Abstract
Radioembolization, also known as selective internal radiation therapy (SIRT), is an established treatment for the management of patients with unresectable liver tumors. Advances in liver dosimetry and new knowledge about tumor dose-response relationships have helped promote the well-tolerated use of higher prescribed doses, consequently transitioning radioembolization from palliative to curative therapy. Lung dosimetry, unfortunately, has not seen the same advances in dose calculation methodology and renewed consensus in dose limits as normal liver and tumor dosimetry. Therefore, the efficacy of curative radioembolization may be compromised in patients where the current lung dose calculations unnecessarily limit the administered activity. The field is thus at a stage where a systematic review and update of lung dose limits is necessary to advance the clinical practice of radioembolization. This work summarizes the historical context and literature for origins of the current lung dose limits following radioembolization, that is, the 25-year-old, single institution, small patient cohort series that helped establish the lung shunt fraction and dose limits. Newer clinical evidence based on larger patient cohorts that challenges the historical data on lung dose limits are then discussed. We conclude by revisiting the rationale for current lung dose limits and by proposing a staged approach to advance the field of lung dosimetry and thus the practice of radioembolization as a whole.
Keywords: lung, microspheres, pneumonitis, radiation dose, radioembolization
Introduction
Overview of radioembolization therapy
Radioembolization, also known as Selective Internal Radiation Therapy (SIRT), is an established treatment for the management of patients with unresectable liver tumors. The treatment involves intrahepatic arterial administration of microspheres (20–60 µm in size) loaded with beta-emitting radionuclides, such as yttrium-90 (90Y) or holmium-166 (166Ho). Because hepatic tumors are preferentially perfused by the hepatic artery, the radioactive microspheres preferentially accumulate in the tumor-feeding arterial vasculature, thereby resulting in a high local radiation dose to tumor(s).
Radioembolization consists of a planning procedure (using a surrogate gamma-emitting radionuclide) followed by the treatment procedure (using the radioactive microspheres) [1–3]. Technetium-99m macro-aggregated albumin (99mTc-MAA), the imaging surrogate for radioactive microspheres, is administered at the catheter position intended for treatment with the microspheres. The patient proceeds to the nuclear medicine clinic for 2D planar scintigraphy and 3D single-photon emission computed tomography/computed tomography (SPECT/CT) imaging of the MAA biodistribution (Fig. 1).
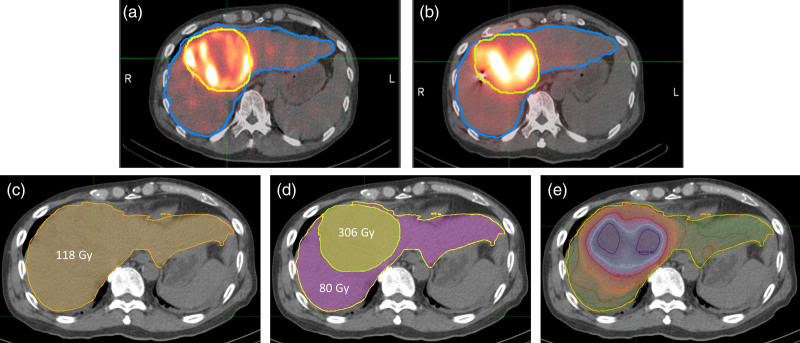
Fig. 1.
Example on the evolution and advances in liver dosimetry. The patient has a solitary large hepatocellular carcinoma (yellow contour) with an estimated volume of 449 mL and underwent whole liver treatment (2036 mL). The patient was treated with 5.51 GBq of glass 90Y-radioembolization. The top panel shows (a) 99mTc-macro-aggregate albumin (MAA) single-photon emission computed tomography/computed tomography (SPECT/CT) images used for planning and (b) 90Y-microspheres SPECT/CT images used for treatment delivery verification. The lower panel shows a transaxial slice of liver CT fused with absorbed dose maps of a patient using (c) single-compartment uniform uptake (instructions for use model) with mean dose to whole liver estimated to be 118 Gy, (d) partition model with mean dose to the tumor and normal liver compartments estimated to be 306 and 80 Gy, respectively, and (e) voxel dosimetry with the actual heterogeneous dose distribution in the tumor and normal liver compartments as evident by the isodose lines shown, with tumor regions receiving >400 Gy, and normal liver dose volumes receiving <50 Gy.
There are three main uses of 99mTc-MAA as part of the planning procedure: (1) the detection of any extrahepatic deposition, (2) the calculation of the prescribed microsphere activity following each device’s instructions for use (IFU) and (3) the assessment of the lung shunt fraction (LSF) and the mean lung dose. Safety considerations related to radiation pneumonitis place a maximum limit on the allowable mean lung dose. Thus, the LSF and lung dose estimated from 99mTc-MAA images can define and even sometimes restrict the allowable prescribed activity/dose for radioembolization.
During the treatment procedure, typically 10–14 days later, hepatic angiography is again performed, where the appropriateness of the catheter location(s) defined during the planning procedure for the intended treatment is re-verified prior to the delivery of the prescribed microspheres activity per the device IFU. The patient proceeds to the nuclear medicine clinic for either SPECT/CT (90Y or 166Ho) or positron emission tomography (PET)/CT (90Y only) imaging to visualize the distribution of microsphere delivery and to perform confirmatory dosimetry (Fig. 1).
Currently, there are three types of microspheres approved for clinical use: 90Y-resin microspheres (SIR-Spheres [4]), 90Y-glass microspheres (TheraSphere [5]) and 166Ho-loaded poly-lactate spheres (QuiremSpheres [6]). The first two devices have both United States Food and Drug Administration approval and European Conformity (CE) mark, whereas the third device currently only has CE-marking. Table 1 summarizes some basic properties of the approved clinical devices.
Table 1.
Summary of radioembolization device properties and instructions for use dosimetry
| Device | NameManufacturerLocation | SIR-Spheres [4]Sirtex MedicalWoburn, MA, USA | TheraSphere [5]Boston ScientificMarlborough, MA, USA | QuiremSpheres [6]Quirem MedicalDeventer, The Netherlands |
|---|---|---|---|---|
| Radionuclide | Radionuclide | Yttrium-90 | Yttrium-90 | Holmium-166 |
| Half-life | 64.1h | 64.1h | 26.8h | |
| Beta Emission Emax | 2.23MeV (100%) | 2.23MeV (100%) | 1.85MeV (50%) 1.77MeV (49%) |
|
| Gamma Emission | N/A | N/A | 81keV (6.7%) | |
| Microspheres | Material | Resin | Glass | Poly(L-lactic acid) |
| Density | 1.6g/cc | 3.3g/cc | 1.4g/cc | |
| Size Range | 20 – 60µm | 20 – 30µm | 15 – 60µm | |
| IFU Liver Dosimetry | Model Overview | Single-Compartment Uniform Dose | ||
| Activity Prescription | BSA and Tumor Burden | Treated Liver Mean Dose | Treated Liver Mean Dose | |
| Nominal Dose Limits | N/A | 80 – 150Gy | <60Gy | |
| IFU Lung Dosimetry | Model Overview | Single-Compartment Uniform Dose | ||
| LSF Limit | 20% | N/A | N/A | |
| Single LD Limit | 30Gy | 30Gy | 30Gy | |
| Cumulative LD (LDc) Limit | 50Gy | 50Gy | N/A | |
IFU, instructions for use; LDc, cumulative lung dose; LSF, lung shunt fraction; BSA, body surface area; N/A, Not Applicable.
Status of liver dosimetry and dose-response
For many years, radioembolization has primarily been a palliative treatment with focus on safety. This is reflected in the simple IFU dosimetry guidelines, which do not calculate the actual spatially variant dose distribution but are instead based on a single compartment uniform dose to treated liver volume (Fig. 1). The partition model improves on IFU dosimetry by estimating doses separately for the total tumor and the normal liver compartments. Voxel dosimetry progresses beyond the partition model by estimating doses at a voxel level and provides information on the heterogeneous and spatially varying dose distribution in the tumor and normal liver compartments. Voxel dosimetry also enables the calculation of the dose-volume histograms, akin to those used in external beam radiotherapy, that are useful for planning and verification dosimetry (Fig. 2).
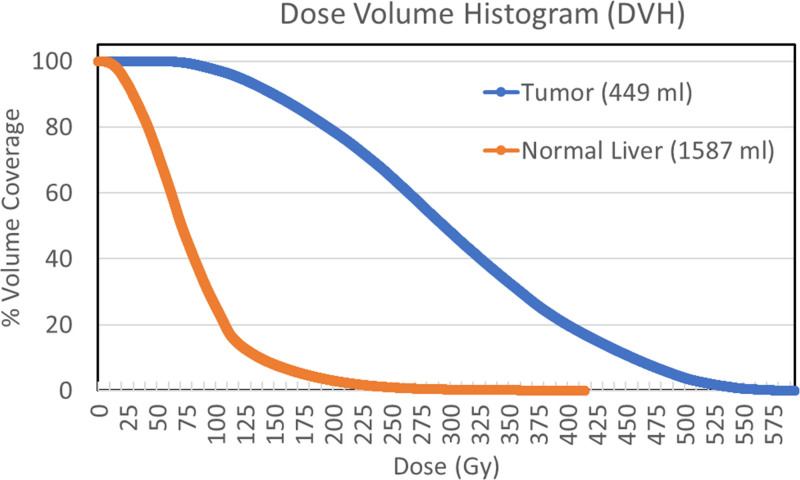
Fig. 2.
The dose-volume histograms (DVH), defined as the volume coverage in the ordinate versus the minimum dose coverage for that volume in the abscissa, for the tumor and normal liver compartments in the clinical example discussed in Fig. 1. The characteristic ‘shoulder on tumor DVH’ demonstrates good dose coverage with 80% and 20% of tumor receiving >200 Gy and >400 Gy, respectively. The ‘steep drop-off for normal liver DVH’ demonstrates good dose sparing with 20% and 75% of normal liver receiving <50Gy and <100Gy, respectively.
Several recent studies have suggested the curative benefit of 90Y-radioembolization beyond palliation using advanced liver dosimetry. These studies have demonstrated tumor dose-response, based on voxel dosimetry with post-therapy 90Y SPECT/CT or 90Y PET/CT imaging, for hepatocellular carcinoma (HCC) [7–11], metastatic colorectal carcinoma (mCRC) [12,13] and metastatic neuroendocrine tumors (mNET) [14,15].
Consequently, there is a growing clinical incentive to use advanced liver dosimetry to prospectively plan for achieving a tumor dose threshold that increases the probability of tumor control following 90Y-radioembolization. Under such a paradigm, clinicians may presently encounter cases where the desired activity for tumor control cannot be administered without exceeding the current IFU lung dose limits. Therefore, it is essential that LSF and lung dose be accurately estimated, not just to prevent radiation pneumonitis but, perhaps more importantly, to avoid unnecessarily limiting the administered activity in patients and delivering a sub-optimal tumor dose.
Status of lung dosimetry and lung dose limits
Lung dosimetry lags liver dosimetry in two primary aspects: (1) the lack of clear algorithms and standardized instructions for the calculations of LSF and lung dose and (2) the incomplete characterization of lung tissue response to lung dose following radioembolization.
The lack of specific details on procedures for the calculation of the LSF and lung dose is evident in the device IFUs. Besides, the ambiguity in view and contouring, the calculation of the LSF from 99mTc-MAA planar imaging has limitations that stem from the extrahepatic signal, differential attenuation, liver shine-through and organ delineation (Fig. 3). While the calculation of the LSF from 99mTc-MAA SPECT/CT imaging can mitigate concerns related to attenuation, scatter and organ delineation, it requires special care with issues related to image co-registration, differences in image resolution, liver shine-through and lung truncation (Fig. 3). Furthermore, studies reporting on patient-specific lung masses have shown that the traditional 1000 g lung mass assumption overestimates the lung mass, on average, by ~20% [16]. The calculation of lung mass from CT likewise requires careful consideration of the impact of respiratory phase on lung volume and lung density estimates as well as possible lung truncation in 99mTc-MAA SPECT/CT.
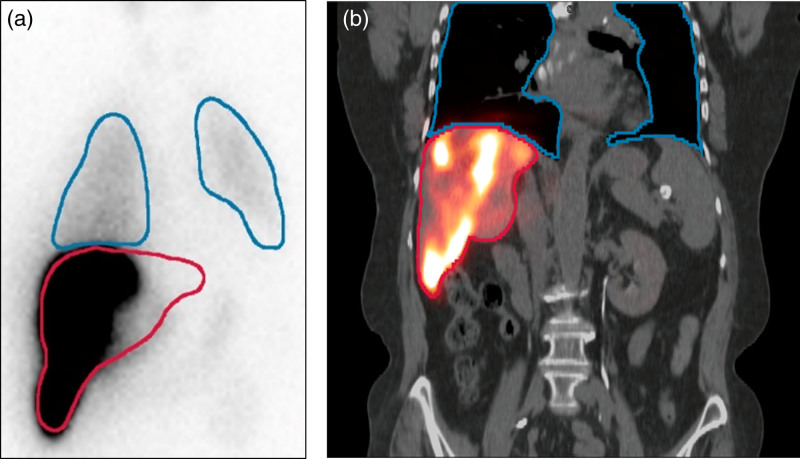
Fig. 3.
(a) An illustration of a typical technetium-99m macro-aggregated albumin (99mTc-MAA) planar scintigraphy of the thorax and abdomen used in the evaluation of the lung shunt fraction and the lung dose demonstrating the poor information for organ delineation on which contours of the lung (‘blue’) and liver (‘red’) are based. (b) The coronal slice through fused 99mTc-MAA single-photon emission computed tomography/computed tomography (SPECT/CT) with corresponding 3D lung (‘blue’) and liver (‘red’) volumes of interest demonstrating superior organ delineation but still subject to issues related to differences in image resolution, liver shine-through and lung truncation.
A recent publication presented a systematic review of the various methods of determining the LSF and the lung dose [17]. The review discussed both 2D planar and 3D SPECT/CT-based calculations and also reviewed pretherapy versus post-therapy assessments of the LSF and lung dose. The advantages and limitations of each of these methods were deliberated with a focus on accuracy and practical considerations. Finally, a lexicon was proposed to minimize the current ambiguity in referencing LSF and lung dose methodologies in literature and in practice with five necessary descriptors: category, agent, modality, contour and algorithm.
Scope of this review
The objective of this work is to systematically review the historical context and literature for origins of the current lung dose limits following radioembolization. We also review more recent literature reporting on large patient cohorts with higher LSF and lung dose and present some case reports of radiation pneumonitis. We end the review with a critical appraisal of the rationale for current lung dose limits and provide a practical proposal for advancing the field of lung dosimetry.
Clinical evidence for lung dose limits
Radiation pneumonitis
Hepatopulmonary (also called arteriovenous) shunts within the tumor vasculature lead to the transfer of microspheres from the arterial to the venous circulation that are eventually trapped in the lung capillaries leading to the deposition of radiation dose in lung tissue. Radiation pneumonitis is an associated complication when high levels of radiation dose are delivered to the lung parenchyma. As shown in Table 1, the IFUs limit the mean lung dose to less than 30 Gy for individual treatment to mitigate the risk of radiation pneumonitis.
Radiation pneumonitis is typically characterized by deteriorating pulmonary function, both clinically (dry cough and progressive exertional dyspnea) and by functional tests (showing restrictive pattern), occurring 1–6 months after radioembolization [18,19]. Prior to its diagnosis, other causes of deteriorating pulmonary function must be confidently excluded, such as cytotoxic chemotherapy, heart failure, chest infection, pulmonary embolism, pleural effusion, pulmonary metastases, chronic obstructive airway disease and pulmonary fibrosis.
Radiologically, radiation pneumonitis manifests 1–2 months after therapy as ill-defined patchy opacities and ground-glass opacities in a symmetric pattern, with relative hilar or perihilar sparing [20]. The chest radiograph typically shows extensive patchy consolidations with well-defined lateral margins. CT scans of the lungs show similar consolidation, the lateral edge of which runs parallel to the lung edge and fissures. Corticosteroids are often used in the treatment of radiation pneumonitis and may reduce the degree of inflammation.
Historical data that established lung dose limits
Table 2 summarizes the available literature reporting both estimated lung dose (single and cumulative treatments, if applicable) and incidence of radiation pneumonitis. There are two seminal publications, both incidentally from the same group reporting on the same patient cohort that have strongly influenced the guidance on lung dose limits (Table 1) following radioembolization. Leung et al. [18] focused on the clinical manifestation of radiation pneumonitis, whereas Ho et al. [23] focused on the LSF and lung dose of the patients. In the adoption of the lexicon proposed for radioembolization lung dosimetry [17], the LSF and lung dose values reported in these two studies can be described as: 99mTc-MAA, planar anterior-view only, separate lungs and liver regions of interest (ROIs) and 1000 g lung mass.
Table 2.
Summary of studies reporting on lung dose and associated radiation pneumonitis (RP). Cases of RP are identified with “*”. LSF values reported were calculated based on planar MAA images with lung and liver ROIs for counts and LD calculations assumed a 1000g lung mass for all patients – Ho et al. (1997) used anterior view counts, while Salem et al. (2008) and Das et al. (2020) used geometric mean counts.
| Reference | SIRT Sessions | Total Patients | RP Incidence | Dose Stratification | Stratified LSFs | Stratified Lung Doses |
|---|---|---|---|---|---|---|
| Ho et al. 1997 [23] | Single | N = 95 | 1/95 | 82/95 | <15% | LD < 20 Gy |
| 10/95 | <15% | 20 Gy < LD < 30 Gy | ||||
| 3/95 | <15% | LD = 32*, 37, 40 Gy | ||||
| Multiple | N = 21 | 1/21 | 16/21 | <15% | LDc < 20 Gy | |
| 5/21 | <15% | LDc = 25, 29, 39, 54, 59* Gy | ||||
| Salem et al. 2008 [22] | Single | N = 403 | 0/403 | 385/403 | NR | LD < 30 Gy |
| 18/403 | 24% ± 8% † | LD > 30 Gy | ||||
| Multiple | NR | 0 | 39 | 18% ± 10% † | 30 Gy < LDc < 50 Gy | |
| 19 | 21% ± 7% † | LDc > 50 Gy | ||||
| Das et al. 2020 [26] | Single | N = 103 | 0/103 | 103/103 | >15% | LD = 23 Gy (15-29 Gy) ‡ |
| Multiple | NR | 0 | ALL | >15% | LDc = 30 Gy (21-44 Gy) ‡ |
SIRT, selective internal radiation therapy; LD, lung dose; LDc, cumulative lung dose; LSF, lung shunt fraction; NR, Not Reported; †, mean ± standard deviation; ‡, median (interquartile range).
In 95 patients with single radioembolization treatment and LSF <15%, Ho et al. [23] found evidence of radiation pneumonitis in one patient (asterisk) out of three that had lung dose >30 Gy (i.e. 32*, 37 and 40 Gy). Otherwise stated, only 1/95 for all lung dose and 1/3 patients with lung dose >30 Gy experienced radiation pneumonitis after a single radioembolization treatment. This clinical evidence led to the recommendation for lung dose limit of 30 Gy for single radioembolization treatments [5].
In 21 patients with multiple radioembolization treatments, Ho et al. [23] found evidence of radiation pneumonitis in one patient (asterisk) out of two that had LDc >50 Gy (i.e. 54, 59* Gy), where LDc is the cumulative lung dose (arithmetic sum of individual lung dose). Otherwise stated, 1/21 for all dose levels and 1/2 patients with LDc >50 Gy experienced radiation pneumonitis after multiple radioembolization treatments. We posit that this clinical evidence led to the recommendation for lung dose limit of 50 Gy for multiple radioembolization treatments.
Ho et al. [23] also reported on five additional patients with an initial LSF >20% leading to lung dose >30 Gy that underwent prophylactic partial embolization with nonradioactive particles to decrease lung dose <30 Gy. Following their single radioembolization treatment, three of the five patients were diagnosed with radiation pneumonitis (lung doses of 11, 25 and 25 Gy). The authors acknowledge the possibility of failure of a prophylactic partial embolization procedure and the high likelihood of patients receiving higher doses than those predicted by the revised lung dose estimates. We theorize that this clinical data, along with the finding that patients with LSF <13% had no lung complications, led to the recommendation of LSF <20% for resin 90Y-radioembolization treatments.
In retrospect, the clinical data that strongly influenced the lung dosimetry limits in terms of single and cumulative treatment doses are not based on large number of patients. In actuality, the data showed that no patients (out of 92) with lung dose <30 Gy and one out of only three patients with lung dose >30 Gy experienced radiation pneumonitis after a single radioembolization treatment. Similarly, one out of only two patients with LDc >50 Gy experienced radiation pneumonitis after multiple radioembolization treatments. The authors acknowledged the limitations of their lung dose work but supported their proposed single-treatment lung dose limit of 30 Gy as being consistent with the ~25 Gy whole-lung dose limit recommended to minimize the incidence of radiation pneumonitis when using external-beam radiation therapy (EBRT) for partial and whole lung irradiation [21,24,25].
Evidence of radiation pneumonitis in current practice
Since the initial work by Ho et al. 23, only two publications, one by Salem et al. [22] and one by Das et al. [26], have focused directly on the incidence of radiation pneumonitis following radioembolization. However, additional published evidence of radiation pneumonitis (or lack thereof) can also be found among the observed adverse events in several prospective and retrospective radioembolization studies and among individual case reports. Of note, in a combined analysis of three multicenter, randomized, phase 3 trials that included over 550 treatments, no incidence of radiation pneumonitis was reported as part of adverse events (lung doses not reported) [27].
The most relevant recent report on the evaluation of radiation pneumonitis comes from the seminal work of Salem et al. [22] that included over 400 patients undergoing glass 90Y-radioembolization during a 4-year-period. Of note, 18 patients received single treatment lung dose >30 Gy (37.1 Gy average lung dose) and 19 patients received a LDc >50 Gy (75.7 Gy average LDc). Overall, they documented no cases of clinical or imaging radiation pneumonitis after treatment. In terms of minor complications, they reported imaging findings in 10 patients that indicated pleural effusions, atelectasis and ground-glass attenuation.
Das et al. [26] have also recently reported a study of 103 patients who had LSF >15% with a median LSF value of 24.4% and with median lung dose and LDc of 22.9 Gy and 29.5 Gy, respectively. Twenty patients (19%) reported nonspecific pulmonary symptoms (cough, shortness of breath and wheezing) in the 1-year post-90Y. However, thoracic imaging demonstrated no pulmonary fibrosis/injury following treatment in any patient. They concluded that, in isolation, LSF >15% should not deter from treatment.
Besides the previously discussed works of Salem et al. [22] and Das et al. [26] that involve large patient cohorts, there are only a handful of case reports (literature review yielded five well-documented case reports) published on the topic of lung complications that occurred after radioembolization treatment. We summarize these five case reports next. Cases 1 and 2 involve lung complications (not radiation pneumonitis) and low lung doses (<10 Gy), suggesting additional comorbidities in these two patients. Case 3 involves a patient receiving high-dose radioembolization in the hepatic dome that irradiated the adjacent lower lung lobes and stimulated the development of organizing pneumonia. Cases 4 and 5 involve the positive case of radiation pneumonitis where treatment with corticosteroids was found to be beneficial.
Case report 1
A case of possible radiation pneumonitis was published by Kesim et al. [19] for an HCC patient who was administered 1.18 GBq resin-microspheres with an LSF (lexicon: pretreatment 99mTc-MAA, planar two-views, separate lung and liver ROIs, no details on the algorithm) of 5% resulting in lung dose (lexicon: no details on lung mass) of 3 Gy. No additional details on MAA mapping or radioembolization treatment were provided. The patient was reported to have a comorbidity of congestive heart failure, which may be complicit in respiratory complications reported because the lung dose was estimated to be very low. They presented detailed manifestations of radiation pneumonitis in a modern clinical setting along with its management, which made the study relevant.
Case report 2
A case of possible radiation pneumonitis was published by Dobrocky et al. [28] for an LSF (lexicon: pretreatment 99mTc-MAA, planar, no details on views and ROIs) of 11.5% with an activity administered of 1.60 GBq resin-microspheres resulting in lung dose (lexicon: 1000 g lung mass) of 9 Gy. Three months post-treatment, multiple patchy solid lung opacities with surrounding ground-glass halo were visualized on CT. The patient was clinically asymptomatic and revealed no symptoms of respiratory discomfort. Histological work-up revealed minimally consolidated lung parenchyma and 90Y-microspheres localized mainly within the interstitium. Six months later the CT showed complete regression of pulmonary consolidations with no additional medical treatment.
Case report 3
A case of confirmed organizing pneumonia was reported by Devcic et al. [29] The patient presented with a 7.2 cm tumor and was treated with segmental radioembolization, where a high tumor dose of ~380 Gy with glass-microspheres was delivered. The LSF (lexicon: pretreatment 99mTc-MAA, planar) was 1% and the lung dose [lexicon: post-treatment 90Y SPECT/CT, volumes of interest (VOIs) on lung and liver, CT-based lung mass] from 90Y SPECT/CT was estimated at 0.5 Gy. At 3-month follow-up, while the tumor demonstrated a modified Response Evaluation Criteria in Solid Tumors complete response, the chest CT demonstrated ground glass and consolidation in the right lower lobe abutting the diaphragm near the treated liver lesions, suggesting that radioembolization in the hepatic dome irradiated the adjacent lung and stimulated the development of organizing pneumonia in the lower lung lobes. The [18] FDG PET/CT at 6 months demonstrated radiographic findings highly suggestive of organizing pneumonia. The patient was asymptomatic and denied shortness of breath, dyspnea on exertion, fever or aspiration. The patient chose to pursue conservative management without steroids. A chest CT 8 months after radioembolization demonstrated near-complete resolution of organizing pneumonia.
Case report 4
Wright et al. [30] described a positive case of radiation pneumonitis following 90Y-glass radioembolization in a patient with liver metastases whose LSF (lexicon: pre-treatment 99mTc-MAA, planar, ROIs on lung and liver, no details on views) was estimated at 9.3%. Two treatments with glass microspheres in short succession were estimated to deliver lung dose (1000 g lung mass) of 8 and 23 Gy (LDc = 31 Gy). Three weeks after the second radioembolization treatment, the patient developed progressive dyspnea and chest tightness without fever, cough or hemoptysis. Chest radiography showed dense opacification of the right lung with peripheral sparing. A CT pulmonary angiogram demonstrated no pulmonary embolus, but confluent bilateral ground-glass opacities. Treatment with corticosteroids was initiated. This patient developed clinical, functional and radiographic findings consistent with radiation pneumonitis, with near-complete pulmonary parenchymal recovery and no clinical evidence of relapse or progressive decline in pulmonary function over a 9-month-period.
Case report 5
A case from one of the author’s (M.G.E.H.L.) clinical experience. A 69-year-old female HCC patient with multifocal HCC with portal vein thrombosis (PVT) in the left lobe (Barcelona Clinic Liver Cancer stage C) was treated with 1.35 GBq glass microspheres in segments 2–4 (3.5 GBq vial; 4 days post-calibration). The LSF (lexicon: pretreatment 99mTc-MAA, planar, geometric mean view, ROIs on liver and lung) and lung dose (lexicon: 1000 g lung mass) were calculated to be 50% and 46 Gy, respectively. Patient-specific calculations of the delivered lung mean dose was estimated from post-therapy 90Y-PET/CT scan of the patient taken the day after the treatment (Fig. 4) and determined to be 17 Gy with total lung VOI captured on PET/CT minus CT-based liver VOI with 2 cm expansion and patient-specific lung mass. The treated liver volume was 483 mL and the prescribed treatment dose per IFU was 120 Gy. With an Eastern Cooperative Oncology Group performance status score of 1, Child-Pugh score A5 and normal baseline lab values for serum bilirubin and serum albumin, there was nothing extraordinary about the case. The patient was admitted to ICU 1.5 months after radioembolization treatment with respiratory insufficiency and was then subsequently treated with steroids. The chest radiography of the thorax at 1.5 months after radioembolization, suggesting fibrosis in all segments of the lungs, is shown in Fig. 4. Even after the partial recovery over the course of 3 months, she remained oxygen-dependent. The images of the thorax in CT at 4 months after radioembolization (Fig. 4) showed ground-glass opacities in all segments of the lungs, most pronounced in the upper lobes with extensive bronchiectasis and fibrosis. CT also revealed progressive disease in the liver, both tumors and PVT. She died of progressive disease at 8 months after treatment.
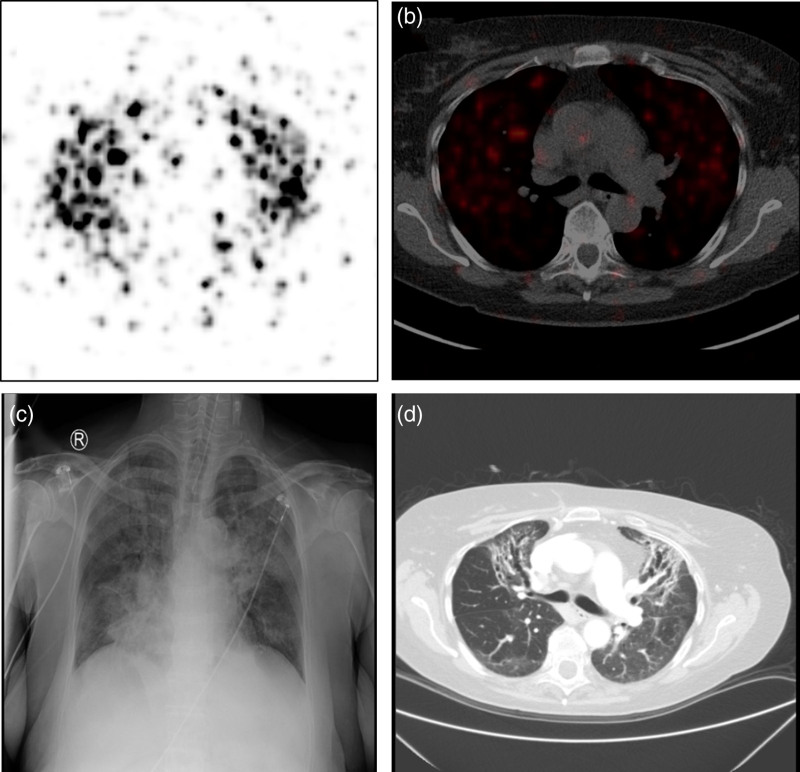
Fig. 4.
A 69-year-old female hepatocellular carcinoma patient was treated with 1.35 GBq 90Y-glass-microspheres in segments 2–4. (a) and (b) shows the post-therapy 90Y-positron emission tomography/computed tomography (PET/CT) scan of the patient, based on which the delivered lung mean dose was estimated to be 17 Gy. (c) The chest radiograph of the thorax at 1.5 months after radioembolization suggesting fibrosis in all segments of the lungs. (d) The CT of the thorax at 4 months after radioembolization showing ground-glass opacities in all segments of the lungs, with extensive bronchiectasis and fibrosis.
Summary of radiation pneumonitis in current practice
In summary, there were no reported cases of radiation pneumonitis in studies of large patient cohort where lung dose and LDc exceeds the IFU limits. In fact, none of the 18 patients with lung dose >30 Gy or 19 patients with LDc >50 Gy documented radiation pneumonitis [22]. SIRT has also been shown to be well tolerated for patients with LSF >15% [26]. There were two published case reports of confirmed radiation pneumonitis in recent literature, one where two treatments were performed within a span of 3 weeks, and the other where the pretreatment LSF and lung dose were high (50% and 46 Gy, respectively). In both these cases, treatment with corticosteroids was shown to be beneficial and radiation pneumonitis was not lethal to the patients. Furthermore, any incidence of lung or respiratory complications reported was likely confounded by the presence of additional comorbidities in patients.
Discussions
Limitations on the determination and interpretation of LSF, lung dose and lung dose limits
Broadly speaking, the limitations on our overall approach and knowledge involving LSF, lung dose and lung dose limits can be grouped as arising from three major themes of concerns: (1) the lack of standardization in reporting of LSF and lung dose in clinical literature, (2) the lack of clinical data on a robust risk model for radiation pneumonitis after radioembolization and (3) the assumption that the distribution of planning MAA mimics that of treatment microspheres.
The issue of lack of standardization in reporting of LSF and lung dose in clinical literature was addressed in a recent review of the procedures for calculation of LSF and lung dose and advocated for the use of a lexicon to describe LSF and lung dose in terms of category, agent, modality, contour and algorithm [17]. They reviewed calculations of LSF and the lung dose with both 2D planar and 3D SPECT/CT based, and evaluated pretherapy calculations compared to post-therapy assessments.
The limitations of MAA as a surrogate for microspheres are well known and efforts are underway to mitigate them. There are ongoing evaluations to bypass MAA altogether and instead use the treatment devices themselves, 90Y- or 166Ho-microsheres, albeit in limited quantity (~10%), for the evaluation of lung shunt. Incidentally, the QuiremSpheres IFU allows for the use of 166Ho-PLLA (or 99mTc-MAA) for assessment of the LSF and lung dose. Nonetheless, the use of MAA will be part of radioembolization practice for some time to come. Therefore, there is a need to incorporate procedures and adopt processes in clinical practice that can potentially improve the reliability of MAA for both lung dose considerations and its inter-hepatic distribution to support advanced liver/tumor dosimetry modeling. Some of these approaches may include matching catheter position between planning and treatment delivery, minimizing the time delay between MAA injection and imaging, and using 3D imaging such as SPECT/CT or PET/CT.
The issue of lack of clinical data on risk model for radiation pneumonitis will be discussed next where we interrogate the current lung dose limits and offer some suggestions. We end with a practical proposal for advancing the field of lung dosimetry based on a staged approach incorporating planar-based LSF and lung dose with 1000 g lung mass and SPECT/CT-based LSF and lung dose with patient-specific lung mass calculations.
Revisiting the rationale for current lung dose limits
No doubt, radiation pneumonitis may not be treatable and can be lethal, if severe; yet, the evidence, or rather the lack of evidence, of radiation pneumonitis in literature warrants reflection. There are no published studies to date that associate the incidence of radiation pneumonitis using current lung dosimetry models or dose limits. The lung dose limits in IFUs were based on clinical data from 25 years ago on two reported cases of radiation pneumonitis: one out of only three patients with lung dose >30 Gy, and one out of only two patients with LDc >50 Gy (lexicon: pretreatment 99mTc-MAA, liver and lung ROIs, anterior view counts, 1 kg lung mass) [23]. In a more recent study, none of the 18 patients with lung dose >30 Gy and none of the 19 patients with LDc >50 Gy had radiation pneumonitis [22]. This recent study, with higher numbers of patients and therefore greater statistical power, cast doubts on the appropriateness of the lung dose limits proposed in the IFU.
Radioembolization has historically accepted the use of inaccurate and imprecise lung dosimetry methods and the use of outdated LSF and lung dose thresholds because these practices err on the side of patient safety, a priority in palliative care. However, as radioembolization is increasingly used for curative intent, these practices cannot continue to be accepted as they could compromise the new priority: tumor control. The lack of evidence of radiation pneumonitis is a concern regarding dosimetry planning guidelines for radioembolization where administered doses may be unnecessarily capped due to excessive concerns over radiation pneumonitis. This implies that efficacy for radioembolization may have been compromised due to the overly conservative lung dose limits. This is especially problematic for the patient population where local tumor control is the treatment intent. There are reports suggesting that the maximum allowed radioembolization activity may be affected by lung dose considerations in about 10–15% of cases [16,31].
A recent survey among the Cardiovascular and Interventional Radiological Society of Europe members performing radioembolization highlighted the critical role lung shunt and lung dose assessments play on patient exclusion or treatment modifications [32]. They reported that lung shunting was the main reason that excluded patients from treatment. Of those centers, the majority considered a lung shunt percentage higher than 20% a contraindication. Exclusion of patients (between 1 and 25%) because of lung shunting was reported in 48% of the centers and activity reduction was reported in 46% of the centers. The use of cutoff values based on the percentage point of LSF is mathematically flawed because of the bias against low treatment volume or activity. Furthermore, a contraindication for radioembolization based solely on a population-wide LSF threshold ignores all other patient-specific factors impacting lung doses, such as lung mass, treatment volumes and administered activities.
The balance between patient safety and treatment efficacy is better established in EBRT, where treatment plans are optimized to achieve a certain tumor control probability while maintaining an acceptable and finite probability of normal tissue complication (NTCP) (i.e. a nonzero risk to the patient). For example, radiation-induced liver disease is dose-limiting toxicity for liver EBRT and occurs with a frequency of about 5–10% when the whole liver is irradiated with up to 30–35 Gy [24]. Therefore, under the radiation oncology paradigm, one could state that the lung NTCP for radiation pneumonitis after radioembolization treatment is currently not known. However, a direct translation of absorbed dose limits for lung between radioembolization and EBRT modalities cannot be wholly justified as evidenced by the observed differences between the limits for whole liver irradiation. The higher whole liver dose limit for radioembolization (e.g. around 60–150 Gy) compared to EBRT (around 40 Gy) may likely stem from differences in the spatial distribution of dose, dose rate and fractionation schema.
Further investigations, and perhaps even dose-escalation studies, may be required to more precisely characterize the radiation response or NTCP of lung parenchyma after radioembolization. 99mTc-MAA SPECT/CT has been shown to provide a more accurate assessment of LSF and of lung dose when used with a patient-specific lung mass. Therefore, there is a need to establish appropriate lung dose limits based on 99mTc-MAA SPECT/CT imaging.
It is well known that some tumor types (e.g. HCC) may, on average, be more vascular than other tumor types (e.g. mCRC), and therefore patients with the former may be prone to present with higher LSF. Advanced disease, high tumor burden and bulky disease may also be prone to higher degrees of the arteriovenous shunt. Therefore, we also need to incorporate disease-specific considerations into the discussion of revising lung dose limits. The use of 90Y-radioembolization in early-stage HCC patients as a bridge to transplantation has been well established. In recent work, Salem et al. [33] investigated the distribution of LSF and lung dose (lexicon: planning 99mTc-MAA, planar geometric-mean, ROIs on the liver and lung VOI, and 1000 g lung mass) in a cohort of 488 patients who underwent radioembolization for downstaging or bridge to transplantation. They reported a median LSF of 3.9% (inter-quartile range 2.4–6%) and a median lung dose of 1.9 Gy (inter-quantile range 1.0–3.3 Gy). They conclude that all 448 patients fell well within the lung safety limit of 30 Gy. Furthermore, if they excluded patients with transjugular intrahepatic portosystemic shunt, the patient population (n = 410) could be safely treated without knowledge of planning LSF and lung dose altogether. Salem et al. [33] has demonstrated a pathway to incorporate considerations on disease and its stage into the discussion of lung dose. Elimination of lung shunt study to such subgroups will lead to shorter time to treatments, reduced costs and improved patient convenience.
It is important to recognize that all oncological patient treatment embraces inherent risks for adverse events, most of which cannot be controlled. Common side-effects for cancer patients include neutropenia, lymphedema, pain, weight loss, to more serious presentations, such as peripheral neuropathy, deep-vein thrombosis, seizures, and so on. Therefore, we need to contextualize the low risk for radiation pneumonitis following radioembolization against the totality of risk for all adverse events in the care of oncology patients. With an estimated number of well over 100 000 cases of radioembolization performed over the past 2 decades, there have only been less than 10 or so cases of radiation pneumonitis reported in the literature. The exceedingly low incidence rate is testimony to the excellent safety record for radioembolization procedures.
Proposals for advancing the field of lung dosimetry
The radioembolization community needs to use more accurate estimates of mean lung doses when treating patients with the intent of tumor control. The community would benefit from curating clinical data, in terms of both planar and SPECT calculations, during planning and post-treatment, together with patient clinical outcomes, that can help establish the true lung dose tolerance following radioembolization. We need to adopt standardized reporting of LSF and lung dose based on a common lexicon – one, for example, based on, category, agent, modality, contour and algorithm [17]. We appeal for the creation of a registry to collect, coalesce and trend data on lung dose and patient follow-up that can then be used to generate evidence-based lung dose limits for radiation pneumonitis following radioembolization.
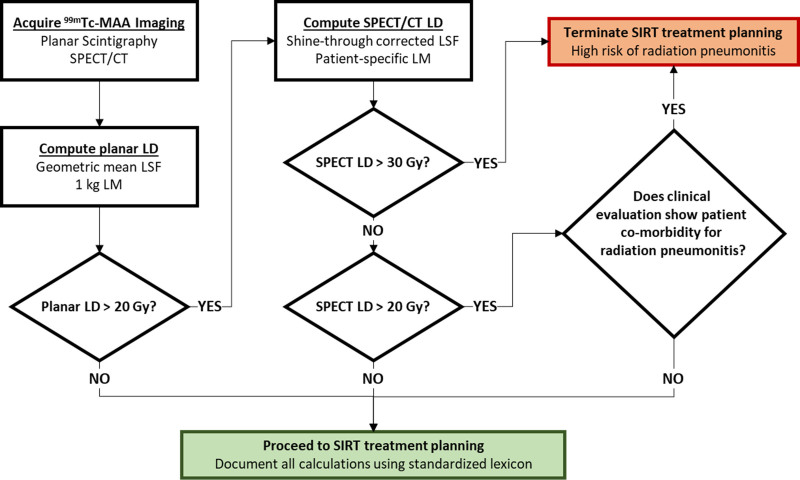
Our recommendation, based on the authors clinical practice and shown in Fig. 5, is that every site acquires both planar (thorax and abdomen) and SPECT/CT (liver and inferior thorax) images after MAA administration. Then lung dose would be calculated using planar images using GM, anterior and posterior view counts along with 1000 g lung mass. The best planar-based estimates of LSF and lung dose, as shown by Lopez et al., [34], has the highest liver counts, and is often (over 80% of the time) the anterior view. If the lung dose based on the above planar algorithm is <20 Gy, then there are no constraints on activity either for this or a potential second radioembolization. If, however, the planar algorithm reports dose >20 Gy then use 99mTc-MAA SPECT/CT and other CT imaging to estimate patient-specific values of LSF, lung mass and lung dose. There are numerous caveats and the systematic approaches for 99mTc-MAA SPECT/CT based LSF and lung dose determination have been reviewed.
Fig. 5.
Proposed algorithm for technetium-99m macro-aggregated albumin (99mTc-MAA)-based lung dose (LD) considerations on treatment planning based on a staged approach incorporating planar based lung shunt fraction (LSF) and LD, starting with simple 1 kg lung mass and then progressing to more complex single-photon emission computed tomography/computed tomography (SPECT/CT) based LSF and LD with patient-specific lung mass (LM) calculations. SIRT, selective internal radiation therapy.
With respect to cumulative doses after multiple treatments, our proposal is to use SPECT/CT based estimations in all those cases and maintain SPECT/CT based cumulative dose <50 Gy. This will be an excellent candidate pool for the registry to track.
It is the authors’ opinion that patients may be safely treated to mean lung dose of 30 Gy when using 99mTc-MAA SPECT/CT with patient-specific lung mass estimates of lung dose, especially if there are no comorbidities for radiation pneumonitis. Patient follow-up for at least 6 months is recommended to assess radiographic or clinical signs of radiation pneumonitis if planned lung doses were greater than 20 Gy (based on planning 99mTc-MAA SPECT/CT). Here, again, the registry can maintain real-time performance data on the national radioembolization cohort so that all practitioners are able to share and review registry outcome results.
Summary
There have been tremendous advances in liver and tumor dosimetry models that enable clinicians to prospectively plan treatments with higher administered activities to increase the probability of local tumor control following radioembolization. Unfortunately, lung dosimetry and dose limits as described by the IFUs are very rudimentary. The clinical data that justified the lung dose and LDc limits of 30 and 50 Gy, respectively, are based on data from 25 years ago. Newer clinical evidence based on larger patient cohorts challenges the historical data and makes a case for higher dose limits. The time has come to update the lung dose algorithm and limits for radioembolization.
The radioembolization community is encouraged to acquire both planar and SPECT/CT imaging and employ a patient-specific algorithm for determining modality and algorithm of choice. We urge the use of a standardized lexicon when describing LSF and lung dose based on category, agent, modality, contour and algorithm, to overcome the ambiguity in the calculation and reporting of LSF and lung dose. We appeal for the creation of a registry to collect, coalesce and trend data on lung dose and patient follow-up that can then be used to generate evidence-based lung dose limits for radiation pneumonitis following radioembolization.
Acknowledgements
S.C.K. has received research support from Boston Scientific and GE Healthcare, and acts as a consultant for Boston Scientific, Sirtex Medical, ABK Biomedical, Varian Medical and Terumo Medical. M.G.E.H.L. acts as a consultant for Boston Scientific, Terumo and Quirem. The Department of Radiology and Nuclear Medicine of the University Medical Center Utrecht receives royalty payments from Quirem and research support from Boston Scientific, Terumo and Quirem. R.S. is an advisor to Boston Scientific and Sirtex Medical.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bradford R. Treatment planning part I: vascular considerations associated with safety and efficacy in radioembolization. Pasciak A, Bradley Y, McKinney J, eds. In: Handbook of radioembolization: Physics, Biology, Nuclear Medicine, and Imaging. Boca Raton, FL: CRC Press; 2017. [Google Scholar]
- 2.Murthy R, Nunez R, Szklaruk J, Erwin W, Madoff DC, Gupta S, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005; 25 (Suppl 1):S41–S55. [DOI] [PubMed] [Google Scholar]
- 3.Srinivas SM, Shrikanthan S, Yu N. Treatment planning part II: procedure simulation and prognostication. Pasciak AS, Bradley Y, McKinney JM, eds. In: Handbook of Radioembolization: Physics, Biology, Nuclear Medicine, and Imaging. Boca Raton, FL: CRC Press; 2017. [Google Scholar]
- 4.Sirtex Medical. SIR-Spheres Instructions For User (IFU). Published online 2017. https://www.sirtex.com/media/155126/ssl-us-13.pdf. [Accessed 29 April 2021] [Google Scholar]
- 5.Boston Scientific. TheraSphere Instructions For User (IFU). Published online 2021. https://www.bostonscientific.com/en-US/products/cancer-therapies/therasphere-y90-glass-microspheres.html. [Accessed 29 April 2021]
- 6.Quirem Medical. QuiremSpheres Instructions for User (IFU). Published online 2020. https://www.quirem.com/download/LS1101-10_06-IFU-QuiremSpheres-Multi-Language.pdf. [Accessed 29 April 2021]
- 7.Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, et al. Prospective trial using internal pair-production positron emission tomography to establish the yttrium-90 radioembolization dose required for response of hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018; 101:358–365. [DOI] [PubMed] [Google Scholar]
- 8.Kappadath SC, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumor dose response after 90Y-radioembolization with glass microspheres using 90Y-SPECT/CT-based voxel dosimetry. Int J Radiat Oncol Biol Phys. 2018; 102:451–461. [DOI] [PubMed] [Google Scholar]
- 9.Garin E, Lenoir L, Edeline J, Laffont S, Mesbah H, Porée P, et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: a new personalized promising concept. Eur J Nucl Med Mol Imaging. 2013; 40:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strigari L, Sciuto R, Rea S, Carpanese L, Pizzi G, Soriani A, et al. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: radiobiologic considerations. J Nucl Med. 2010; 51:1377–1385. [DOI] [PubMed] [Google Scholar]
- 11.Hermann AL, Dieudonné A, Ronot M, Sanchez M, Pereira H, Chatellier G, et al. ; SARAH Trial Group. Relationship of tumor radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90Y in the SARAH study. Radiology. 2020; 296:673–684. [DOI] [PubMed] [Google Scholar]
- 12.Flamen P, Vanderlinden B, Delatte P, Ghanem G, Ameye L, Van Den Eynde M, Hendlisz A. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with Yttrium-90 labeled resin microspheres. Phys Med Biol. 2008; 53:6591–6603. [DOI] [PubMed] [Google Scholar]
- 13.van Roekel C, Bastiaannet R, Smits MLJ, Bruijnen RC, Braat AJA, de Jong HWAM, et al. Dose-effect relationships of holmium-166 radioembolization in colorectal cancer. J Nucl Med. 2021; 62:272–279. [DOI] [PubMed] [Google Scholar]
- 14.Chansanti O, Jahangiri Y, Matsui Y, Adachi A, Geeratikun Y, Kaufman JA, et al. Tumor dose response in yttrium-90 resin microsphere embolization for neuroendocrine liver metastases: a tumor-specific analysis with dose estimation using SPECT-CT. J Vasc Interv Radiol. 2017; 28:1528–1535. [DOI] [PubMed] [Google Scholar]
- 15.Braat AJAT, Kappadath SC, Ahmadzadehfar H, Stothers CL, Frilling A, Deroose CM, et al. Radioembolization with 90 Y resin microspheres of neuroendocrine liver metastases: International Multicenter Study on Efficacy and Toxicity. Cardiovasc Intervent Radiol. 2019; 42:413–425. [DOI] [PubMed] [Google Scholar]
- 16.Lopez B, Mahvash A, Lam MGEH, Kappadath SC. Calculation of lung mean dose and quantification of error for 90 Y-microsphere radioembolization using 99m Tc-MAA SPECT/CT and diagnostic chest CT. Med Phys. 2019; 46:3929–3940. [DOI] [PubMed] [Google Scholar]
- 17.Kappadath SC, Lopez BP, Salem R, Lam MGEH. Review of lung shunt and lung dose calculation methods for radioembolization treatment planning. Q J Nucl Med Mol Imaging. 2021; 65:32–42. [DOI] [PubMed] [Google Scholar]
- 18.Leung TW, Lau WY, Ho SK, Ward SC, Chow JH, Chan MS, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995; 33:919–924. [DOI] [PubMed] [Google Scholar]
- 19.Kesim S, Ones T, Eryuksel E, Baltacioglu F, Tureli D, Ozguven S, Erdil TY. Unexpected radiation pneumonitis after SIRT with significant decrease in DLCO with internal radiation exposure: a case report. BMC Med Imaging. 2020; 20:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spina JC, Hume I, Pelaez A, Peralta O, Quadrelli M, Garcia Monaco R. Expected and unexpected imaging findings after 90Y transarterial radioembolization for liver tumors. Radiographics. 2019; 39:578–595. [DOI] [PubMed] [Google Scholar]
- 21.Margolis LW, Phillips TL. Whole-lung irradiation for metastatic tumor. Radiology. 1969; 93:1173–1179. [DOI] [PubMed] [Google Scholar]
- 22.Salem R, Parikh P, Atassi B, Lewandowski RJ, Ryu RK, Sato KT, et al. Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol. 2008; 31:431–438. [DOI] [PubMed] [Google Scholar]
- 23.Ho S, Lau WY, Leung TW, Chan M, Johnson PJ, Li AK. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med. 1997; 24:293–298. [DOI] [PubMed] [Google Scholar]
- 24.Emami B, Lyman J, Brown A, Goitein M, Munzenrider JE, Shank B, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991; 21:109–122. [DOI] [PubMed] [Google Scholar]
- 25.Marks LB, Bentzen SM, Deasy JO, Kong F-MS, Bradley JD, Vogelius IS, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010; 76(3 Suppl.):S70–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das A, Riaz A, Gabr A, Ali R, Mora R, Al Asadi A, et al. Safety and efficacy of radioembolization with glass microspheres in hepatocellular carcinoma patients with elevated lung shunt fraction: analysis of a 103-patient cohort. Eur J Nucl Med Mol Imaging. 2020; 47:807–815. [DOI] [PubMed] [Google Scholar]
- 27.Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global ): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017; 18:1159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobrocky T, Fuerstner M, Klaeser B, Lopez-Benitez R, Wälti YB, Kara L. Regional radiation pneumonitis after SIRT of a subcapsular liver metastasis: what is the effect of direct beta irradiation? Cardiovasc Intervent Radiol. 2015; 38:1025–1030. [DOI] [PubMed] [Google Scholar]
- 29.Devcic Z, Rojas CA, Elboraey M, Toskich B. Organizing pneumonia induced by ablative radioembolization for the treatment of hepatic metastatic renal cell carcinoma. J Clin Imaging Sci. 2019; 9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright CL, Werner JD, Tran JM, Gates VL, Rikabi AA, Shah MH, Salem R. Radiation pneumonitis following yttrium-90 radioembolization: case report and literature review. J Vasc Interv Radiol. 2012; 23:669–674. [DOI] [PubMed] [Google Scholar]
- 31.Dittmann H, Kopp D, Kupferschlaeger J, Feil D, Groezinger G, Syha R, et al. A prospective study of quantitative SPECT/CT for evaluation of lung shunt fraction before SIRT of liver tumors. J Nucl Med. 2018; 59:1366–1372. [DOI] [PubMed] [Google Scholar]
- 32.Reinders MTM, Mees E, Powerski MJ, Bruijnen RCG, van den Bosch MAAJ, Lam MGEH, Smits MLJ. Radioembolisation in Europe: a survey amongst CIRSE members. Cardiovasc Intervent Radiol. 2018; 41:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabr A, Kulik L, Mouli S, Riaz A, Ali R, Desai K, et al. Liver transplantation following yttrium-90 radioembolization: 15-year experience in 207-patient cohort. Hepatology. 2021; 73:998–1010. [DOI] [PubMed] [Google Scholar]
- 34.Lopez BP, Mahvash A, Long JP, Lam MGEH, Kappadath SC. Improving accuracy of predicted lung dosimetry in 90Y-microsphere radioembolization with 99mTc-MAA planar scintigraphy. Med Phys. 2020; 47:e627. [Google Scholar]