal functions.80
UAS-TRPA1 is a thermosensitive tool and its expression leads to activation of neurons at elevated temperatures because TRPA1 is a heat-activated nonselective cation channel.84
Method details
Laser vibrometry
Laser vibrometry of courting pairs were performed at a temperature of around 23°C. Video and laser vibrometer recordings were conducted on a vibration-damped table in a soundproof room. Virgin female/male progeny from wild-caught males and females were collected upon eclosion from the pupal case and directly filmed while courting within 1 week of collection so as not to habituate the wild stock to lab conditions. In all other cases, flies were filmed and courted when 4 days old upon eclosion from the pupal case. Flies were filmed with a Stingray F-33B camera (Allied Vision) on either the recording membrane, other artificial substrates, or on a sample of fruit or plant. All natural and artificial substrates were prepared with similar thickness (around 5mm) and size (around 10mm × 9mm). To record, the beam of a laser Doppler vibrometer (Polytec OFV 5000 controller, OFV 534 sensor head; Waldbronn, Germany) was directed perpendicular to the surface of a square of reflective tape (3M, 0.5mm2; Scotchlite, Neuss, Germany) placed in the center onto the surface of the fruit or artificial substrates. Reflective tapes are commonly used in biotremology studies that involve laser vibrometry on non-reflective substrates;55,118, 119, 120 the physics of mechanical waves allows for their energy to be transferred from one solid (i.e., the substrate) to another (i.e., the tape) by wave motion.121 Signals were digitised with 12bit amplitude resolution with a PCI MIO-16-E4 card (Analog Devices; Norwood, MA) and with LabView (National Instruments; Austin, TX) on a PC. Signals were transformed into .wav data with Cool Edit Pro (Adobe Systems) or Neurolab software.122 Video and laser vibrometer recordings were synchronized at the start by brief interruption of the laser path; this produces both a momentary peak in the oscillogram and a black frame in the video.
Behavioral courtship assays
Video-imaging of courting pairs were performed at a temperature of around 23°C. Male–virgin female fly pairs were tested at 4 days old. Their behavior was recorded with a 100mm macro lens and a Stingray F-033B camera (Allied Vision Technologies; Stadtroda, Germany) and acquired with the Debut Video Capture (Pro Edition) software into a iMac computer. Apart from experiments on substrates, courting pairs were filmed in transparent plexiglass chambers (10mm diameter and 6 mm height) as in Fabre et al.8 For experiments on substrates, the substrates used were ripened fruits: apple, banana, prickly-pear; layer of bark from an apple tree; gravel stone; an isolating foam (open-cell type foam characterized by low conductivity and produced for thermal insulation49). The foam selected did not appear to produce repellent artificial smells to the flies as courtship index was high (not shown). Cylindrical holes (around 10mm diameter and 9mm depth) were carved within fruit and foam substrates. We also filmed flies on our original recording membrane; a thermal foil, made of silver metallized polyester material, with an albedo of approximately 0.8 (Sub Zero Technology; Leicester, UK). A piece of transparent plexiglass was placed between the flies and the camera to contain the flies. Movies were only taken into account for analysis if the flies spent more than 95% of the time on the substrate as opposed to the plexiglass. Recording was started at the initiation of courtship and for approximately 600 s, or until copulation occurred. Each pair was tested only once.
Courtship behavior annotations
Movies were annotated with semi-automated Annotation software (Peter Brodsky, version 1.3) while watched on a large 27-inch desktop screen, thus allowing behaviors to be detected with great sensitivity and accuracy. We registered male courting behaviors such as orientation toward the female, tremulation of the abdomen (i.e., rapid up-and-down movements of the abdomen, as described in Fabre et al.8), extension and vibration of the wings, and also whether the female was moving or stationary (stationary being defined as the female not walking in any direction). Annotations were performed in a randomized and blind manner (movie files were randomly numbered so that the identity of the pair annotated was not identified), and approximately 10% of the annotations were performed twice for comparison (i.e., by two different annotators), with the resulting consensus annotation used when necessary. Each behavior was annotated independently from the others. For experiments testing substrates, annotation was only recorded when both animals were localized on the substrate (instances when either or both animals were localized on the transparent plexiglass top were not included in the analysis). Note, when monitoring abdominal tremulations and female immobility it is very difficult to judge whether the tremulations start just before or just after female stopping, as the tremulations and stopping occur almost simultaneously.
Negative geotaxis climbing assays
Virgin females (aged 4 days old) were inserted into a capped graded tube. The flies were tapped to the bottom and the number of flies crossing a target line localized 4 cm above the bottom of the tube was recorded using a camera.
Principal component analysis
11 walking variables (similar to variables used in Tsai and Chou123) were used to perform principal component analysis (PCA) on 4-7 day old individual female flies carrying the constructs 86D09-Gal4, UAS-dpiezoRNAi, UAS-nanRNAi, 86D09-Gal4 > UAS-dpiezoRNAi or 86D09-Gal4 > UAS-nanRNAi, and filmed walking for 30 minutes in Petri dishes (diameter 5cms) using a webcam (Logitech); Debut Video Capture (Pro Edition) software was used with an iMac computer for video acquisition. Tracking of fly and analysis of locomotion was performed using the plugin MtrackJ124 in Fiji.125 The variables used for PCA in each 5 s time bin were: average and standard deviation of speed, average orientation, average angular velocity, average and standard deviation of horizontal velocity, average and standard deviation of vertical velocity, straightness, magnitude of displacement, weighted average of orientations by distances. Data were standardized prior to computation using the Pearson Correlation treatment. Standardized values were then subjected to PCA123 using XLSTAT software in Excel (Data Analysis and Statistical Solution for Microsoft Excel, Addinsoft, Paris, France 2017).
Immunohistochemistry and microscopy
In preparation for dissection under the binocular microscope, flies were anaesthetised and placed in a Petri dish with phosphate-buffered saline (PBS) where brain and ventral nerve cords were dissected out of the cuticle, fixed (4% paraformaldehyde, Electron Microscopy Science) and stained. The following antibodies were used: Goat FITC-conjugated anti-GFP (1:1000, Abcam), mouse anti-NC82 (1:20, Hybridoma bank), anti-mouse Cy5 (1:1000; JacksonImmunoResearch). Samples were mounted in Fluoromount mounting medium (SigmaAldrich) with the anterior part of the brains and ventral side of the ventral nerve cords oriented upward. The samples were imaged with a confocal microscope (Leica SP5) run by LAS AF software. Legs were dissected out and mounted with Fluoromount for confocal imaging or for imaging on a Leica DMi8 microscope mounted with a BSI express camera (Teledyne Photometrics). The software Fiji125 was used to process the .lif and .tif files. To compare the expression driven by several Gal4 driver lines, we used Fiji to overlay the images based on morphological landmarks (femur-coxa joint, femur-tibia joint, shape of the cuticle).
Quantification and statistical analysis
Statistical details of experiments can be found below and in figures. p < 0.05 is the threshold of significance. On all graphs, red bars are used to indicate mean and 95% confidence intervals.
Laser Vibrometry
Oscillograms were analyzed with Amadeus Pro (HairerSoft) and Raven Pro (The Cornell Lab of Ornithology, Bioacoustics Research Program) software. Frequency analysis on different substrates was performed using Amadeus Pro (HairerSoft) on 1-3 pulses. The values in decibels (dB) obtained in the y axis were scaled relative to the minimum laser output, with −100 dB corresponding to the minimum laser output velocity of 0.04 μm/s and the amplitudes measured increasing relative to this minimal value. The repetition rate of a vibratory signal is defined as the number of pulses per second, i.e., 1/IPI, converted in Herz (Hz).
Courtship Behavior Annotations
Data for each annotated movie were imported into Excel and into Rstudio [147]. As in [9], the resulting file obtained for each annotated movie showed for each period of 1 s, whether the male was moving or stationnary, and whether the male abdomen was tremulating or not (or, in Figure S1B, whether the male wing was fluttering or not). For each movie we calculated the percentage of time that the female was immobile when the male was tremulating its abdomen (or, in Figure S1B, when the male wing was fluttering). We generated the plots using Prism (GraphPad). For statistical analysis and generation of diagrams, we used Microsoft Excel macros, the R programming language and software environment126 and Prism (GraphPad).
Negative Geotaxis Climbing Assay
The percentage of females having passed the threshold line after 3 s is represented. Each climbing assay tested around 20 flies.
Quantification of copulation success
Mating was considered and represented as successful if male and female copulated within 10 minutes of courtship.
Details for each figure
Figure 1: Dunnett’s T3 multiple comparisons tests were used to calculate p values that can be found in the figure and in the figure legend. n-numbers are given in figure legend and represent number of video clips; n-numbers are given in figure legend; each video clip is made from one unique courtship pair. Each data point corresponds to the data from one video clip. In figure legend, averages are given as the mean ± standard error of the mean.
Figure 2A: The y axis shows the relative amplitude of the signals (in decibels, on a logarithmic scale) and the x axis shows increasing frequencies in Herz. Vertical black lines are present if the frequency was recorded in the signal. Analysis of pulses was performed using Amadeus Pro (hairerSoft).
Figure 2B: Dunnett’s T3 multiple comparisons test was used to calculate p values that can be found in the figure. n-numbers are given in figure legend and represent number of pulses recorded across multiple courtship pairs (see legend). Each interpulse interval (IPI) value is represented by a circle on the graph.
Figure 4: Dunnett’s T3 multiple comparisons tests were used to calculate p values that can be found in the figure. n-numbers are given in figure legend and represent number of video clips; each video clip (each circle) is made from one unique courtship pair.
Figures S1A and S1B: Two-tailed unpaired t test was used to calculate p values that can be found in the figure legend. n-numbers are given in the figure and represent number of video clips; each video clip is made from one unique courtship pair. In figure legend, averages are given as the mean ± standard error of the mean.
Figure S1C: Dunnett’s T3 multiple comparisons tests were used to calculate p values that can be found in the figure. n-numbers are given in figure legend and represent number of video clips; each video clip is made from one unique courtship pair.
Figure S1D: Two-tailed unpaired t test was used to calculate p values that can be found in the figure. n-numbers are given in figure legend and represent number of pulses recorded across multiple courtship pairs (see figure legend). Each pulse is represented by a circle on the graph.
Figure S1E: Two-tailed unpaired t test was used to calculate p values that can be found in the figure. n-numbers are given in the figure and represent number of video clips; each video clip is made from one unique courtship pair.
Figure S1F: Dunnet’s T3 multiple comparison tests was used to calculate p values that can be found in the figure. For n-numbers see legend of Figures 1D–1F.
Figures S2B and S2D: Dunnett’s T3 multicomparison tests were used to calculate p values that can be found in the figure. n-numbers can be found in legend of Figures 4A and 4B and represent number of video clips (each video clip is made from one unique courtship pair).
Figure S2C: Dunnett’s T3 multicomparison tests were used to calculate p values that can be found in the figure. n-number can be found in figure legend and represent the number of climbing assays performed (each circle represent one climbing assay – see sections above for method and below for statistical details).
Figures S2E and S2F: Dunnett’s T3 multiple comparisons tests were used to calculate p values that can be found in the figure. n-number can be found in legend of Figures 4C and 4D.
Figure S2G: Dunnett’s T3 multiple comparisons test was used to calculate p values that can be found in the figure. n-number can be found in figure legend.
Table S1: Dunnett’s T3 multiple comparisons test was used to calculate p values that can be found in the figure.
Acknowledgments
We thank Paul Conduit, Corinne Tovey, Jose Casal, Peter Lawrence, and Jonathan Lee for critical reading of the manuscript; the reviewers for their constructive feedback on our manuscript; Berthold Hedwig for help with the laser vibrometer; and Matt Wayland for help with confocal microscopy. We thank P. Lawrence, B. Hedwig, J. Casal, and P. Conduit for continual support. This reresearch was funded by the Wellcome Trust (grant number 107060/Z/15/Z to P. Lawrence), a Sir Isaac Newton Trust Research Grant (16.24(i) to B. Hedwig and C.C.G.F.), the G.C. Grindley fund (to V.B.P.), the Balfour-Browne Trust fund (to K.M.), and The Department of Zoology and the School of Biology of the University of Cambridge (to C.C.G.F.). The work benefited from use of the Imaging Facility, Department of Zoology, supported by a Sir Isaac Newton Trust Research Grant (18.07ii(c)). This work is licensed under a Creative Commons Attribution 4.0 International License.
Author contributions
Conceptualization, supervision, validation, project administration, funding acquisition, C.C.G.F.; investigation, formal analysis, C.C.G.F., E.G.Z.M., J.P.G., K.M., V.B.P., L.S., L.E.K., and A.C.; funding acquisition, K.M. and V.B.P.; writing – original draft, C.C.G.F.; writing – review and editing, C.C.G.F. and E.G.Z.M.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list. The author list of this paper includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Published: June 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2021.06.002.
Supplemental information
(start-11 s) Audio clip including one bout of tremulations performed by an OrR D. melanogaster male to an OrR D. melanogaster female, as recorded on the apple substrate. The bout contains 36 pulses. (12 s-20 s) Audio clip including one bout of tremulations performed by an OrR D. melanogaster male to an OrR D. melanogaster female, as recorded on banana. The bout contains 27 pulses. (21 s-end) Audio clip including a series of bouts of tremulations performed by an OrR D. melanogaster male to an OrR D. melanogaster female, as recorded on the cactus fruit. The first bout contains 33 pulses, then a few very short bouts follow, and the second long bout contains 39 pulses.
References
- 1.Hill P.S.M. How do animals use substrate-borne vibrations as an information source? Naturwissenschaften. 2009;96:1355–1371. doi: 10.1007/s00114-009-0588-8. [DOI] [PubMed] [Google Scholar]
- 2.Hoch H., Deckert J., Wessel A. Vibrational signalling in a Gondwanan relict insect (Hemiptera: Coleorrhyncha: Peloridiidae) Biol. Lett. 2006;2:222–224. doi: 10.1098/rsbl.2006.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cocroft R.B., Rodríguez R.L. The behavioral ecology of insect vibrational communication. Bioscience. 2005;55:323–334. [Google Scholar]
- 4.Hill P.S.M. Harvard University; 2008. Vibrational Communication in Animals. [Google Scholar]
- 5.Hill P.S.M. Vibration and animal communication: a review. Am. Zool. 2001;41:1135–1142. [Google Scholar]
- 6.Hill P.S.M., Wessel A. Biotremology. Curr. Biol. 2016;26:R187–R191. doi: 10.1016/j.cub.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 7.Virant-Doberlet M., Čokl A. Vibrational communication in insects. Neotrop. Entomol. 2004;33:121–134. [Google Scholar]
- 8.Fabre C.C.G., Hedwig B., Conduit G., Lawrence P.A., Goodwin S.F., Casal J. Substrate-borne vibratory communication during courtship in Drosophila melanogaster. Curr. Biol. 2012;22:2180–2185. doi: 10.1016/j.cub.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzoni V., Anfora G., Virant-Doberlet M. Substrate vibrations during courtship in three Drosophila species. PLoS ONE. 2013;8:e80708. doi: 10.1371/journal.pone.0080708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busnel R.G., Pasquinelly F., Dumortier C. La trémulation du corps et la transmission aux supports des vibrations en résultant comme moyen d’information à courte portée des Éphippigères femelle et mâle. Ibid. Bull. Soc. Zool. Fr. 1955;80:18–22. [Google Scholar]
- 11.Medina I., Casal J., Fabre C.C.G. Do circadian genes and ambient temperature affect substrate-borne signalling during Drosophila courtship? Biol. Open. 2015;4:1549–1557. doi: 10.1242/bio.014332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bussell J.J., Yapici N., Zhang S.X., Dickson B.J., Vosshall L.B. Abdominal-B neurons control Drosophila virgin female receptivity. Curr. Biol. 2014;24:1584–1595. doi: 10.1016/j.cub.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markow T.A., Hanson S.J. Multivariate analysis of Drosophila courtship. Proc. Natl. Acad. Sci. USA. 1981;78:430–434. doi: 10.1073/pnas.78.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw K.L., Herlihy D.P. Acoustic preference functions and song variability in the Hawaiian cricket Laupala cerasina. Proc. Biol. Sci. 2000;267:577–584. doi: 10.1098/rspb.2000.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhardt H.C., Huber F., Simmons A.M. Acoustic communication in insects and anurans: Common problems and diverse solutions. Physiol. Entomol. 2003;28:62–63. [Google Scholar]
- 16.Ewing A.W., Bennet-Clark H.C. The courtship songs of Drosophila. Behaviour. 1968;31:288–301. [Google Scholar]
- 17.Doherty J.A. Song recognition and localization in the phonotaxis behavior of the field cricket, Gryllus bimaculatus (Orthoptera: Gryllidae) J. Comp. Physiol. A. 1991;168:213–222. [Google Scholar]
- 18.Doherty J.A. Phonotaxis in the cricket, Gryllus bimaculatus DeGeer: comparisons of choice and no-choice paradigms. J. Comp. Physiol. A. 1985;157:279–289. [Google Scholar]
- 19.Doherty J.A., Storz M.M. Calling song and selective phonotaxis in the field crickets, Gryllus firmus and G. pennsylvanicus (Orthoptera: Gryllidae) J. Insect Behav. 1992;5:555–569. [Google Scholar]
- 20.Amrein H. Pheromone perception and behavior in Drosophila. Curr. Opin. Neurobiol. 2004;14:435–442. doi: 10.1016/j.conb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Aranha M.M., Vasconcelos M.L. Deciphering Drosophila female innate behaviors. Curr. Opin. Neurobiol. 2018;52:139–148. doi: 10.1016/j.conb.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Bastock M., Manning A. The courtship of Drosophila melanogaster. Behaviour. 1955;8:85–111. [Google Scholar]
- 23.Ellendersen B.E., von Philipsborn A.C. Neuronal modulation of D. melanogaster sexual behaviour. Curr. Opin. Insect Sci. 2017;24:21–28. doi: 10.1016/j.cois.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Greenspan R.J., Ferveur J.F.F. Courtship in Drosophila. Annu. Rev. Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 25.Spieth H.T. Courtship behavior in Drosophila. Annu. Rev. Entomol. 1974;19:385–405. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- 26.Tauber E., Eberl D.F. Acoustic communication in Drosophila. Behav. Processes. 2003;64:197–210. [Google Scholar]
- 27.Hall J.C. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 28.Ferveur J.F. Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Curr. Opin. Neurobiol. 2010;20:764–769. doi: 10.1016/j.conb.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Billeter J.C., Rideout E.J., Dornan A.J., Goodwin S.F. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr. Biol. 2006;16:R766–R776. doi: 10.1016/j.cub.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto D., Jallon J.-M., Komatsu A. Genetic dissection of sexual behavior in Drosophila melanogaster. Annu. Rev. Entomol. 1997;42:551–585. doi: 10.1146/annurev.ento.42.1.551. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez R.L., Sullivan L.M., Snyder R.L., Cocroft R.B. Host shifts and the beginning of signal divergence. Evolution. 2008;62:12–20. doi: 10.1111/j.1558-5646.2007.00256.x. [DOI] [PubMed] [Google Scholar]
- 32.Mortimer B. Biotremology: do physical constraints limit the propagation of vibrational information? Anim. Behav. 2017;130:165–174. [Google Scholar]
- 33.Michelsen A., Fink F., Gogala M., Traue D. Plants as transmission channels for insect vibrational songs. Behav. Ecol. Sociobiol. 1982;11:269–281. [Google Scholar]
- 34.Hebets E.A., Elias D.O., Mason A.C., Miller G.L., Stratton G.E. Substrate-dependent signalling success in the wolf spider, Schizocosa retrorsa. Anim. Behav. 2008;75:605–615. [Google Scholar]
- 35.Elias D.O., Mason A.C., Hoy R.R. The effect of substrate on the efficacy of seismic courtship signal transmission in the jumping spider Habronattus dossenus (Araneae: Salticidae) J. Exp. Biol. 2004;207:4105–4110. doi: 10.1242/jeb.01261. [DOI] [PubMed] [Google Scholar]
- 36.Elias D.O., Mason A.C., Hebets E.A. A signal-substrate match in the substrate-borne component of a multimodal courtship display. Curr. Zool. 2010;56:370–378. [Google Scholar]
- 37.Čokl A., Zorović M., Žunič A., Virant-Doberlet M. Tuning of host plants with vibratory songs of Nezara viridula L (Heteroptera: Pentatomidae) J. Exp. Biol. 2005;208:1481–1488. doi: 10.1242/jeb.01557. [DOI] [PubMed] [Google Scholar]
- 38.Čokl A., Virant-Doberlet M. Communication with substrate-borne signals in small plant-dwelling insects. Annu. Rev. Entomol. 2003;48:29–50. doi: 10.1146/annurev.ento.48.091801.112605. [DOI] [PubMed] [Google Scholar]
- 39.Elias D.O., Mason A.C. In: Cocroft R.B., Gogala M., Hill P.S.M., Wessel A., editors. Volume 3. Springer; 2014. The role of wave and substrate heterogeneity in vibratory communication: Practical issues in studying the effect of vibratory environments in communication; pp. 215–247. (Studying Vibrational Communication. Animal Signals and Communication). [Google Scholar]
- 40.Hernández M.V., Fabre C.C.G. The elaborate postural display of courting Drosophila persimilis flies produces substrate-borne vibratory signals. J. Insect Behav. 2016;29:578–590. doi: 10.1007/s10905-016-9579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soto-Yéber L., Soto-Ortiz J., Godoy P., Godoy-Herrera R. The behavior of adult Drosophila in the wild. PLoS ONE. 2018;13:e0209917. doi: 10.1371/journal.pone.0209917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markow T.A. Behavioral and sensory basis of courtship success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1987;84:6200–6204. doi: 10.1073/pnas.84.17.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Pino F., Jara C., Pino L., Godoy-Herrera R. The neuro-ecology of Drosophila pupation behavior. PLoS ONE. 2014;9:e102159. doi: 10.1371/journal.pone.0102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grosjean Y., Rytz R., Farine J.P., Abuin L., Cortot J., Jefferis G.S.X.E., Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–240. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- 45.Yack J. In: Pollack G., Mason A., Popper A., Fay R., editors. Volume 55. Springer; 2016. Vibrational signaling; pp. 99–123. (Insect Hearing. Springer Handbook of Auditory Research). [Google Scholar]
- 46.Röhrig A., Kirchner W.H., Leuthold R.H. Vibrational alarm communication in the African fungus-growing termite genus Macrotermes (Isoptera, Termitidae) Insectes Soc. 1999;46:71–77. [Google Scholar]
- 47.Kirchner W.H., Broecker I., Tautz J. Vibrational alarm communication in the damp-wood termite Zootermopsis nevadensis. Physiol. Entomol. 1994;19:187–190. [Google Scholar]
- 48.Howse P.E. An investigation into the mode of action of the subgenual organ in the termite, Zootermopsis angusticollis Emerson, and in the cockroach, Periplaneta americana L. J. Insect Physiol. 1964;10:409–416. [Google Scholar]
- 49.Ashby M.F. The properties of foams and lattices. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006;364:15–30. doi: 10.1098/rsta.2005.1678. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz A., Naveira H., Fontdevila A. The evolutionary history of Drosophila buzzatii. V. Differential survivorship on Opuntia between D. buzzatii and D. serido. Experientia. 1985;41:129–131. [Google Scholar]
- 51.Markow T.A., O’Grady P. Reproductive ecology of Drosophila. Funct. Ecol. 2008;22:747–759. [Google Scholar]
- 52.Haouas S., Carton Y., Marrakchi M., David J. Reproductive strategy of D. buzzati and D. melanogaster associated with the prickly pear of Opuntia in Tunisisa. Oecol. Gener. 1984;5:175–179. [Google Scholar]
- 53.Hrabar N., Virant-Doberlet M., Cokl A. Species specificity of male southern green stink bug Nezara viridula (L.) reactions to the female calling song. Dong Wu Xue Bao. 2004;50:566–575. [Google Scholar]
- 54.Miklas N., Stritih N., Čokl A., Virant-Doberlet M., Renou M. The influence of substrate on male responsiveness to the female calling song in Nezara viridula. J. Insect Behav. 2001;14:313–332. [Google Scholar]
- 55.Elias D.O., Mason A.C., Maddison W.P., Hoy R.R. Seismic signals in a courting male jumping spider (Araneae: Salticidae) J. Exp. Biol. 2003;206:4029–4039. doi: 10.1242/jeb.00634. [DOI] [PubMed] [Google Scholar]
- 56.Žunič A., Virant-Doberlet M., Čokl A. Species recognition during substrate-borne communication in Nezara viridula (L.) (Pentatomidae: Heteroptera) J. Insect Behav. 2011;24:468–487. [Google Scholar]
- 57.Žunič A., Doberlet M.V., Čokl A. Preference of the southern green stink bug (Nezara viridula) males for female calling song parameters. Bull. Insectol. 2008;61:183–184. [Google Scholar]
- 58.Cocroft R.B., Hamel J., Su Q., Gibson J. In: Cocroft R., Gogala M., Hill P., Wessel A., editors. Volume 3. Springer; 2014. Vibrational playback experiments: challenges and solutions; pp. 249–276. (Studying Vibrational Communication: Animal Signals and Communication). [Google Scholar]
- 59.Michelsen A. In: Cocroft R.B., Gogala M., Hill P.S.M., Wessel A., editors. Volume 3. Springer; 2014. Physical aspects of vibrational communication; pp. 199–213. (Studying Vibrational Communication. Animal Signals and Communication). [Google Scholar]
- 60.Hill P.S.M., Shadley J.R. Talking back: sending soil vibration signals to lekking prairie mole cricket males’. Am. Zool. 2001;41:1200–1214. [Google Scholar]
- 61.Čokl A., Zorović M., Žunič A., Virant-Doberlet M. Tuning of host plants with vibratory songs of Nezara viridula L (Heteroptera: Pentatomidae) J. Exp. Biol. 2005;208:1481–1488. doi: 10.1242/jeb.01557. [DOI] [PubMed] [Google Scholar]
- 62.Čokl A. Stink bug interaction with host plants during communication. J. Insect Physiol. 2008;54:1113–1124. doi: 10.1016/j.jinsphys.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 63.McNett G.D., Cocroft R.B. Host shifts favor vibrational signal divergence in Enchenopa binotata treehoppers. Behav. Ecol. 2008;19:650–656. [Google Scholar]
- 64.Gordon S.D., Uetz G.W. Multimodal communication of wolf spiders on different substrates: evidence for behavioural plasticity. Anim. Behav. 2011;81:367–375. [Google Scholar]
- 65.Sullivan-Beckers L., Hebets E.A. Tactical adjustment of signalling leads to increased mating success and survival. Anim. Behav. 2014;93:111–117. [Google Scholar]
- 66.Gray B., Bailey N.W., Poon M., Zuk M. Multimodal signal compensation: do field crickets shift sexual signal modality after the loss of acoustic communication? Anim. Behav. 2014;93:243–248. [Google Scholar]
- 67.Stritih Peljhan N., Strauß J. The mechanical leg response to vibration stimuli in cave crickets and implications for vibrosensory organ functions. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2018;204:687–702. doi: 10.1007/s00359-018-1271-3. [DOI] [PubMed] [Google Scholar]
- 68.Strauß J., Stritih N., Lakes-Harlan R. The subgenual organ complex in the cave cricket Troglophilus neglectus (Orthoptera: Rhaphidophoridae): comparative innervation and sensory evolution. R. Soc. Open Sci. 2014;1:140240. doi: 10.1098/rsos.140240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strauß J., Lomas K., Field L.H. The complex tibial organ of the New Zealand ground weta: sensory adaptations for vibrational signal detection. Sci. Rep. 2017;7:2031. doi: 10.1038/s41598-017-02132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stein W., Sauer A.E. Physiology of vibration-sensitive afferents in the femoral chordotonal organ of the stick insect. J. Comp. Physiol. A. 1999;184:253–263. [Google Scholar]
- 71.Menzel J.G., Tautz J. Functional morphology of the subgenual organ of the carpenter ant. Tissue Cell. 1994;26:735–746. doi: 10.1016/0040-8166(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 72.Lakes-Harlan R., Strauß J. In: Cocroft R., Gogala M., Hill P., Wessel A., editors. vol. 3. Springer; 2014. Functional morphology and evolutionary diversity of vibration receptors in insects; pp. 277–302. (Studying Vibrational Communication. Animal Signals and Communication). [Google Scholar]
- 73.Howse P.E. The structure of the subgenual organ and certain other mechanoreceptors of the termite Zootermopsis angusticollis (Hagen) Proc. R. Entomol. Soc. Lond., Ser. A Gen. Entomol. 1965;40:137–146. [Google Scholar]
- 74.Shanbhag S.R., Singh K., Naresh Singh R. Ultrastructure of the femoral chordotonal organs and their novel synaptic organization in the legs of Drosophila melanogaster Meigen (Diptera : Drosophilidae) Int. J. Insect Morphol. Embryol. 1992;21:311–322. [Google Scholar]
- 75.Mamiya A., Gurung P., Tuthill J.C. Neural coding of leg proprioception in Drosophila. Neuron. 2018;100:636–650.e6. doi: 10.1016/j.neuron.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nottebohm E., Ramaekers A., Dambly-Chaudière C., Ghysen A. The leg of Drosophila as a model system for the analysis of neuronal diversity. J. Physiol. Paris. 1994;88:141–151. doi: 10.1016/0928-4257(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 77.Agrawal S., Dickinson E.S., Sustar A., Gurung P., Shepherd D., Truman J.W., Tuthill J.C. Central processing of leg proprioception in Drosophila. eLife. 2020;9:e60299. doi: 10.7554/eLife.60299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jenett A., Rubin G.M., Ngo T.T.B., Shepherd D., Murphy C., Dionne H., Pfeiffer B.D., Cavallaro A., Hall D., Jeter J. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsubouchi A., Caldwell J.C., Tracey W.D. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr. Biol. 2012;22:2124–2134. doi: 10.1016/j.cub.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sweeney S.T., Broadie K., Keane J., Niemann H., O’Kane C.J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 81.Kamikouchi A., Inagaki H.K., Effertz T., Hendrich O., Fiala A., Göpfert M.C., Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 82.Tsubouchi A., Yano T., Yokoyama T.K., Murtin C., Otsuna H., Ito K. Topological and modality-specific representation of somatosensory information in the fly brain. Science. 2017;358:615–623. doi: 10.1126/science.aan4428. [DOI] [PubMed] [Google Scholar]
- 83.Phillis R., Statton D., Caruccio P., Murphey R.K. Mutations in the 8 kDa dynein light chain gene disrupt sensory axon projections in the Drosophila imaginal CNS. Development. 1996;122:2955–2963. doi: 10.1242/dev.122.10.2955. [DOI] [PubMed] [Google Scholar]
- 84.Hamada F.N., Rosenzweig M., Kang K., Pulver S.R., Ghezzi A., Jegla T.J., Garrity P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Field L.H., Matheson T. Chordotonal organs of insects. Adv. Insect Physiol. 1998;27:1–228. [Google Scholar]
- 86.Field L.H., Pflüger H.-J. The femoral chordotonal organ: a bifunctional orthopteran (Locusta migratoria) sense organ? Comp. Biochem. Physiol. Part A. Physiol. 1989;93:729–743. [Google Scholar]
- 87.BUSchges A. The physiology of sensory cells in the ventral scoloparium of the stick insect femoral chordotonal organ. J. Exp. Biol. 1994;189:285–292. doi: 10.1242/jeb.189.1.285. [DOI] [PubMed] [Google Scholar]
- 88.Kittmann R., Schmitz J. Functional specialization of the scoloparia of the femoral chordotonal organ in stick insects. J. Exp. Biol. 1992;173:91–108. [Google Scholar]
- 89.Sauer A.E., Stein W. Sensorimotor pathways processing vibratory signals from the femoral chordotonal organ of the stick insect. J. Comp. Physiol. A. 1999;185:21–31. [Google Scholar]
- 90.Omoto J.J., Keleş M.F., Nguyen B.M., Bolanos C., Lovick J.K., Frye M.A., Hartenstein V. Visual input to the Drosophila central complex by developmentally and functionally distinct neuronal populations. Curr. Biol. 2017;27:1098–1110. doi: 10.1016/j.cub.2017.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolff T., Iyer N.A., Rubin G.M. Neuroarchitecture and neuroanatomy of the Drosophila central complex: A GAL4-based dissection of protocerebral bridge neurons and circuits. J. Comp. Neurol. 2015;523:997–1037. doi: 10.1002/cne.23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seelig J.D., Jayaraman V. Neural dynamics for landmark orientation and angular path integration. Nature. 2015;521:186–191. doi: 10.1038/nature14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vega J.A., García-Suárez O., Montaño J.A., Pardo B., Cobo J.M. The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microsc. Res. Tech. 2009;72:299–309. doi: 10.1002/jemt.20651. [DOI] [PubMed] [Google Scholar]
- 94.Chalfie M. Neurosensory mechanotransduction. Nat. Rev. Mol. Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- 95.Christensen A.P., Corey D.P. TRP channels in mechanosensation: direct or indirect activation? Nat. Rev. Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 96.Syntichaki P., Tavernarakis N. Genetic models of mechanotransduction: the nematode Caenorhabditis elegans. Physiol. Rev. 2004;84:1097–1153. doi: 10.1152/physrev.00043.2003. [DOI] [PubMed] [Google Scholar]
- 97.Kim J., Chung Y.D., Park D.Y., Choi S., Shin D.W., Soh H., Lee H.W., Son W., Yim J., Park C.S. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 98.Ramdya P., Lichocki P., Cruchet S., Frisch L., Tse W., Floreano D., Benton R. Mechanosensory interactions drive collective behaviour in Drosophila. Nature. 2015;519:233–236. doi: 10.1038/nature14024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akitake B., Ren Q., Boiko N., Ni J., Sokabe T., Stockand J.D., Eaton B.A., Montell C. Coordination and fine motor control depend on Drosophila TRPγ. Nat. Commun. 2015;6:7288. doi: 10.1038/ncomms8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gong Z., Son W., Chung Y.D., Kim J., Shin D.W., McClung C.A., Lee Y., Lee H.W., Chang D.J., Kaang B.K. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J. Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu L., Li Y., Wang R., Yin C., Dong Q., Hing H., Kim C., Welsh M.J. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 102.Shao L., Chung P., Wong A., Siwanowicz I., Kent C.F., Long X., Heberlein U. A neural circuit encoding the experience of copulation in female Drosophila. Neuron. 2019;102:1025–1036.e6. doi: 10.1016/j.neuron.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 103.Kim S.E., Coste B., Chadha A., Cook B., Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu X.Z.S., Chien F., Butler A., Salkoff L., Montell C. TRPgamma, a Drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron. 2000;26:647–657. doi: 10.1016/s0896-6273(00)81201-5. [DOI] [PubMed] [Google Scholar]
- 105.Jourjine N., Mullaney B.C., Mann K., Scott K. Coupled sensing of hunger and thirst signals balances sugar and water consumption. Cell. 2016;166:855–866. doi: 10.1016/j.cell.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang W., Yan Z., Jan L.Y., Jan Y.N. Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc. Natl. Acad. Sci. USA. 2013;110:13612–13617. doi: 10.1073/pnas.1312477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang M., Wang Y., Geng J., Zhou S., Xiao B. Mechanically activated Piezo channels mediate touch and suppress acute mechanical pain response in mice. Cell Rep. 2019;26:1419–1431.e4. doi: 10.1016/j.celrep.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 108.Ranade S.S., Woo S.H., Dubin A.E., Moshourab R.A., Wetzel C., Petrus M., Mathur J., Bégay V., Coste B., Mainquist J. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516:121–125. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chesler A.T., Szczot M., Bharucha-Goebel D., Čeko M., Donkervoort S., Laubacher C., Hayes L.H., Alter K., Zampieri C., Stanley C. The role of Piezo2 in human mechanosensation. N. Engl. J. Med. 2016;375:1355–1364. doi: 10.1056/NEJMoa1602812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woo S.H., Ranade S., Weyer A.D., Dubin A.E., Baba Y., Qiu Z., Petrus M., Miyamoto T., Reddy K., Lumpkin E.A. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang L., Yu J., Guo X., Wei J., Liu T., Zhang W. Parallel mechanosensory pathways direct oviposition decision-making in Drosophila. Curr. Biol. 2020;30:3075–3088.e4. doi: 10.1016/j.cub.2020.05.076. [DOI] [PubMed] [Google Scholar]
- 112.He L., Si G., Huang J., Samuel A.D.T., Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555:103–106. doi: 10.1038/nature25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gou B., Liu Y., Guntur A.R., Stern U., Yang C.H. Mechanosensitive neurons on the internal reproductive tract contribute to egg-laying-induced acetic acid attraction in Drosophila. Cell Rep. 2014;9:522–530. doi: 10.1016/j.celrep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang P., Jia Y., Liu T., Jan Y.-N., Zhang W. Visceral mechano-sensing neurons control Drosophila feeding by using Piezo as a sensor. Neuron. 2020;108:640–650.e4. doi: 10.1016/j.neuron.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Min S., Oh Y., Verma P., Whitehead S.C., Yapici N., Van Vactor D., Suh G.S., Liberles S. Control of feeding by Piezo-mediated gut mechanosensation in Drosophila. eLife. 2021;10:e63049. doi: 10.7554/eLife.63049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kefauver J.M., Ward A.B., Patapoutian A. Discoveries in structure and physiology of mechanically activated ion channels. Nature. 2020;587:567–576. doi: 10.1038/s41586-020-2933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Markow T.A., O’Grady P. Academic; 2006. Drosophila: A Guide to Species Identification and Use. [Google Scholar]
- 118.Gemeno C., Baldo G., Nieri R., Valls J., Alomar O., Mazzoni V. Substrate-borne vibrational signals in mating communication of Macrolophus bugs. J. Insect Behav. 2015;28:482–498. [Google Scholar]
- 119.Endo J., Takanashi T., Mukai H., Numata H. Egg-cracking vibration as a cue for stink bug siblings to synchronize hatching. Curr. Biol. 2019;29:143–148.e2. doi: 10.1016/j.cub.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 120.Prešern J., Polajnar J., de Groot M., Zorović M., Virant-Doberlet M. On the spot: utilization of directional cues in vibrational communication of a stink bug. Sci. Rep. 2018;8:5418. doi: 10.1038/s41598-018-23710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Achenbach J.D., Thau S.A. Elsevier Science; 1975. Wave Propagation in Elastic Solids. [Google Scholar]
- 122.Hedwig B., Knepper M. NEUROLAB, a comprehensive program for the analysis of neurophysiological and behavioural data. J. Neurosci. Methods. 1992;45:135–148. doi: 10.1016/0165-0270(92)90051-e. [DOI] [PubMed] [Google Scholar]
- 123.Tsai K.T., Chou Y.H. Random walk revisited: quantification and comparative analysis of Drosophila walking trajectories. iScience. 2019;19:1145–1159. doi: 10.1016/j.isci.2019.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meijering E., Dzyubachyk O., Smal I. Methods for cell and particle tracking. Methods Enzymol. 2012;504:183–200. doi: 10.1016/B978-0-12-391857-4.00009-4. [DOI] [PubMed] [Google Scholar]
- 125.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.RStudio Team . RStudio; 2019. RStudio: integrated development for R. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The male chases the female while fluttering his wing. There is one very short bout of tremulation associated with wing fluttering, the female stops briefly. Finally, a long bout of male tremulation (first, male tremulation is associated with wing fluttering, and then tremulation is performed on its own) coincides with female immobility; this is soon followed by copulation.3
The first video clip shows a control female 86D09-Gal4 paired with an OrR male. Male tremulation coincides with female immobility. The second video clip shows a female 86D09-Gal4>UAS-nanRNAi paired with an OrR male. Male tremulations are not associated with female immobility, and on the contrary the female walks away.4
(start-11 s) Audio clip including one bout of tremulations performed by an OrR D. melanogaster male to an OrR D. melanogaster female, as recorded on the apple substrate. The bout contains 36 pulses. (12 s-20 s) Audio clip including one bout of tremulations performed by an OrR D. melanogaster male to an OrR D. melanogaster female, as recorded on banana. The bout contains 27 pulses. (21 s-end) Audio clip including a series of bouts of tremulations performed by an OrR D. melanogaster male to an OrR D. melanogaster female, as recorded on the cactus fruit. The first bout contains 33 pulses, then a few very short bouts follow, and the second long bout contains 39 pulses.
Data Availability Statement
Excel macros, R code and datasets used to analyze the overlapping behaviors of pairs of flies during courtship are available in the public repository at the following links:
The Excel Macro used to build ethograms from annotated movies is available at: https://github.com/CarolineFabre/Excel-Macro-for-ethograms-.
The R algorithm used to analyze overlapping courtship behaviors script_behavior_R is available at: https://github.com/CarolineFabre/Script_behaviour.R.
Raw data can be found at: https://github.com/CarolineFabre/Raw-data-from-McKelvey-et-al-...Fabre.