Abstract
Aquired apraxia of speech is a disorder that impairs speech production, despite intact peripheral neuromotor function. Its pathomechanism remains to be established. Neurodegenerative lesion models provide an unequalled opportunity to explore the neural correlates of apraxia of speech, which is present in a subset of patients diagnosed with non-semantic variants of primary progressive aphasia. The normalized pairwise variability index, an acoustic measure of speech motor programming, has shown high sensitivity and specificity for apraxia of speech in cross-sectional studies. Here, we aimed to examine the strength of the pairwise variability index and overall word duration (i.e. articulation rate) as markers of progressive motor programming deficits in primary progressive aphasia with apraxia of speech. Seventy-nine individuals diagnosed with primary progressive aphasia (39 with non-fluent variant and 40 with logopenic variant) and 40 matched healthy controls participated. Patients were followed-up annually (range 1–6 years, median number of visits = 2). All participants completed a speech assessment task and a high-resolution MRI. Our analyses investigated trajectories of speech production (e.g. pairwise variablity index and word duration) and associations with cortical atrophy in the patients. At first presentation, word duration differentiated the nonfluent and logopenic cases statistically, but the range of scores overlapped substantially across groups. Longitudinally, we observed progressive deterioration in pairwise variability index and word duration specific to the non-fluent group only. The pairwise variability index showed particularly strong associations with progressive atrophy in speech motor programming brain regions. Of novelty, our results uncovered a key role of the right frontal gyrus in underpinning speech motor programming changes in non-fluent cases, highlighting the importance of right-brain regions in responding to progressive neurological changes in the speech motor network. Taken together, our findings validate the use of a new metric, the pairwise variability index, as a robust marker of apraxia of speech in contrast to more generic measures of speaking rate. Sensitive/specific neuroimaging biomarkers of the emergence and progression of speech impairments will be useful to inform theories of the pathomechanisms underpinning impaired speech motor control. Our findings justify developing more sensitive measures of rhythmic temporal control of speech that may enable confident detection of emerging speech disturbances and more sensitive tracking of intervention-related changes for pharmacological, neuromodulatory and behavioural interventions. A more reliable detection of speech disturbances has relevance for patient care, with predominance of progressive apraxia of speech a high-risk factor for later diagnosis of progressive supranuclear palsy or corticobasal degeneration.
Keywords: primary progressive aphasia, progressive nonfluent aphasia, logopenic progressive aphasia, apraxia of speech, cortical thickness
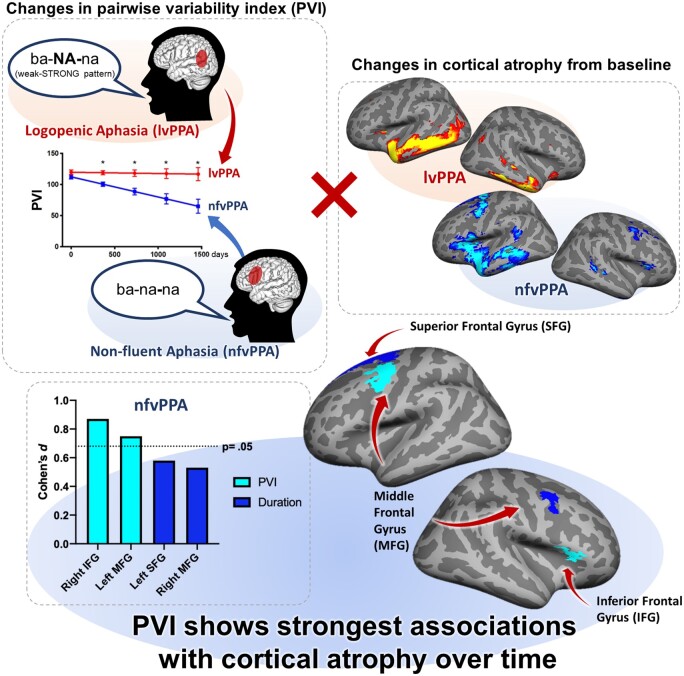
Landin-Romero et al. combined speech and neuroimaging analyses to validate the pairwise variability index, a simple temporal acoustic measure, in the assessment and monitoring of apraxia of speech in primary progressive aphasia. Additionally, findings highlight the role of established and new brain regions in supporting speech motor control.
Graphical Abstract
Graphical Abstract.
Introduction
Speech motor control is a complex process that relies on a network of brain regions across frontal lobes, including primary, premotor, supplementary motor areas and inferior frontal gyrus as well as insula, somatosensory and auditory cortices, subcortical and cerebellar regions.1–3 Not surprisingly, given the number of brain regions involved, a range of speech motor control disorders have been identified, that include apraxia of speech (AOS) and dysarthria. Unlike in dysarthria, peripheral neuromotor function is intact in AOS. Apraxic speech errors arise from disrupted feedforward (or predictive) control of the spatio-temporal parameters of speech movements.4,5 It manifests as increased duration of syllables in words, particularly unstressed syllables, increased duration of transitions between syllables and spatio-temporal articulatory errors (i.e. distortions) on production of speech sounds6–10; also see Ballard et al.11 for a review. It is of specific interest as it represents the interface of the linguistic and the motor control systems, providing a window into the processes(es) for converting thoughts into the movement programs that drive the articulatory system. Unlike the dysarthrias, AOS arises from left hemisphere-lateralized brain disturbances, with evidence implicating a central role of the left premotor cortex.2,7,12–15 Detecting presence or predominance of AOS is of central importance due to its association with emergence of Parkinson’s plus syndrome16,17 and selection of efficacious motor learning interventions to prolong speech function.18 Importantly, no specific and objective behavioural markers of AOS have been adopted. Addressing this issue would strengthen the current research on behavioural outcomes of pharmacological, neuromodulatory, and behavioural interventions, and would increase our understanding of the neural underpinnings of speech motor control.
The neurodegenerative brain condition of frontotemporal dementia (FTD) provides an ideal opportunity to identify the pathomechanisms of AOS. AOS is present in 70–90% of patients diagnosed with nonfluent variant primary progressive aphasia (nfvPPA), one of the main FTD syndromes.19,20 Pathologically, nfvPPA is usually a tauopathy, but recent meta-analysis evidence suggests it is also associated with TDP-43 subtypes, including type C in up to 68% of cases21 and more rarely, type A inclusions.22 Its clinical presentation, however, can often be confused with another PPA, the logopenic variant (lvPPA), in which apraxia of speech is absent. In contrast to nfvPPA, lvPPA is an atypical presentation of Alzheimer pathology initially focussed in left temporal-parietal cortex.23 This region is associated with processing and retrieval of abstract phonological representations for subsequent articulation and holding phonological information in working memory.24 The differentiation of speech errors arising from phonological/working memory versus motor programming impairment has been confounded by the need to examine both components through the articulation of speech. This has motivated recent efforts to identify objective acoustic measures that correlate with expert perceptual judgments of apraxic speech features.6–9,25,26
Objective acoustic signatures of articulatory timing or rhythm have shown promise for the detection of AOS.6–9,25–28 For example, speech or articulation rate deteriorates disproportionately faster with disease progression in nfvPPA compared with lvPPA.25,26 Speech rate refers to the number of syllables produced per second and is often measured over one or more sentences. Speech rate can be slowed for many reasons, including language-related factors such as impaired lexical access or grammatical processing.25 To control for these influences, articulation rate is usually measured from single, often multisyllabic, words. It is thought to reflect motor planning/programming and execution processes and can be affected by damage to any region within the bilateral speech motor network.
The normalized pairwise variability index (PVI) for vowel duration is a measure that has high sensitivity and specificity for nfvPPA and AOS.6,7,9 This measure captures a feature of AOS where very brief syllables become protracted while others are not.10 PVI measures the relative duration of the first two vowels in three-syllable words. Words with a strong–weak stress (e.g. dinosaur) have high positive PVI values and words with a weak–strong stress (e.g. banana) have high negative PVI values. Individuals with AOS tend to lengthen weak syllables at word onset, driving the negative PVI values towards zero.6,7,9 While this change slows the overall articulation rate, it is specific to the apraxic disruption to programming and to producing time/rhythmically-constrained movement transitions. To date, PVI has only been explored in cross-sectional samples of patients with stroke-related or progressive AOS.6,7,9 In stroke, PVI was found to be a better predictor of AOS than overall articulation rate.6 If deterioration in specific acoustic metrics is found to correlate with changes in regional cortical integrity in specific PPA variants, we are a step closer to identifying causative mechanisms for the unique profile of speech deficits in AOS. Such insight can direct the development of a more sensitive and standardized tool for identifying signs of AOS earlier in the disease course, for reliable diagnosis at any point along the disease trajectory, and for clinical monitoring of change in response to progression or intervention.
Here, we aimed to test the strength of the PVI metrics, as well as overall word duration (i.e. articulation rate), as markers of nfvPPA with AOS by investigating (i) whether these measures differentiate this group from lvPPA throughout the disease course and (ii) whether changes in PVI are associated with cortical changes over time in one or both patient groups. We hypothesized that the deterioration in PVI towards zero over time, specific to the nfvPPA group and particularly affecting weak–strong words, would be strongly associated with changes in cortical atrophy in the left inferior frontal regions, regions classically associated with speech motor planning/programming. Similarly, we hypothesized that word duration would increase (i.e. slower rate) with disease progression in nfvPPA, relative to lvPPA, but associations with cortical atrophy would be less specific; that is, diffusely distributed throughout the speech network and therefore weaker, given that slowed articulation rate is not specific to speech praxis.
Materials and methods
Participants
Seventy-nine individuals diagnosed with non-semantic PPA (40 lvPPA and 39 nfvPPA) from FRONTIER, the multidisciplinary frontotemporal dementia research clinic in Sydney, were included in this study. These PPA patient groups were matched to 40 healthy controls (HC) according to age, sex and education (Table 1). Both PPA groups were also matched on disease duration, calculated from symptom onset. All patients were assessed by the multidisciplinary team and diagnosis was reached after a consensus meeting between the behavioural neurologists (JRH and CL). Diagnosis followed current clinical and diagnostic criteria for PPA29 based on a comprehensive clinical assessment, review of the clinical file and history, cognitive examination, informant report and structural MRI brain scans. The Frontotemporal Dementia Rating Scale (FRS) was used to measure overall disease severity.30 Patients underwent clinical, cognitive and neuroimaging annual follow-up (range 1–6 years, median number of visits = 2). Clinical diagnosis of PPA in all patients was confirmed at the latest point of follow-up available. AOS was reported for 77% (30/39) of nfvPPA cases, consistent with previous studies;19,20 with AOS diagnosis based on the neurologists’ perceptual identification of AOS signs, including effortful or halting speech, speech sound distortions, changes in stress and intonation in speech, and/or reduced speech intelligibility.29,31 Further details of the speech assessements are provided in Supplementary materials.
Table 1.
Demographic and clinical characteristics at baseline of nfvPPA, lvPPA and healthy controls
| nfvPPA | lvPPA | HC | F | P | |
|---|---|---|---|---|---|
| n = 39 | n = 40 | n = 40 | |||
| Sex (M:F) | 17:22 | 15:25 | 20:20 | 1.27# | 0.53 |
| Age (years) | 65.5 ± 9.5 | 66.9 ± 7.4 | 64.9 ± 6.6 | 0.66 | 0.51 |
| Education (years)a | 13.0 ± 3.2 | 12.2 ± 3.3 | 13.3 ± 3.0 | 1.39 | 0.25 |
| Handedness (L:R) | 3:36 | 3:37 | 0:40 | 3.20# | 0.20 |
| English as first languageb | 28 | 39 | 39 | 17.55# | <0.001 |
| Disease duration (years) | 3.3 ± 2.2 | 3.5 ± 2.5 | – | 0.20 | 0.65 |
| FRS (Rasch score)c | 2.3 ± 1.8 | 1.5 ± 1.8 | – | 3.71 | 0.06 |
| Number of assessmentsd | 2 [1–6] | 2 [1–4] | – | 0.51^ | 0.52 |
| Baseline | 39 | 40 | 40 | – | – |
| Year 2 | 25 | 22 | – | – | – |
| Year 3 | 11 | 12 | – | – | – |
| Year 4 | 3 | 2 | – | – | – |
| Year 5 | 1 | 0 | – | – | – |
| Year 6 | 1 | 0 | – | – | – |
| Total | 80 | 76 | 40 | – | – |
Values are mean ± standard deviation, with the exception of dwhere values are expressed as median [range]. #Chi-square value. ^t-statistic value. FRS, Frontotemporal dementia Rating Scale; HC, healthy controls; lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non-fluent variant primary progressive aphasia. Missing data: aYears of education missing for 1 control; bFirst language in non-native English speakers; nfvPPA (n = 11): Slavic (3; Hungarian, Latvian/Russian, Croatian); Romance (2, Italian); Semitic (2; Arabic, Maltese); Germanic (2, German, Dutch); African (1; Afrikaans); Dravidian (1, Mayalayam); lvPPA (n = 1) = Germanic (1; Dutch). English as first language missing for 1 control; cFRS score missing for 3 nfvPPA and 2 lvPPA; dNumber of MRI and clinical assessments.
Healthy controls were selected from the FRONTIER volunteer database and local community clubs. Controls scored ≥88/100 on the Addenbrooke’s Cognitive Examination (ACE-R or ACE-III)32–34 and 0 on the Sum of Boxes score of the Clinical Dementia Rating Scale.35 Exclusion criteria for all participants included the presence of concurrent primary psychiatric disturbance, other neurodegenerative conditions or neurological disorders, history of significant traumatic brain injury (with LOC > 5 min) and/or history of substance abuse.
The South Eastern Sydney Local Health District and the University of New South Wales ethics committees approved the study. Participants and/or their primary caregiver provided informed consent in accordance with the Declaration of Helsinki. Participants volunteered their time and were reimbursed for travel costs.
Speech assessment
All participants completed the 30-item word repetition task of the Sydney Language Battery (SYDBAT)36 during the comprehensive diagnostic assessment. From this task, responses to six 3-syllable words were extracted for analysis: two with strong–weak (SW) stress (bicycle and dinosaur) and four with weak–strong (WS) stress (banana, computer, pagoda, potato). Six additional words were presented to participants to increase the sample size for each stress type (SW: cardigan, motorbike, barbecue; WS: tomato, pyjamas, zucchini).
Two measures were made on each word production: normalized PVI for vowel duration and whole word duration. PVI is a measure of lexical stress contrastiveness and reveals impaired temporal control of sequential speech segments that characterizes AOS [See PVI equation (PVI = 100 × ABS [(d1 − d2)/(d1 + d2) × 0.5]; where ‘d’ is duration in milliseconds of the first (1) and second (2) vowel, ABS, absolute value); e.g.7,9]. All selected words fit recommended criteria for PVI measurement of (i) having a tense (i.e. long) vowel in the stressed syllable and (ii) vowels being adjacent to plosive (e.g./p/,/t/), fricative (/f/,/s/,/th/), and nasal (/m/,/n/) phonemes, with clear boundaries in the acoustic waveform to allow reliable identification of vowel onset and offset.28,37 For each participant at each time point, their median PVI value for each word type (SW, WS) was used for statistical analyses. In prior work using these stimulus characteristics, an absolute value for median PVI_WS smaller than 100 is strongly predictive of expert judgement of AOS presence.6,7
The second measure, word duration, is a global measure of the rate of articulation, which is affected by damage to any components of the bilateral speech motor network.38 Word duration is measured in milliseconds from the onset of the initial sound of the word to the offset of the final sound, determined using the vocal pitch and loudness trajectories calculated using Praat software.39
All speech samples were analysed by the same primary rater (PAM), trained by an experienced speech scientist (KJB), and following procedures described elsewhere.6,37 Raters were blinded to PPA variant (i.e. nfvPPA, lvPPA), case history, medical records, any brain imaging findings, and results of the speech and language tests performed. Inter-rater reliability of PVI measurements has been performed previously in samples of randomly selected AOS cases, see Refs.6,37 and reported to be high (Intra-class correlation coefficients of 0.90, P < 0.0001, consistency agreement on single measures, 95% CI).
For the behavioural analyses, poor audio quality prevented reliable acoustic analysis on a small number of samples. For SW words, 10 timepoint measurements were missing in nfvPPA and 6 in lvPPA patients. For WS words, 10 timepoint measurements were missing for 10 nfvPPA and 4 in lvPPA patients. One lvPPA patient had no behavioural data available but one MRI. This patient was excluded from the behavioural analyses but included in the neuroimaging analyses.
Neuroimaging assessment
MRI acquisition
Participants underwent whole-brain structural MRI in a 3 T Phillips scanner with a standard 8-channel head coil. Two 3D high-resolution turbo field echo T1-weighted sequences were acquired using the following parameters: coronal orientation, matrix 256 × 256, 200 slices, 1 mm2 in-plane resolution, slice thickness 1 mm, echo time/repetition time 2.6/5.8 ms, flip angle α = 8°. Among the PPA groups, 22/40 lvPPA patients and 25/39 nfvPPA patients underwent annual follow-up MRI (range 1–6 years, median number of MRI scans = 2). A total of 196 MRI scans (40 HC, 76 lvPPA and 80 nfvPPA) were included in the study. MRI scans were obtained on average within 1 month of the clinical data acquisition.
Pre-processing
At each time point, the two T1 volumes were merged and averaged to increase the signal-to-noise ratio and grey-white matter contrasts. FreeSurfer software, version 5.3.0 (http://surfer.nmr.mgh.harvard.edu.au/) was used to perform surface-based cortical processing40,41 using standard methods.42
The longitudinal pre-processing included the following steps. First, an unbiased within-subject template43 was created using robust, inverse consistent registration between the two points for each individual.44 Next, skull stripping, Talairach transformations, atlas registration, spherical surface maps and parcellations were initialized with common information from the within-subject template to increase reliability and statistical power.45 Cortical thickness was smoothed with a 20 mm full-width at half-height Gaussian kernel for all analyses to reduce the impact of imperfect alignment between cortices and thereby improve the signal-to-noise ratio.46
Subcortical structures were automatically segmented and extracted for both hemispheres. For these subcortical structures, measurements from both hemispheres were averaged and adjusted for total intracranial volume, in line with published methods.47 All images were visually inspected and manually corrected to remove artefacts and tissue segmentation errors. No MRI scans were excluded from the imaging analyses.
Statistical analyses
Baseline demographic and clinical variables
Analyses were conducted using IBM SPSS statistics (version 24.0). One-way analyses of variance were conducted to examine demographic (age, education) and clinical (disease duration, FRS, ACE and SYDBAT scores) variables followed by Sidak post hoc tests where relevant. Categorical variables (sex, handedness) were analysed using chi-square tests.
Baseline and longitudinal speech analysis
Baseline PVI and word duration were analysed using one-way ANOVA to determine patterns of speech motor impairment across PPA groups (lvPPA, nfvPPA) and healthy controls (HC). Pair-wise post hoc tests were conducted with a Sidak correction for multiple comparisons.
Linear mixed effects (LME) models were fitted to model the time-related changes in outcome variables according to diagnosis. LME modelling is a powerful and flexible approach for analysing longitudinal data, as it can handle variable rates of missing data, uneven timing of events and single time points. The fixed effects in the model included diagnosis, time, and the interaction between diagnosis and time. The only random effect included was the individual variability associated with the patients at baseline (where we used the random intercept model). Residual errors of the model and the random intercepts for each participant at baseline were assumed to be normally distributed. Post hoc analyses examined pairwise mean comparisons according to diagnosis in outcome variables across all time-points. All participants were assumed to be independent. Longitudinal analyses compared nfvPPA and lvPPA, as we assumed no change in HC groups over time.
Baseline and longitudinal comparisons of cortical thickness and subcortical volumes
For cross-sectional analyses, whole-brain differences in cortical thickness at baseline were examined using vertex-wise general linear models (GLM) including cortical thickness as a dependent variable and group (nfvPPA, lvPPA and healthy controls) as an independent variable. For subcortical structures, one-way ANOVAs followed by Sidak post hoc tests were used to identify group differences for lvPPA, nfvPPA and healthy controls.
For the longitudinal analyses, vertex-wise comparisons of annual rate of change in cortical thickness were analysed using the Spatiotemporal LME Matlab tools (FreeSurfer version 5.3.0).48,49 These analyses were conducted in nfvPPA and lvPPA.
The mean trend in cortical thickness over time was assessed across PPA groups. Visual inspection of the plots revealed linear trajectories of cortical thickness over time. Therefore, a spatiotemporal LME model of cortical atrophy was fitted, with (i) diagnosis (nfvPPA and lvPPA); (ii) follow-up time (expressed in days from baseline MRI acquisition); (iii) the interaction between diagnosis and follow-up time as fixed effects; (iv) the intercept; and (v) time from baseline MRI acquisition as random effects. As both groups were matched at baseline for age, sex, disease duration and education, no covariates were included in the model. The null hypotheses of no change in cortical thickness over time and no diagnosis × time interaction (i.e. no group-specific atrophy rate) were tested. Statistical significance was first set at an FDR <0.05 threshold50,51 to correct for multiple comparisons. A disadvantage of the FDR correction approach is that different statistical thresholds are automatically applied within group (i.e. per hemisphere) and between group, which limits the ability to infer patterns of spread of cortical changes across time within and between groups. We controlled for these confounds by manually setting the statistical threshold at P < 0.001 uncorrected, with a conservative cluster extent threshold of k > 50 mm2 to minimize Type I error while balancing the risk of Type II error.52 This approach resulted in more stringent and consistent statistical maps than the FDR correction. In line with previous methodology,53,54 an uncorrected threshold of P < 0.01 was used in the between-group longitudinal analyses to account for the increased number of time-varying parameters and the increased degrees for freedom that would limit the power to detect underlying effects. The significance maps for each contrast were visualized and post-processed using tksurfer included in the FreeSurfer software suite.
For the subcortical data, a random-intercept LME model with fixed effects of group, time and the interaction between group × time was used to compare changes in subcortical structures over time in the lvPPA and nfvPPA groups.
Associations between changes in speech measures and cortical thickness over time
Brain-behaviour analyses were conducted to examine the contribution of different cortical regions to changes in speech measures over time in nfvPPA and lvPPA. First, cortical regions of interest (ROIs) were selected based on the whole-brain within- and between-group longitudinal analyses and extracted from the respective significance maps. For within-group changes, 14 cortical ROIs (9 for nfvPPA and 5 for lvPPA) were identified from the phenotype-specific cortical thinning maps. For between-group changes, 9 ROIs (6 for nfvPPA and 3 for lvPPA) were identified from the time × diagnosis interaction cortical thinning maps. These ROIs were then mapped for cortical thickness extraction in all individuals across all time points in nfvPPA and lvPPA separately (Supplementary materials). Next, relations between changes in the speech measures and progressive cortical thinning in the ROIs were explored fitting independent random intercept models using IBM SPSS statistics (version 24.0). Briefly, these LME models tested associations between changes in cortical thickness of these ROIs, the speech measurements (PVI and word duration) and their time varying interaction both within and between groups. This approach enabled us to identify both phenotype-specific and differential (i.e. greater in one group versus the other) correlates of brain-motor speech associations. Detailed information on the ROI selection procedure and LME model fitting is available in the Supplementary Materials.
Data availability
Anonymized data will be shared by reasonable request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with Australian legislation on the general data protection regulation and decisions by the Ethical Review Board of The University of Sydney, which should be regulated in a material transfer agreement.
Results
Demographics and clinical data
Groups were matched for basic demographic variables (age, sex, education; all P-values > 0.05) (Table 1). In addition, the patient groups were matched for disease duration and functional impairment/severity as measured by the FRS. The proportion of native English speakers was significantly greater in lvPPA and HC compared to nfvPPA. Patients with lvPPA and nfvPPA were also matched with regards to the number of follow-up assessments (P = 0.52).
Different cognitive profiles were found between patient groups (Table 2). Briefly, both patient groups performed worse than healthy controls across all measures of ACE and SYDBAT subdomains (all P-values < 0.001). Comparing the PPA groups, lvPPA performed worse than nfvPPA across all ACE subdomains (attention, memory and visuospatial all P-values ≤ 0.01; language P = 0.03) except for fluency (no differences between groups; P = 0.35). On the SYDBAT, lvPPA performed worse than nfvPPA on the naming and comprehension subtests (both P-values ≤ 0.01), whereas nfvPPA performed worse than lvPPA on the repetition subtest (P = 0.01). No differences between groups were seen on the semantic association subtest (P = 0.51).
Table 2.
Baseline performance on cognition and language in nfvPPA, lvPPA and healthy controls
| nfvPPA | lvPPA | HC | F | P | Post-hoc | |
|---|---|---|---|---|---|---|
| (n = 39) | (n = 40) | (n = 40) | ||||
| ACE Total | 74.2 ± 14.7 | 59.4 ± 16.8 | 95.5 ± 3.6 | 77.466 | <0.001 |
|
| Attention | 15.7 ± 2.7 | 12.9 ± 3.8 | 17.5 ± 1.0 | 28.639 | <0.001 |
|
| Memory | 19.9 ± 5.9 | 12.5 ± 6.4 | 24.5 ± 1.8 | 56.426 | <0.001 |
|
| Fluency | 5.4 ± 3.0 | 4.5 ± 2.8 | 12.6 ± 1.2 | 130.76 | <0.001 | Patients < HC |
| Language | 19.2 ± 4.2 | 16.9 ± 5.5 | 25.4 ± 1.1 | 46.967 | <0.001 |
|
| Visuospatial | 14.1 ± 2.2 | 12.6 ± 3.2 | 15.6 ± 0.7 | 17.044 | <0.001 |
|
| SYDBAT | ||||||
| Naming | 20.3 ± 6.0 | 14.9 ± 6.8 | 27.4 ± 2.0 | 53.669 | <0.001 |
|
| Semantic | 25.2 ± 4.0 | 24.1 ± 5.2 | 28.8 ± 1.1 | 15.435 | <0.001 | Patients < HC |
| Comprehension | 27.5 ± 2.6 | 26.0 ± 3.0 | 29.5 ± 1.0 | 20.978 | <0.001 |
|
| Repetition | 21.6 ± 8.7 | 25.5 ± 5.5 | 29.8 ± 0.5 | 18.941 | <0.001 |
|
Values are mean ± standard deviation. ACE, Addenbrooke’s Cognitive Examination; lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non-fluent variant primary progressive aphasia; SYDBAT, Sydney Language Battery. Missing data: SYDBAT Total missing for 2 lvPPA, 2 Controls; SYDBAT Naming missing for 1 lvPPA; SYDBAT Semantic missing for 1 lvPPA, 2 controls; SYDBAT Comprehension missing for 1 lvPPA.
Baseline and longitudinal speech analyses
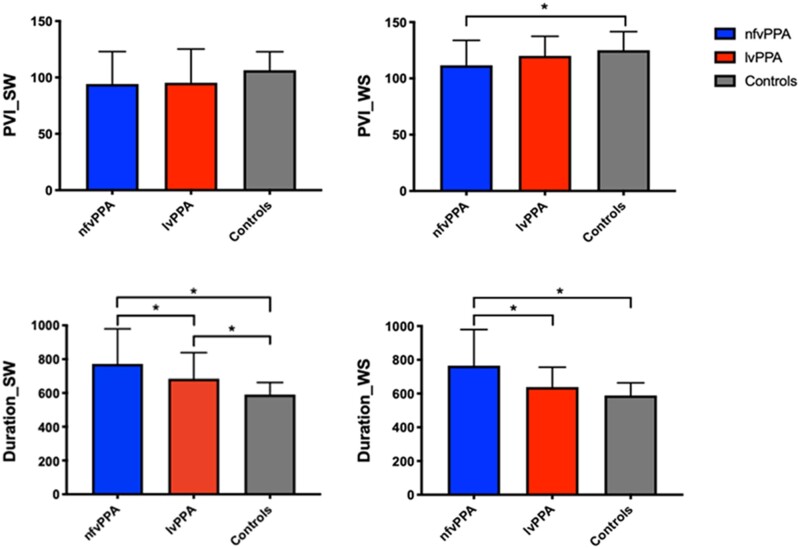
The differences in speech measures between groups at baseline are shown in Fig. 1 and Table 3. Briefly, nfvPPA patients performed worse than healthy controls in PVI for WS words but not for SW words. The two clinical groups did not differ from each other. With regards to word duration, the nfvPPA group performed worse than lvPPA and HC for both SW and WS words. In contrast, lvPPA were only impaired for SW words compared with HC.
Figure 1.
Baseline temporal speech measures in nfvPPA, lvPPA and healthy controls. lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non-fluent variant primary progressive aphasia; PVI, pairwise variability index; Duration is measured in milliseconds; SW, strong–weak stressed words (e.g. dinosaur); WS, weak–strong stressed words (e.g. banana). *P < 0.05.
Table 3.
Baseline temporal speech measures in nfvPPA, lvPPA and healthy controls
| nfvPPA | lvPPA | HC | F | P | Post-hoc | |
|---|---|---|---|---|---|---|
| PVI_SW | 94.2 ± 28.7 | 95.3 ± 29.9 | 106.4 ± 16.4 | 2.436 | 0.09 | – |
| PVI_WS | 111.7 ± 22.1 | 120.1 ± 17.33 | 125.0 ± 16.6 | 4.643 | 0.01 | nfvPPA < HC |
| Duration_SW | 772.1 ± 207.6 | 684.5 ± 154.1 | 590.2 ± 72.1 | 12.209 | <0.001 | nfvPPA > lvPPA > HC |
| Duration_WS | 765.0 ± 214.7 | 638.6 ± 117.5 | 588.6 ± 75.5 | 13.437 | <0.001 | nfvPPA > lvPPA, HC |
PVI (Pairwise variability index) and duration (milliseconds) values are shown as mean ± standard deviation. lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non-fluent variant primary progressive aphasia; SW, strong–weak stressed words (e.g. dinosaur); WS, weak–strong stressed words (e.g. banana). Missing data: PVI_SW missing for 2 nfvPPA, 2 lvPPA, 5 HC; PVI_WS missing for 2 nfvPPA, 1 lvPPA, 5 HC; Duration_SW missing for 2 nfvPPA; 2 lvPPA; 5 HC; Duration_WS missing for 2 nfvPPA; 1 lvPPA and 6 HC. For indivduals tested before July 2012, only the six words from the SYDBAT were available (two SW, four WS).
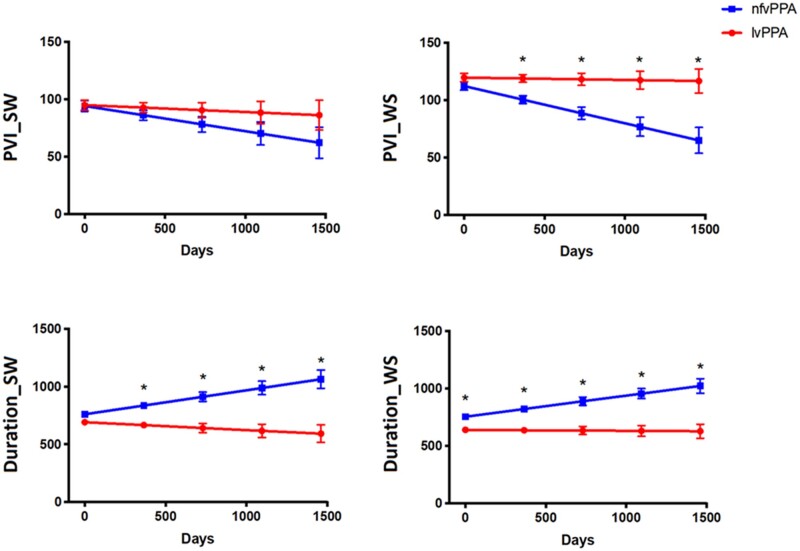
Overtime, a significant decline in PVI for WS words was found in nfvPPA compared with lvPPA (Fig. 2 and Table 4). In addition, the nfvPPA group also showed a significant increase in word duration for both SW and WS words compared with lvPPA over time.
Figure 2.
Longitudinal changes in temporal speech measures in nfvPPA and lvPPA groups. lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non-fluent variant primary progressive aphasia; PVI, pairwise variability index; Duration is measured in milliseconds; SW, strong–weak stressed words (e.g. dinosaur); WS, weak–strong stressed words (e.g. banana). See Supplementary materials for data separated by presence of AOS and dysarthria within diagnostic groups.
Table 4.
Annual change of longitudinal temporal speech measures for strong–weak (SW) and weak–strong (WS) words in nfvPPA and lvPPA
| Follow-up |
Diagnosis interaction |
Parameter coefficientsa |
||||
|---|---|---|---|---|---|---|
| F | P | F | P | nfvPPA | lvPPA | |
| PVI_SW | 3.825 | 0.05 | 1.249 | 0.26 | −7.99 | −2.18 |
| PVI_ WS | 8.496 | 0.004 | 6.554 | 0.01 | −11.83 | −0.76 |
| Duration_SW | 2.918 | 0.09 | 11.287 | 0.001 | 75.90 | −24.73 |
| Duration_WS | 7.597 | 0.007 | 9.176 | 0.003 | 67.05 | −3.16 |
Parameter estimates of annual change within each group in milliseconds. lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non-fluent variant primary progressive aphasia; PVI, pairwise variability index; SW, strong–weak stressed words; WS, weak–strong stressed words. Missing data: SW words data missing for 6 lvPPA and 10 nfvPPA time points, WS words missing for 4 lvPPA and 10 nfvPPA time points.
Neuroimaging results
Baseline and longitudinal neuroimaging analyses
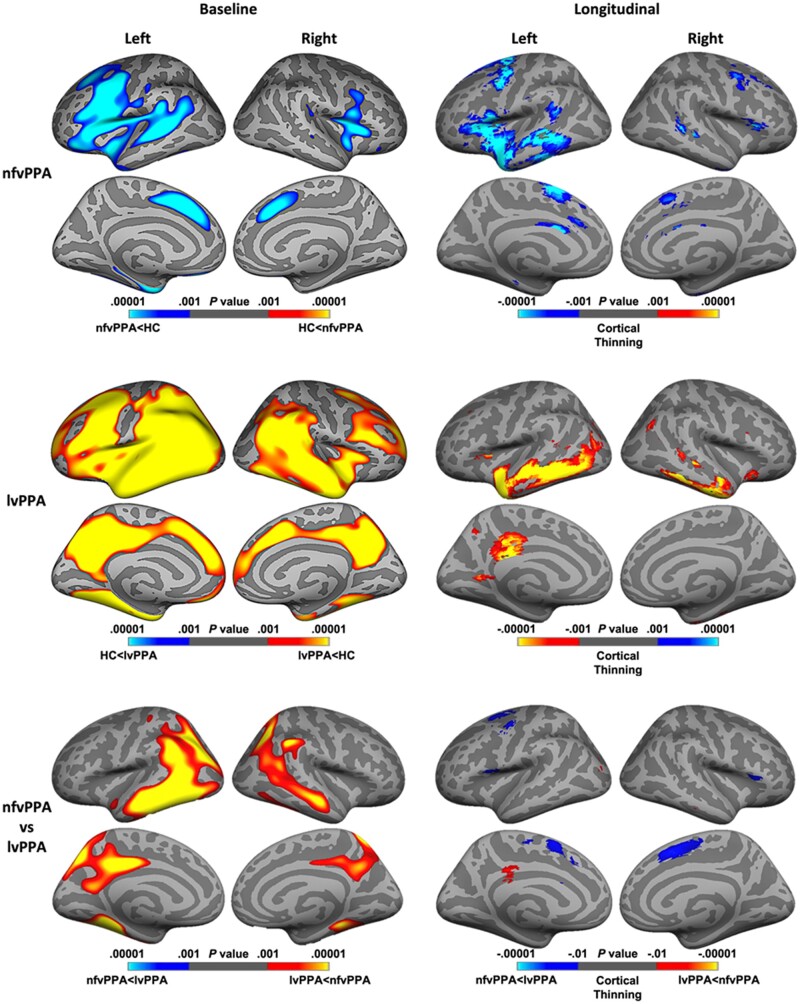
Baseline neuroimaging results comparing nfvPPA and lvPPA to healthy controls are shown in the left column of Fig. 3 (top and middle panels). In brief, the nfvPPA group (shown in blue) showed widespread atrophy in the frontal regions, surrounding the insula, caudal middle frontal and superior frontal gyrus. The lvPPA group (shown in red-yellow) demonstrated widespread atrophy encompassing both posterior and anterior regions at baseline, surrounding the temporoparietal junction, posterior cingulate cortex, and caudal middle frontal regions compared to healthy controls. Comparisons between nfvPPA and lvPPA are shown in the left side of Fig. 3 (bottom panel). Patients with lvPPA showed greater atrophy in bilateral posterior cingulate cortex, temporal lobes, fusiform and posterior parietal regions (shown in red-yellow), whereas nfvPPA patients showed no regions of greater atrophy than lvPPA patients. Regions of greater atrophy in lvPPA patients were seen across both hemispheres, with more significant and widespread atrophy in the left hemisphere.
Figure 3.
Baseline and longitudinal patterns of cortical thinning in nfvPPA and lvPPA. Regions of greater cortical atrophy are depicted in blue for nfvPPA and in red-yellow for lvPPA. The left panel shows atrophy findings at baseline in PPA groups compared with controls (top and middle row), and between PPA groups (bottom row). Baseline results are thresholded at P = 0.001, uncorrected for multiple comparisons. The right panel shows regions of cortical thinning over time within (top and middle trow) and between (bottom row) PPA groups. Longitudinal results are thresholded at P = 0.001 for within-group analysis, and at P = 0.01 for between-group analysis, uncorrected for multiple comparisons. lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non-fluent variant primary progressive aphasia.
Longitudinal imaging findings within (top and middle panels) and between syndromes (bottom panel) are shown in the right column of Fig. 3. In nfvPPA, the left hemisphere atrophy identified at baseline continued to spread in the left temporal and inferior frontal lobes, including the pars opercularis and triangularis, insula, caudal middle frontal, superior frontal, and the anterior cingulate gyrus, with symmetric, but less significant, regions of cortical thinning in the right hemisphere (shown in blue). In lvPPA, additional cortical thinning was predominanty seen in the left hemisphere, with bilateral atrophy in inferior temporal gyrus, the left middle and superior temporal gyrus, and the posterior cingulate cortex (shown in yellow). Longitudinal findings comparing between nfvPPA and lvPPA are shown in the right side of Fig. 3 (bottom panel). In nfvPPA, patients showed greater atrophy over time in bilateral superior frontal gyrus, and the left caudal middle frontal gyrus and the right pars triangularis, whereas in lvPPA patients showed greater cortical thinning in the left posterior cingulate cortex.
Results of the subcortical analyses are available in the Supplementary materials. At baseline, both lvPPA and nfvPPA showed volume reductions in the hippocampus compared with controls, with no differences between patient groups. Additionally, lvPPA showed greater atrophy in the amygdala compared to nfvPPA and controls and reduced thalamus and caudate compared with controls. Over time, significant reductions in the thalamus, caudate and hippocampus were observed across nfvPPA and lvPPA groups. No diagnosis × time interactions were found indicating that both groups declined at comparable rates.
Associations between speech temporal measures for WS words and cortical thickness over time
These analyses examined associations between the PVI, duration and cortical thickness both within and between nfvPPA and lvPPA over time. The analyses focussed on WS words due to their discriminative power to separate speech production trajectories in nfvPPA and lvPPA.
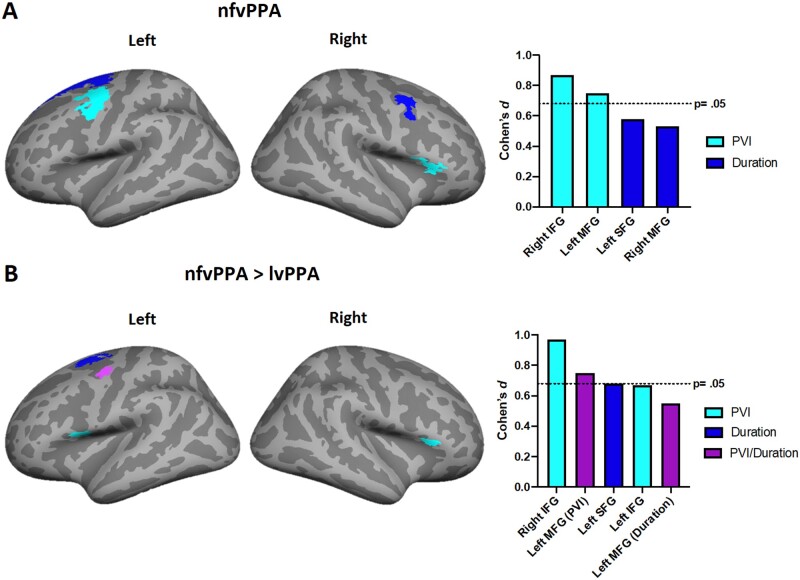
In nfvPPA, both within group and in direct comparisons to lvPPA, changes in PVI over time were associated with significant and greater progressive cortical thinning in the right inferior frontal and left middle frontal cortices with large and significant effect sizes (Fig. 4A and B).
Figure 4.
Brain regions showing associations between temporal speech measures and cortical thinning in nfvPPA and lvPPA. Coloured regions depict associations between cortical atrophy of ROIs and changes in PVI (light blue) or word duration (dark blue). The cortical region represented in purple was independently associated with changes in PVI and duration in nfvPPA compared to lvPPA. Panel A depict changes in nfvPPA over time. Panel B depict greater changes in nfvPPA compared with lvPPA. The Y-axis indicates the effect size (Cohen’s d: small = 0.20; medium = 0.50; large = 0.80). The X-axis indicates the anatomical regions underpinning the effect. Longitudinal results are thresholded at P =0.05 corrected for multiple comparisons. lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non-fluent variant primary progressive aphasia.
In nfvPPA, increased word duration over time was associated with progressive thining on the left superior and right middle frontal gyrus with a moderate effect size, although this effect did not reach statistical significance (Fig. 4 A). Word duration was also moderately associated with greater left midde frontal cortex thinning in nfvPPA compared to lvPPA, but this did not reach statistical significance (Fig. 4B).
In lvPPA, no significant associations were found within or between groups for either speech measure with cortical thickness changes over time.
Discussion
Using longitudinal analyses of speech and cortical integrity measures, we identified a network of robust cortical biomarkers of progressive AOS specific to nfvPPA and strongly associated with motor speech deterioration. A measure of relative timing across polysyllabic words with weak initial stress (PVI_WS; e.g. banana) was strongly associated with changes in cortical atrophy in the left premotor area, an area critical for speech motor planning/programming. Importantly, the right inferior frontal gyrus showed the greatest sentitivity to PVI deterioration in nfvPPA compared to lvPPA with disease progression. This highlights an important yet poorly understood role of this region in speech motor control2,13 and in the changing profile of speech impairment with nfvPPA progression. Another measure, word duration (i.e. articulation rate) also deteriorated over time in the nfvPPA group relative to lvPPA and was only weakly associated with more distributed cortical changes over time.
Rhythmic structure of words is affected in nfvPPA but not lvPPA
The PVI clearly differentiated between nfvPPA and lvPPA both behaviourally and anatomically. The nfvPPA group showed worsening of this symptom over time. Furthermore, worsening of PVI for weak–strong words was significantly correlated with progression of left-lateralized cortical thinning affecting the ventral premotor cortex in the middle frontal gyrus. These results expand the findings from previous cross-sectional studies that reported association of damage to the left ventral premotor cortex with apraxic speech disturbance.2,13,14,20
Notably, the PVI measure was only informative for words with a weak–strong stress pattern. This highlights the specificity of the PVI measure, over and above a global measure of articulation or speaking rate. PVI, as implemented here, captures relative timing over adjacent syllables within words. Rhythmic patterns are distributed unequally in English, with over 90% of two-syllable nouns having the strong–weak (i.e. trochaic) pattern.55 This imbalance in frequency and opportunity for practice over a lifetime has consequences. Infants as young as 7–8 months are sensitive to the metrical patterns of speech, which assist them in learning to find the boundaries of words in the speech of others.56,57 In typical development, production of the highly frequent strong–weak pattern (e.g. dinosaur) presents little challenge and is mastered by 3 years of age58; however, the weak–strong pattern (e.g. banana) is still not mastered by 11 years.59 Furthermore, Ziegler and Aichert60 have demonstrated that, for individuals with AOS, articulation errors are more frequent in words with weak–strong stress. Their work provides compelling support for the notion of AOS as ‘a problem of assembling vocal tract gestures into larger syllabic and metrical structures’ (p. 37). They liken damage to the left ventrolateral frontal speech regions as ‘dissolving the glue’ that binds learned motor plans. Invoking the ‘last in, first out’ concept,61,62 rhythmic control of the later acquired weak–strong metrical pattern might be disproprortionately vulnerable to damage in the underpinning neurological network. Consistent with this, several studies have reported beneficial effects of restorative speech interventions that employ rhythmic cueing strategies, such as pacing or speech entrainment, for speech apraxia across multiple aetiologies.18,63–66
Overall articulation rate is affected in nfvPPA but not lvPPA
Progressive slowing of articulation rate for both strong–weak and weak–strong words was strongly associated with the nfvPPA group only; however, associations with atrophic changes over time were relatively weak (P-values ≥ 0.05 and moderate magnitude of effects). Slowed speech is a feature of most types of motor speech disorder, which include dysarthrias and AOS. While slow articulation rate is commonly noted in AOS, unlike dysarthria, the slowness is not related to reduced movement velocities.67–70 On the other hand, articulatory slowing in cortical and subcortical dysarthrias tends to affect absolute timing across all segments of speech due to hypertonia or rigidity of the speech musculature.71 The nonspecificity of articulation rate undermines its value as a marker for any one type of motor speech disorder. However, data reported here and elsewhere suggest that articulation is an important baseline indicator of general motor speech disorder and, in the context of frontotemporal dementias, signals posterior frontal or subcortical disease. In contrast to articulation rate, the disruption to relative timing captured by the PVI measure and specific to words with weak–strong stress is likely associated with the more challenging motor planning demands of this movement sequence, as noted above. This explanation is supported by Ballard et al.6 who reported that PVI_WS was associated with presence of AOS but not cortical dysarthria, in a cohort of stroke patients. Furthermore, our analyses revealed that word duration was not significantly linked to neurological deterioration in any specific cortical region over time. While the measure of polysyllable word duration, as an index of articulation rate (i.e. syllables per second), may be powerful in differentiating nfvPPA from lvPPA early in the disease course, it is unlikely to aid in detection of AOS versus the dysarthrias that can herald the onset of other linked neurological conditions such as motor neuron disease and corticobasal syndrome. It is likely that other instrumental speech metrics are needed for reliable positive detection of dysarthria(s) in nfvPPA.72,73
Uncovering the role of right inferior frontal gyrus in speech motor control
A strong relationship was also observed between worsening PVI_WS and thinning in the right inferior frontal gyrus (IFG) for individuals with nfvPPA. The nfvPPA cases already had extensive left frontal atrophy in baseline (see Fig. 3) and so the stronger correlation between right frontal atrophy and speech measures over time likely reflects the greater potential for deterioration on the right; but, also supports recent proposals of contralateral involvement of right inferior frontal gyrus in speech praxis.2
The right IFG region has not been associated with AOS, a condition almost exclusively triggered by left-lateralized damage.71 In nfvPPA, AOS is an early clinical feature associated with left hemisphere cortical atrophy. However, studies in stroke have shown that patients who recover language skills after a left hemisphere stroke can re-present with aphasia after stroke or neuromodulatory suppression to the right homologous area74,75 also argued for a facilitative role of right IFG in adapting to left IFG hemisphere damage. Using a virtual lesion method, they found that damage to left IFG was associated with reduced activation in the damaged ROI during pseudo-word repetition; and a corresponding increase in activity in the homologous area was associated with a facilitatory drive to left IFG and faster responding. Importantly, however, spread of pathology to the right hemisphere and to the right frontal cortex over time in dementia and PPA is common.54,76–79 Progression of pathology into the right IFG could undermine any facilitatory effect from right IFG and explain the correlation between worsening PVI_WS and right IFG thinning observed here.
Little is currently known about the implications of contralateral spread of pathology on the behavioural symptoms of nfvPPA, or AOS specifically. Further work is needed to identify the associations with symptom severity, prognosis in behavioural rehabilitation, or potential sites for noninvasive neuromodulation. This study provides a valuable metric to quantify the effects of extent of left and right IFG involvement on speech production throughout the course of nfvPPA.
Clinical implications
Our findings further validate the use of the PVI metric as a robust marker of the context-specific speech timing disruption that characterizes AOS. Word duration, as an index of articulation rate, also emerges as a powerful baseline marker of motor speech disorder in general, but is not specific to AOS. Further study is required to determine whether PVI is also affected in cases of frontotemporal dementia with dysarthria, and no AOS, or whether other acoustic metrics more accurately capture the specific speech changes associated with different dysarthria types. Objective biomarkers associated with the emergence and/or progression of AOS will be useful for clinical trials, and towards theoretical accounts of the pathomechanisms underpinning impaired speech motor control. They can assist with the prediction of neuropathological type and, therefore, prediction of rate and course of disease progression and selection for intervention trials. The different trajectories and timelines for nfvPPA and lvPPA require different support strategies to be instigated, and different foci of follow-up assessments towards, for example, anticipated rapid Alzheimer-type cognitive decline in lvPPA31,80 and potential conversion to conditions, such as progressive supranuclear palsy or motor neurone disease in nfvPPA.20,81
A more developed neurobiological account of AOS can inform appropriate design of behavioural or neuromodulatory treatments for targeting or compensating for the specific impairment and give insight into mechanisms of action for effective interventions. Here, the specific impairment in control of relative timing, quantified by the PVI_WS measure, identifies candidates for promising rhythmic entrainment treatments. Rhythmic entrainment protocols that stimulate sychronisation of speech production and neural oscillatory speech networks, using either nonverbal or verbal rhythmic stimuli, have triggered marked and persistent improvement in speech fluency and accuracy in both stroke-related and progressive nonfluent aphasia and AOS.18,63,65 Little is yet known about the impact of neurodegeneration and subsequent rhythmic behavioural training on the neural oscillatory networks that underpin speech processing and production. Future exploration of these acoustic metrics for patient selection and as outcome measures, alongside longitudinal neural imaging will contribute to mechanistic accounts of disease-related loss and treatment-related preservation and restoration of motor speech skill.
Finally, while the PVI_WS metric used here was not sufficiently powerful to differentiate the two PPA groups at baseline, future work can determine testing contexts to improve sensitivity and specificity. For example, in individuals with stroke-related AOS, PVI is more discriminating in a more challenging speaking context where the stimulus words are embedded within a sentence.28
Conclusions
This study provides further compelling evidence for the critical role of the left premotor cortex in speech praxis, as well as a role of the right IFG in responding to neurological changes in the speech motor network with disease progression. Findings validate the use of the PVI_WS metric as a robust marker of disease progression and atrophic change in regions underpinning speech praxis, for individuals with nfvPPA. The analysis here was limited to observation and correlation with imaging metrics, although the longitudinal approach strengthens the main findings beyond previous cross-sectional methods. Nonetheless, methods that can address causality are encouraged. The findings justify developing more sensitive measures of rhythmic control of speech that may enable confident detection of AOS as it emerges and more sensitive tracking of intervention-related changes. Identification of such biomarkers of AOS could lead to better stratification of patients for clinical trials, informed models of disease, and insight into the mechanisms of action for behavioural and noninvasive brain stimulation interventions.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We are grateful to the research participants and their families for their continued support of our research. The authors acknowledge the support and technical assistance of the University of Sydney’s Imaging Data Service, co-developed by Sydney Imaging and Sydney Informatics Hub (ICT) at the University of Sydney. The authors wish to acknowledge Dr Zac Chatterton for his assistance writing the R code to compute PVI and word duration measurements. This code is available upon reasonable request.
Funding
This study was supported in part by funding to ForeFront, a large collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease funded by the National Health and Medical Research Council of Australia Program Grant (#1132524), Dementia Research Team Grant (#1095127), and the Australian Research Council Centre of Excellence in Cognition and its Disorders (CE11000102). R. Landin-Romero is supported by the Appenzeller Neuroscience Fellowship in Alzheimer’s Disease and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Program (CE110001021). C.T. Liang is supported by an Australian Research Training Program stipend scholarship. O. Piguet is supported by a National Health and Medical Research Council Senior Research Fellowship (GNT1103258). C. Leyton is supported by a National Health and Medical Research Council-Australian Research Council dementia development fellowship (APP1102969). J. R. Hodges, Y. Higashiyama, P.A. Monroe and K. J. Ballard report no disclosures.
Competing interests
The authors report no competing interests.
Glossary
- AOS =
apraxia of speech
- LME =
linear mixed effects
- lvPPA =
logopenic variant primary progressive aphasia
- nfvPPA =
non-fluent variant primary progressive aphasia
- PPA =
primary progressive aphasia
- PVI =
pairwise variability index
References
- 1.Ackermann H, Ziegler W.. Brain mechanisms underlying speech motor control. In: Hardcastle WJ, Laverand J, Gibbons FE, eds. The handbook of phonetic sciences, Vol. 2. Hoboken, NJ: Wiley-Blackwell; 2010:202–250. [Google Scholar]
- 2.Guenther FH.Neural control of speech. Cambridge, MA: The MIT Press; 2016. [Google Scholar]
- 3.Jurgens U.Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26(2):235–258. [DOI] [PubMed] [Google Scholar]
- 4.Maas E, Mailend ML, Guenther FH.. Feedforward and feedback control in apraxia of speech: Effects of noise masking on vowel production. J Speech Lang Hear Res. 2015;58(2):185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard KJ, Halaki M, Sowman P, et al. An investigation of compensation and adaptation to auditory perturbations in individuals with acquired apraxia of speech. Front Hum Neurosci. 2018;12:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballard KJ, Azizi L, Duffy JR, et al. A predictive model for diagnosing stroke-related apraxia of speech. Neuropsychologia. 2016;81:129–139. [DOI] [PubMed] [Google Scholar]
- 7.Ballard KJ, Savage S, Leyton CE, Vogel AP, Hornberger M, Hodges JR.. Logopenic and nonfluent variants of primary progressive aphasia are differentiated by acoustic measures of speech production. PLoS One. 2014;9(2):e89864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basilakos A, Yourganov G, den Ouden DB, et al. A multivariate analytic approach to the differential diagnosis of apraxia of speech. J Speech Lang Hear Res. 2017;60(12):3378–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy JR, Hanley H, Utianski R, et al. Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain Lang. 2017;168:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent RD, Rosenbek JC.. Acoustic patterns of apraxia of speech. J Speech Hear Res. 1983;26(2):231–249. [DOI] [PubMed] [Google Scholar]
- 11.Ballard KJ, Granier JP, Robin DA.. Understanding the nature of apraxia of speech: Theory, analysis, and treatment. Aphasiology. 2000;14(10):969–995. [Google Scholar]
- 12.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain. 2012;135(5):1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.New AB, Robin DA, Parkinson AL, et al. Altered resting-state network connectivity in stroke patients with and without apraxia of speech. Neuroimage Clin. 2015;8:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robin DA, Jacks A, Ramage AE.. The neural substrates of apraxia of speech as uncovered by brain imaging: A critical review. In: Ingham RJ, ed. Neuroimaging in Communication Sciences and Disorders. San Diego, CA: Plural Publishing, Inc; 2008:129–154. [Google Scholar]
- 15.Ziegler W.Apraxia of speech. In: Goldenberg G, Miller BL, eds. Handbook of clinical neurology. Elsevier; 2008:269–285. [DOI] [PubMed] [Google Scholar]
- 16.Josephs KA, Duffy JR.. Apraxia of speech and nonfluent aphasia: A new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol. 2008;21(6):688–692. [DOI] [PubMed] [Google Scholar]
- 17.Seckin ZI, Duffy JR, Strand EA, et al. The evolution of parkinsonism in primary progressive apraxia of speech: A 6-year longitudinal study. Parkinsonism Relat Disord. 2020;81:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry ML, Hubbard HI, Grasso SM, et al. Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain. 2018;141(6):1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amici S, Gorno-Tempini ML, Ogar JM, Dronkers NF, Miller BL.. An overview on primary progressive aphasia and its variants. Behav Neurol. 2006;17(2):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephs KA, Duffy JR, Strand EA, et al. The evolution of primary progressive apraxia of speech. Brain. 2014;137(Pt 10):2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-beta pathology in distinct variants of primary progressive aphasia. Ann Neurol. 2018;84(5):729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams-Carr KL, Bocchetta M, Neason M, et al. A case of TDP-43 type C pathology presenting as nonfluent variant primary progressive aphasia. Neurocase. 2020;26(1):1–6. [DOI] [PubMed] [Google Scholar]
- 23.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leyton CE, Piguet O, Savage S, Burrell J, Hodges JR.. The neural basis of logopenic progressive aphasia. J Alzheimers Dis. 2012;32(4):1051–1059. [DOI] [PubMed] [Google Scholar]
- 25.Ash S, Nevler N, Phillips J, et al. A longitudinal study of speech production in primary progressive aphasia and behavioral variant frontotemporal dementia. Brain Lang. 2019;194:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordella C, Quimby M, Touroutoglou A, Brickhouse M, Dickerson BC, Green JR.. Quantification of motor speech impairment and its anatomic basis in primary progressive aphasia. Neurology. 2019;92(17):e1992–e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matias-Guiu JA, Suarez-Coalla P, Pytel V, et al. Reading prosody in the non-fluent and logopenic variants of primary progressive aphasia. Cortex. 2020;132:63–78. [DOI] [PubMed] [Google Scholar]
- 28.Vergis MK, Ballard KJ, Duffy JR, McNeil MR, Scholl D, Layfield C.. An acoustic measure of lexical stress differentiates aphasia and aphasia plus apraxia of speech after stroke. Aphasiology. 2014;28(5):554–575. [Google Scholar]
- 29.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR.. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74(20):1591–1597. [DOI] [PubMed] [Google Scholar]
- 31.Leyton CE, Villemagne VL, Savage S, et al. Subtypes of progressive aphasia: Application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain. 2011;134 (Pt 10):3030–3043. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR.. Validation of the Addenbrooke's Cognitive Examination III in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2013;36(3-4):242–250. [DOI] [PubMed] [Google Scholar]
- 33.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR.. The Addenbrooke's Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21(11):1078–1085. [DOI] [PubMed] [Google Scholar]
- 34.So M, Foxe D, Kumfor F, et al. Addenbrooke's Cognitive Examination III: Psychometric characteristics and relations to functional ability in dementia. J Int Neuropsychol Soc. 2018;24(8):854–863. [DOI] [PubMed] [Google Scholar]
- 35.Morris JC.The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 36.Savage S, Hsieh S, Leslie F, Foxe D, Piguet O, Hodges JR.. Distinguishing subtypes in primary progressive aphasia: Application of the Sydney language battery. Dement Geriatr Cogn Disord. 2013;35(3-4):208–218. [DOI] [PubMed] [Google Scholar]
- 37.Ballard KJ, Djaja D, Arciuli J, James DGH, van Doorn J.. Developmental trajectory for production of prosody: Lexical stress contrastivity in children ages 3 to 7 years and in adults. J Speech Lang Hear Res. 2012;55(6):1822–1835. [DOI] [PubMed] [Google Scholar]
- 38.Duffy JR, Josephs KA.. The diagnosis and understanding of apraxia of speech: Why including neurodegenerative etiologies may be important. J Speech Lang Hear Res. 2012;55(5):S1518–S1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Praat 5.2.0.1. http://www.fon.hum.uva.nl/praat/ Accessed January 2020.
- 40.Fischl B, Sereno MI, Tootell RB, Dale AM.. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dale AM, Fischl B, Sereno MI.. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 42.Fischl B, Dale AM.. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reuter M, Schmansky NJ, Rosas HD, Fischl B.. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reuter M, Rosas HD, Fischl B.. Highly accurate inverse consistent registration: A robust approach. Neuroimage. 2010;53(4):1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuter M, Fischl B.. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lerch JP, Evans AC.. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24(1):163–173. [DOI] [PubMed] [Google Scholar]
- 47.Voevodskaya O, Simmons A, Nordenskjold R, et al. Alzheimer's Disease Neuroimaging Initiative. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer's disease. Front Aging Neurosci. 2014;6(264):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernal-Rusiel JL, Greve DN, Reuter M, Fischl B, Sabuncu MR, Alzheimer's Disease Neuroimaging Initiative. Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. Neuroimage. 2013;66:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernal-Rusiel JL, Reuter M, Greve DN, Fischl B, Sabuncu MR, Alzheimer's Disease Neuroimaging Initiative. Spatiotemporal linear mixed effects modeling for the mass-univariate analysis of longitudinal neuroimage data. Neuroimage. 2013;81:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamini Y, Yekutieli D.. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165–1188. [Google Scholar]
- 51.Benjamini Y, Hochberg Y.. Controlling the false discovery rate - A practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 52.Lieberman MD, Cunningham WA.. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4(4):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landin-Romero R, Piguet O.. Recent advances in longitudinal structural neuroimaging of younger-onset dementias. Neurodegener Dis Manag. 2017;7(6):349–352. [DOI] [PubMed] [Google Scholar]
- 54.Landin-Romero R, Kumfor F, Leyton CE, Irish M, Hodges JR, Piguet O.. Disease-specific patterns of cortical and subcortical degeneration in a longitudinal study of Alzheimer's disease and behavioural-variant frontotemporal dementia. Neuroimage. 2017;151:72–80. [DOI] [PubMed] [Google Scholar]
- 55.Kelly MH, Bock JK.. Stress in time. J Exp Psychol Hum. 1988;14(3):389–403. [DOI] [PubMed] [Google Scholar]
- 56.Echols CH.A role for stress in early speech segmentation. In: Signal to syntax: Bootstrapping from speech to grammar in early acquisition, Milton Park, Oxfordshire: Taylor and Francis Group; 1996:151–170. [Google Scholar]
- 57.Jusczyk PW, Houston DM, Newsome M.. The beginnings of word segmentation in English-learning infants. Cogn Psychol. 1999;39(3-4):159–207. [DOI] [PubMed] [Google Scholar]
- 58.James DGH, Ferguson WA, Butcher A.. Assessing children's speech using picture-naming: The influence of differing phonological variables on some speech outcomes. Int J Speech Lang Pathol. 2016;18(4):364–377. [DOI] [PubMed] [Google Scholar]
- 59.Arciuli J, Ballard KJ.. Still not adult-like: Lexical stress contrastivity in word productions of eight- to eleven-year-olds. J Child Lang. 2017;44(5):1274–1288. [DOI] [PubMed] [Google Scholar]
- 60.Ziegler W, Aichert I.. How much is a word? Predicting ease of articulation planning from apraxic speech error patterns. Cortex. 2015;69:24–39. [DOI] [PubMed] [Google Scholar]
- 61.Douaud G, Groves AR, Tamnes CK, et al. A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci U S A. 2014;111(49):17648–17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whalley K.Last in, first out? Nat Rev Neurosci. 2015;16(1):2. [Google Scholar]
- 63.Brendel B, Ziegler W.. Effectiveness of metrical pacing in the treatment of apraxia of speech. Aphasiology. 2008;22(1):77–102. [Google Scholar]
- 64.Ballard KJ, Robin DA, McCabe P, McDonald J.. A treatment for dysprosody in childhood apraxia of speech. J Speech Lang Hear Res. 2010;53(5):1227–1245. [DOI] [PubMed] [Google Scholar]
- 65.Fridriksson J, Hubbard HI, Hudspeth SG, et al. Speech entrainment enables patients with Broca's aphasia to produce fluent speech. Brain. 2012;135(12):3815–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray E, McCabe P, Ballard KJ.. A randomized controlled trial for children with childhood apraxia of speech comparing rapid syllable transition treatment and the nuffield dyspraxia programme-third edition. J Speech Lang Hear Res. 2015;58(3):669–686. [DOI] [PubMed] [Google Scholar]
- 67.McNeil M, Adams S. A comparison of speech kinematics among apraxic, conduction aphasic, ataxic dysarthria, and normal geriatric speakers, In Prescott TE, (Ed.), Clinical aphasiology, Vol. 19; 1991:279–294. [Google Scholar]
- 68.McNeil M, Caligiuri M, Rosenbek JC. A comparison of labiomandibular kinematic durations, displacements, velocities, and dysmetrias in apraxic and normal adults, In Prescott TE, (Ed.), Clinical aphasiology, Vol. 18; 1989:173–194. [Google Scholar]
- 69.Robin DA, Bean C, Folkins JW.. Lip movement in apraxia of speech. J Speech Hear Res. 1989;32(3):512–523. [DOI] [PubMed] [Google Scholar]
- 70.Walsh B, Smith A.. Basic parameters of articulatory movements and acoustics in individuals with Parkinson's disease. Mov Disord. 2012;27(7):843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duffy JR.Motor speech disorders: Substrates, differential diagnosis, and management. Maryland Heights, Missouri: Elsevier Mosby; 2019. [Google Scholar]
- 72.Hlavnička J, Čmejla R, Tykalová T, Šonka K, Růžička E, Rusz J.. Automated analysis of connected speech reveals early biomarkers of Parkinson's disease in patients with rapid eye movement sleep behaviour disorder. Sci Rep. 2017;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nevler N, Ash S, McMillan C, et al. Automated analysis of natural speech in amyotrophic lateral sclerosis spectrum disorders. Neurology. 2020;95(12):E1629–E1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartwigsen G, Saur D, Price CJ, Ulmer S, Baumgaertner A, Siebner HR.. Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proc Natl Acad Sci U S A. 2013;110(41):16402–16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marsh EB, Hillis AE.. Recovery from aphasia following brain injury: The role of reorganization. Prog Brain Res. 2006;157:143–156. [DOI] [PubMed] [Google Scholar]
- 76.Leyton CE, Landin-Romero R, Liang CT, et al. Correlates of anomia in non-semantic variants of primary progressive aphasia converge over time. Cortex. 2019;120:201–211. [DOI] [PubMed] [Google Scholar]
- 77.Landin-Romero R, Tan R, Hodges JR, Kumfor F.. An update on semantic dementia: Genetics, imaging, and pathology. Alzheimers Res Ther. 2016;8(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumfor F, Landin-Romero R, Devenney E, et al. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain. 2016;139 (Pt 3):986–998. [DOI] [PubMed] [Google Scholar]
- 79.Lam BY, Halliday GM, Irish M, Hodges JR, Piguet O.. Longitudinal white matter changes in frontotemporal dementia subtypes. Hum Brain Mapp. 2014;35(7):3547–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63(6):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumfor F, Ballard KJ, Burrell JR, Hodges JR, Piguet O.. Language indicators of change of diagnosis in nonfluent-variant primary progressive aphasia. Front Hum Neurosci. 2019;13:85–86.30890926 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared by reasonable request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with Australian legislation on the general data protection regulation and decisions by the Ethical Review Board of The University of Sydney, which should be regulated in a material transfer agreement.