Abstract
Bacteriophage phiC31 makes the enzyme integrase that allows the insertion of the phage genome into its bacterial host. The enzyme recognizes a specific DNA sequence in the phage (attP) and a different sequence in the bacteria (attB). Recombination between these sites leads to integration in a reaction that requires no accessory factors. Seminal studies carried out by M. Calos and co-workers demonstrated that the phiC31 integrase was capable of integrating plasmid with an attB site into mammalian genomes at sites that approximated the attP site. We describe the use of attB containing plasmids with insulated reporter genes for successful integration of DNA into Xenopus embryos. The method offers a way to produce transgenic embryos without manipulation of sperm nuclei using microinjection methods standard for experiments in Xenopus laevis. The method aims to allow the non-mosaic controlled expression of new genetic material in the injected embryo and compares favorably with the time normally taken to analyze embryos injected with mRNAs, plasmids, morpholinos or oligonucleotides.
INTRODUCTION
The insertion of new DNA into the genome of an organism can be mediated in a number of ways. One way is to take advantage of the mechanisms that viruses have developed to insert their genome into the genome of their hosts. Depending on the mechanism, insertion can be random (or nearly so), or occur in a more sequence-specific manner.
The protocol described here takes advantage of the mechanism used by the bacteriophage phiC31. phiC31 is a 41,491 bp temperate phage that infects Streptomyces1. phiC31 encodes an integrase that catalyzes recombination into the bacterial genome. The integrase needs two different DNA sequences, the attP site in the phage and the attB site in the bacteria. Integration results in two new sequences attR and attL. Figure 1 schematizes integration mediated by phiC31 integrase. phiC31 integrase does not require any accessory proteins to mediate integration but cannot catalyze the reverse reaction (excision) in the absence of additional factors. The minimal size of the attP and attB sites is 39 and 34 bp respectively and, although absolute conservation of the sites is not required, integration rates are reduced when sequence changes are made2-4
Figure 1.
A schematic of integrase-mediated insertion of a plasmid with antibiotic resistance (light purple) containing an attB site (light blue) into genomic DNA (dark blue) with an attP site (yellow). The integrase (dark purple) binds to the attB and attP sites and creates the hybrid attR and attL sites upon integration. In the protocol described, the attB site on the plasmid lies outside both insulator sequences (red) and the reporter gene (green).
If recombinant phiC31 integrase and a plasmid that contains the attB site are introduced into mammalian cells, the attB containing plasmid is integrated into the genome5. Because, without accessory factors, phiC31 integrase catalyzes integration and not excision the plasmids are stably inserted. Integration is not random; but because the integrase does not demand absolute conservation of the attP site multiple potential insertion sites exist in mammalian (and other organisms) genomes. Integration occurs at “pseudo attP” sites that contain between 24-56% identity to a true attP site. Subsequent work has shown that phiC31 integrase works in cells from a variety of organisms3, 6-15.
Existing methods of Xenopus transgenesis
Xenopus laevis has a long history of use in developmental biology studies. Even without integration into the genome, plasmid DNA with a promoter and a reporter gene can be injected into a Xenopus embryo and usually the promoter behaves in a temporally and tissue appropriate way. However, many of the cells that should activate the promoter do not. This leads to a mosaic pattern of expression. In addition, extrachromosomal plasmids have a limited half-life in the developing embryo. To address this problem, several methods of transgenesis have been reported.
(i) The Kroll and Amaya approach
Kroll and Amaya16 published the first broadly practiced protocol for making transgenic Xenopus using a restriction enzyme mediated insertion (REMI) approach to integrate plasmid into sperm nuclei followed by injection of the sperm nuclei into eggs. They used a lysolecithin treatment to demembranate sperm and release their nuclei. The nuclei were subsequently mixed with linearized plasmid, restriction enzyme and a cell cycling extract made from crushed eggs17. The protocol makes the nuclei somewhat permeable, produces breaks in the genomic DNA of the sperm and allows plasmid to ligate into the breaks. Enzymatic activities of the cell cycling extract assist with membrane permeability, DNA decondensation and religation of the cut DNA. Using a large bore needle, single nuclei are delivered to unfertilized eggs. Many of the injected eggs fail to develop normally, but by injecting large numbers and monitoring development transgenic embryos can be isolated. This method has been quite successful and generated many of the existing transgenic lines of both Xenopus laevis and tropicalis. However, many of the injected eggs fail to develop normally, multiple insertions are usually generated, preparation of an active cell cycling extract is required, large bore needles are needed to prevent shearing of the sperm nuclei (increasing the chances of damaging the eggs) and it can be technically challenging, especially if it is practiced infrequently
(ii) A modification of the Kroll and Amaya method.
Sparrow et al.18 modified the Kroll and Amaya method by eliminating the use of cell cycle extracts and the treatment of the sperm nuclei with restriction endonuclease. They proposed that the increase in fragility of the sperm nuclei after decondensation by the cell cycling extracts complicates the procedure and leads to fewer viable embryos. Further, they found that treatment of the nuclei with restriction enzymes was unnecessary, presumably due to a sufficient number of breaks being generated in the DNA during sperm nuclei isolation, to incorporate linear plasmid. Their protocol had similar efficiency with less reagents preparation. In addition, the sperm nuclei were less fragile allowing the use of a smaller bore needle for injection. Like the Kroll and Amaya method, multiple copies of the plasmid are generally inserted. The method they developed is used by many laboratories making transgenic Xenopus embryos.
(iii) The meganuclease approach.
Pan et al.19and Ogino et al.20 recently reported the effective use of the restriction enzyme I-SceI, a meganuclease with an 18 bp recognition site, to generate transgenic Xenopus laevis. This approach is a modification of a procedure first used in medaka fish21, which involves co-injection of I-Sce1 with I-SceI-linearized plasmid into fertilized eggs. They reported excellent survivorship, with as high as 14% to 30% of the surviving embryos expressing tissue and temporal appropriate expression of the transgene, and germline transmission. The protocol is promising and appears to be simpler and more efficient than previous methods. They report insertion of between 1 to eight copies of the transgene.
Advantages of the phiC31 integrase approach
We were the first to report the use of phiC31 integrase to mediate integration of an attB containing plasmid into the genome of Xenopus laevis22. The approach involves the co-injection of mRNA encoding phiC31 integrase with a plasmid containing an attB site, resulting in the integration of the plasmid into the Xenopus genome. Circular, primarily supercoiled, plasmid DNA should be injected, as linear plasmid is less effective for generating transgenic embryos (Allen and Weeks, unpublished).
In our trials we found that integration of the attB GFP reporter plasmid was not sufficient to achieve a fully normal pattern of expression. We attributed this problem to chromatin position effects and found that by surrounding our reporter gene with insulator sequences23, inserted DNA expression was more normal. Insulator sequences serve cells by limiting the distance effect of enhancers, and also by establishing boundaries that stop the spread of inhibitory (silencing) chromatin. Essentially, insulators allow the integration of new genetic material that contains regulatory borders. This means that enhancers or regulatory elements in the transgene will not “accidentally” affect the expression of genes around the insertion site and the transgene itself will not be activated or silenced due to its site of insertion. Trials where only one side of the reporter was protected by insulator sequences failed to consistently relieve chromatin effects (Allen and Weeks, unpublished).
Unlike the other methods mentioned above, the phiC31 integrase protocol offers facilitated insertion of DNA, and generally produces an embryo with a single copy of the inserted gene. Like the meganuclease method, the injection protocol does not require special handling of nuclei and starts with fertilized eggs, making it technically more accessible than traditional approaches.
Applications and Limitations
Our preliminary analysis suggests that although there is not a single insertion site in the Xenopus genome, insertion is not random. This observation suggests that the phiC31 integrase method described below would be better suited to studies where the goal is to insert a single regulated copy of a gene. Thus, studies aimed at analysis of a promoter to determine regulatory sequences or expression of a protein at predictable levels could profitably use this approach. Studies designed as enhancer traps, where a random pattern of insertion is required, or studies that depend upon multiple insertions, for use as a lineage marker, for example, may be less well suited for the method.
We detail the procedure using GFP as a reporter. The advantage of using GFP is that expression can be monitored without sacrificing the embryos. The disadvantage is that sufficient protein must accumulate for green fluorescence to be visible, and many GFP proteins (including the ones we use) have a rather long half-life. Studies that seek to study either promoters that are activated in early embryos, or weak promoters, could assay expression using fixed embryos and in situ hybridization, or alternative reporters like beta-galactosidase or one of the new generation of fluorescence proteins. These alternatives are not detailed here, but protocols can be found in the literature24,25
Importantly, although we expect that the transgenes produced by this method will be present in the germline, we have not yet shown that transgenic embryos generated by this technique will pass the gene along to their offspring. We note that the other three methods have all been used to create breeding lines of Xenopus.
The protocol described below co-injects mRNA encoding phiC31 integrase with a plasmid containing the attB site and an insulated GFP reporter. The goal is to generate transgenic embryos at a high enough frequency to study the behavior of the reporter gene during development.
MATERIALS
Xenopus laevis: Xenopus I (http://www.xenopusone.com) or Nasco (http://www.nascofa.com).
REAGENTS
Plasmid DNA (see Reagent setup)
Marc’s Modified Ringers Solution (1 X MMR): 100 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES at pH 7.4
Injection buffer
Tricaine (3-Aminobenzoic Acid Ethyl Ester) (Cat# A-5040, Sigma Chemical Company)
Human Chorionic Gonadotropin (Cat #CG-10, Sigma Aldrich, http://www.sigmaaldrich.com)
Bacterial growth reagents: Bacto-Yeast Extract, Bacto- Tryptone powder and Bacto-agar (Becton, Dickinson and Company (http://www.bd.com)
Antibiotics: Ampicillin and Kanamycin (Sigma Aldrich, http://www.sigmaaldrich.com/)
LB with 50μg/ml Kanamycin
LB with 50μg/ml Ampicillin
LB agar (15g/L) plates supplemented with 50μg/ml Kanamycin
LB agar (15g/L) plates supplemented with 50μg/ml Ampicillin
Plasmid Purification Kit, (e.g. Qiagen, www.Qiagen.com)
Genomic DNA Purification kit (e.g. DNeasy from Qiagen, www.Qiagen.com)
In vitro RNA transcription kit (e.g. mMessage machine from Ambion, www.ambion.com)
Redivue alpha 32P deoxycytidine (Cat# aa0005, Amersham, http://www1.amershambiosciences.com) CAUTION: Radioactive material should be handled with care to prevent contamination. Work area should be monitored pre and post use of radioactive compounds. 32P should be shielded with approved plexiglass shields.
Rediprime II DNA Labeling System (Cat # RPN1633, Amersham)
Whatmann 3MM paper
Hybond-N+ nylon membrane (Amersham)
RapidHyb solution (Ambion, www.ambion.com)
X-ray film, Kodak Biomax XAR (Eastman Kodak Co., http://www.kodak.com)
Restriction Enzymes (multiple suppliers)
Ethidium Bromide (Sigma Aldrich, http://www.sigmaaldrich.com) CAUTION: Ethidium Bromide is a mutagen; always wear gloves when handling solutions or gels containing ethidium bromide.
0.3X MMR with 3% ficoll 400
0.3X MMR
0.1X MMR
1% Tricaine (in dH2O)
0.02% Tricaine (in dH2O)
2% cysteine made in dH2O. Bring to pH 7.9 with NaOH. CRITICAL: Should be made fresh.
Injection buffer: 88 mM NaCl, 10 mM HEPES, pH 6.8, made with RNase free water.
1% Agarose, TAE gel
1X TAE
10X non-denaturing DNA loading buffer
1% Agarose, 2.2 M formaldehyde MOPS gel
1X MOPS running buffer
10X denaturing RNA loading buffer
20X SSC: 3.0 M NaCl and 0.3 M sodium citrate at pH 7.0
Depurination solution: 0.25 M HCl
Denaturation Buffer: 1.5 M NaCl with 0.5 M NaOH
Neutralization Buffer: 1 M Tris-Cl, pH 8.0 with 1.5 M NaCl
EQUIPMENT
Dissecting microscope (Nikon SMZ and a Zeiss Stemi SV 11 fluorescent dissecting microscope or similar instrument.)
Microinjection needle puller
Micromanipulator (Singer MK-1, http://www.singerinst.co.uk/mk1.html or similar instrument.)
Microinjector (inject+matic, Geneve or similar instrument.)
Glass capillary tubes : Singer Instrument Company, Glass tubes for micropipettes
0.85mm O.D. X 0.34mm I.D.
18° C incubator
Gel electrophoresis apparatus for flat bed agarose gel electrophoresis
Spectrophotometer (Nanodrop ND1000 or similar instrument)
Camera for photo documentation (SPOT Camera, Diagnostic Instruments, Zeiss Axiocam or similar instrument.)
Compound microscope with fluorescence (Zeiss Axioplan 2 or similar instrument).
UV- light trans-illuminator for viewing gels (Fotodyne, http://www.fotodyne.com or equivalent
Hybridization oven (Hybaid, Thermo corporation, http://www.thermohybaid.com or equivalent)
Reagent Setup
Plasmids
All plasmids used in this protocol have been made available by the scientists noted, but require completion of materials transfer agreements with their home institutions.
The design for integration plasmids contains, in addition to antibiotic resistance and an origin of replication for growth in E. coli, the attB site followed by two sets of insulators. A reporter gene, or other gene of interest, controlled with desired regulatory sequences is placed between the insulators. This general plasmid design is indicated in figure 1. The reporter plasmids described here are Green Fluorescent Protein (GFP)- based, to allow observation in living embryos. However, more sensitive reporters could replace GFP for some applications.
The production of phiC31 integrase, as described below, requires a plasmid with the phiC31 integrase protein encoding sequence under the control of a promoter suitable for in vitro transcription. Commonly used promoters/RNA polymerases are the T7, T3 or SP6.
Details of the sequence and construction of pET11-phiC31poly(A), and the parent vector for the integration plasmids pEGFPB2 can be found in Hollis et al.26. [These plasmids were made available through Dr. M. Calos, Stanford University, calos@stanford.edu].
The insulators used in this protocol are duplicated 250bp core elements derived from the chicken ß-globin HS4 core and plasmid pNI-CD. A description of this sequence can be found in Bell et al.27. [This plasmid was made available through Dr. Gary Felsenfeld, NIDDK, NIH, gary.felsenfeld@nih.gov].
The two reporters designed for Xenopus studies are CMV-EGFP DI attB and CL-EGFP DI attB. Both are derived from pEGFPB2 and details of construction can be found in Allen and Weeks22. Briefly a duplicated copy of the HS4 core element was inserted into the Bmg BI site between the attB site and the 5’ end of the CMV promoter in pEGFPB2 and a second duplicated copy of the HS4 core element in the same orientation as the first was inserted into the Pci I site 3’ of pEGFPB2.
CL-EGFP DI attB replaces the CMV promoter with a minimal gamma-crystallin lens promoter driving GFP reporter plasmid after cutting CMV-EGFP DI attB with Ase I and Age I. The Xenopus gamma crystallin lens promoter28 was kindly provided by Paul Krieg (University of Arizona). A 551 base-pair region of the gamma crystallin lens promoter was PCR amplified with Ase I and Age I ends and inserted into the reporter plasmid. [These plasmids can be obtained from (Dan Weeks, University of Iowa, daniel-weeks@uiowa.edu]
Determining the amount of DNA required for injection
Studies that inject expression plasmids into one-cell embryos often deliver 100 pg of DNA into each embryo. We first examine the ability of the reporter construct (promoter:GFP) to express by looking at the ability of unintegrated plasmid to generate GFP by monitoring the expression of embryos injected with 50-100pg of plasmid Although expression will be mosaic, this control establishes the transcriptional competence of the plasmid.
To avoid confusing the expression of unintegrated plasmid with integrated plasmid we nest titrate down the amount of plasmid injected to a concentration where no reporter gene activity is detected without co-injection with integrase mRNA. In the case of the CMV-GFP reporter and the Crystallin lens-GFP reporter plasmids about 5ng of plasmid is used. We would suggest that this is a good starting point for integrase-mediated insertion of DNA in Xenopus embryos.
Equipment setup
Preparing needles for injection
The needles used for injection are drawn from hollow glass capillaries. We use a one stage horizontal needle puller no longer commercially available, but any needle puller that allows the generation of an injection shaft of 5 to 10 μm should be sufficient. We break the tip of the needle to make a slightly beveled opening using a pair of Dumont No. 5 forceps. Samples are centrifuged prior to use to pellet particulates, and needles are loaded as appropriate for the microinjector available, ours allows aspiration through the tip. We measure and adjust injection volume by using calibrated micro-capillary tubes to collect and quantify the liquid that will be expelled from the needle. In order to optimize this protocol for individual laboratory use, the amount of material being injected should be known and standardized. We aim to inject a volume of 10 nl. This protocol was developed using Xenopus laevis embryos, which are roughly spherical with a diameter of 1mm. Adaptation of the technique to other embryos or cells may require adjustments to the amount of material used
Embryo injection
Investigators that have never microinjected Xenopus embryos would benefit from consulting sources such as Methods in Cell Biology Vol. 36 edited by Kay and Peng24 or Early Development of Xenopus laevis - A Laboratory Manual edited by Sive, Grainger and Harland25.
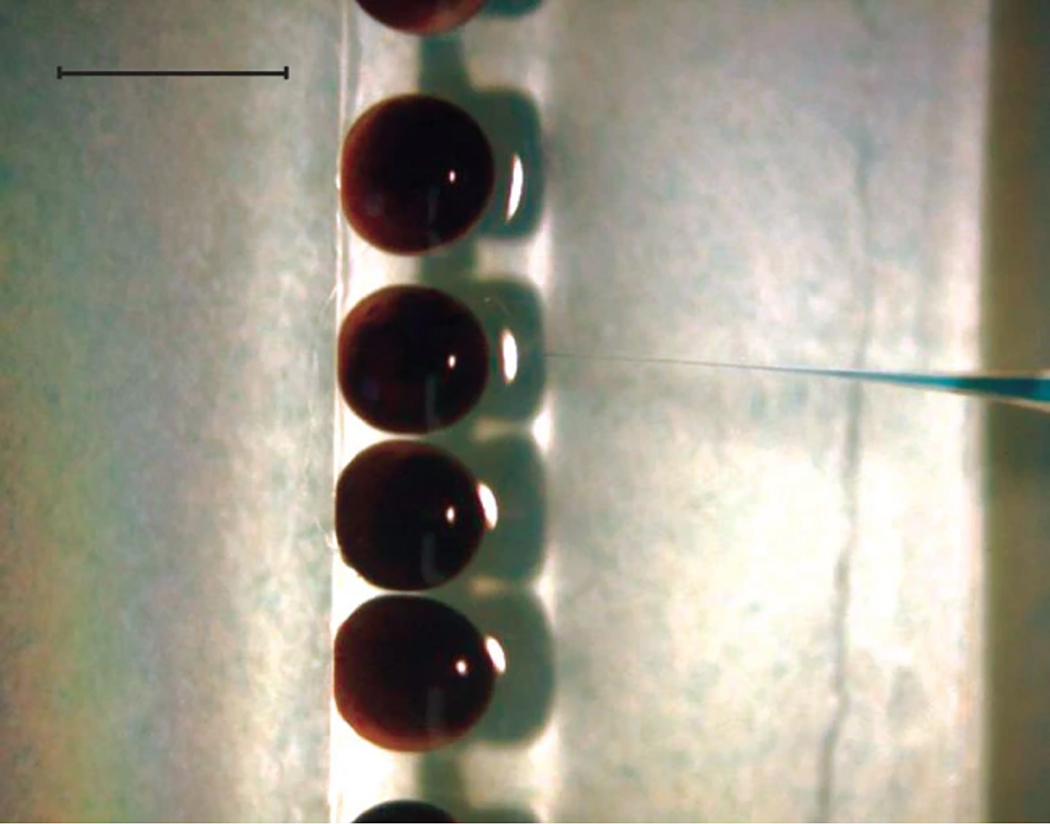
Injections are carried out while viewing the embryos through a dissecting microscope at room temperature (20-22°C); we have not investigated if temperature affects the efficiency of this method. We normally inject 100 embryos per sample and carry out experiments in triplicate. There are several methods of holding embryos for injection, but we fasten two 2 inch by 3 inch glass slides together, slightly offset, making a step. A line of embryos, minimally covered with 0.3X MMR containing 3% ficoll, is placed on the lower slide next to the step. About 60 embryos fit on a slide, and we try to orient them animal pole up. This allows rapid injection of the embryos. We routinely inject into the animal hemisphere, placing the needle near the middle of the animal hemisphere, which corresponds to the site in the embryo where egg and sperm nuclei come into contact. However, we have not rigorously tested the consequences of altering the injection site.
Fluorescence microscopy
Analyze embryos for GFP fluorescence fluorescent Zeiss dissecting microscope (Stemi SV 11) or for higher magnification (or weaker expression) with a Zeiss Axioplan 2 microscope. Light from a 100 W fluorescent source was passed through a GFP filter with an emission filter limit of 535 nanometers.
PROCEDURE
Plasmid preparation
-
1
Clone the DNA of interest into the appropriate plasmid by standard methods. For example, we replaced the ubiquitously expressed CMV promoter in CMV-EGFP DI attB with the minimal crystallin lens promoter to drive lens specific expression.
TROUBLESHOOTING
-
2
Use standard methods to individually transform the kanamycin-resistant plasmid carrying the DNA of interest and the ampicillin-resistant pET11-phiC31poly(A) plasmid (containing the integrase gene) into E. coli. Plate on appropriate selective plates and grow overnight at 37 °C.
CRITICAL STEP: In our hands, plasmids that have the reporter bordered by the HS4 insulators can be difficult to grow and are prone to rearrangements. We recommend using Stbl2 cells (Invitrogen), which are designed to grow recombination-prone plasmids. We have also used DH5-alpha cells, but observe rearrangements more frequently.
TROUBLESHOOTING
-
3
Pick individual colonies from each plate and grow 3 ml cultures at 37 °C overnight with aeration in LB supplemented with 50 μg /ml Kan or 50 μg/ml Amp.
-
4
Use 1 ml of the culture for a mini-prep of plasmid DNA to check for appropriate insertions and lack of recombination. Hold the other 1 ml at 4 °C. When the integrity of the plasmid has been verified, use this 1 ml to inoculate a larger volume (between 100 and 500 ml depending on expected need for the plasmid) of the appropriate antibiotic-containing growth medium. Grow inoculated medium at 37 °C overnight with vigorous aeration.
TROUBLESHOOTING
-
5
Harvest the bacterial cells and use to prepare plasmid DNA for injection, using the HiSpeed Plasmid purification kit (Qiagen). Typical yield is between 1-4 μg of plasmid/ml of culture media.
CRITICAL STEP: We omit the addition of RNase A to buffer P1, which is used to resuspend the bacterial pellet, as this may lead to contamination of the plasmid DNA with sufficient levels of RNase A to degrade integrase mRNA. As a result, a small amount of RNA is purified with the plasmid DNA, but it does not affect the suitability of the plasmid DNA for injection into embryos. We have not comprehensively compared different plasmid prep procedures, however, whatever plasmid purification method is used it is critical that the resulting DNA is RNase-free.
-
6
Check each large preparation of plasmid prior to use in transgenesis assays by either restriction digestion or by sequencing to ensure that no recombination has occurred. Quantitate DNA and assess purity spectrophotometrically by determination of the A260/A280 ratio (should be 1.6-1.8). PAUSE POINT: The purified plasmid DNA can be stored at -20 °C until required.
Integrase mRNA synthesis
-
7
Linearize pET11phiC31polyA in preparation for in vitro transcription by digesting with either Bam HI or Eco RI. We normally cut 5μg of DNA in 20 μl. Check an aliquot (1 or 2 μl) of the reaction on an agarose gel to ensure linearization has occurred then heat-inactivate the enzyme at 65 °C for 20 minutes. Add 1/10th volume 5 M NH4Acetate and 2 volumes of ethanol, hold at -20 °C for 15 minutes and centrifuge at 10,000 X g for 15 minutes. Decant the supernatant and resuspend pellet in RNase free TE buffer (pH 7.4).
PAUSE POINT: The linear DNA can be stored at -20 °C until required.
-
8
Synthesize integrase mRNA in vitro using the T7 mMessage Machine Kit following the manufacturer’s instructions. A total of 1 μg of digested DNA is used as a template for mRNA production.
CRITICAL STEP: The quality of the integrase mRNA is critical to the success of the method. Since this protocol includes DNase treatment of the transcription reaction mix care should be taken to remove or inactivate the DNase. We follow the LiCl precipitation protocol provided by the kit’s manufacturer. LiCl precipitates the RNA which is then resuspended in RNase free water at a concentration of 1 mg/ml.
-
9
Examine an aliquot (we routinely recover about 15μg of RNA and examine 1μg on the gel) of the product on a 1% agarose, formaldehyde MOPS gel in 1X MOPS buffer to ensure that the transcript is the appropriate size and that only one size of product is generated. We add ethidium bromide to a final concentration of 5 μg/ml after resuspending the RNA in loading buffer, and heating the sample at 75 °C for 3-5 minutes prior to loading. We typically run the gel at 90 volts for about 30 minutes. The RNA can be viewed using a UV transilluminator.
CAUTION: Ethidium bromide is a mutagen; gloves should be worn while using this compound or handling the gel. Eyes should be shielded from direct exposure to the UV light from the transilluminator.
TROUBLESHOOTING:
PAUSE POINT: The mRNA is stored at -80 °C until required. If mRNA is stored for longer than a month, samples are rechecked by repeating step 9.
ANTICIPATED RESULTS
Harvesting Xenopus eggs for injection
-
10
Hormonally-induce female Xenopus (either Xenopus I or Nasco) by injecting 500 to 1000 units of Human Chorionic Gonadotropin into the dorsal lymph sac the evening prior to egg collection.
-
11
The next morning, the cloaca on the females is swollen and eggs are often seen in the water tanks where the females are kept. Induce egg laying into a dry plastic Petri plate by firmly, but gently, applying pressure around the pelvic girdle of the frog while the legs are held apart.
-
12
Immediatesly fertilize the eggs using testes that have been surgically removed from the male. Prior to surgery, inject a lethal dose (1 ml of a 1% solution) of Tricaine into the dorsal lymph sac of the male. Hold the testes in 1X MMR, separate and crush a small piece with forceps, and drag it through the eggs
-
13
After exposure to the testes for about a minute flood the eggs with 0.1X MMR and keep at room temperature. Successful fertilization can be observed if the eggs all align with the animal pole (the pigmented side of the egg) facing up.
CRITICAL STEP: As with all experiments using Xenopus, poor egg quality leads to poor fertilization rates and development. Eggs should have relatively uniform size, smooth pigmentation of the animal hemisphere and a spherical shape. Successful fertilization is indicated by having the animal (pigmented) hemisphere orient up, and should approach 100%.
-
14
30 minutes after a successful fertilization remove the MMR solution and treat the one-cell embryos for 1 to 2 minutes with 2% cysteine, pH 7.9 to remove the jelly coats of the embryos. Successful removal of the jelly coat allows close packing of the embryos.
-
15
Pour off the cysteine solution and wash the embryos in 3 changes of 0.3X MMR.
CRITICAL STEP: Care should be taken to remove remaining cysteine, as it can damage the developing embryos.
-
16
Prior to injection, remove the 0.3X MMR and replace with 0.3X MMR, 3% ficoll.
Embryo injection
-
17
Inject single-cell embryos, as described in Equipment setup, with either: 10 nl of water (mock injection); 5 pg of reporter plasmid; or 5 pg of reporter plasmid and 1 ng of ϕC31 integrase mRNA. The concentration of nucleic acids should be determined using a spectrophotometer. All material to be injected should be resuspended in injection buffer at a concentration that allows an injection volume of approximately 10nl.
CRITICAL STEP: In our hands, the highest success rates are achieved using 1 ng of integrase RNA/injection. Reduction of integrase mRNA leads to decreased integration in a dose dependent manner. See Reagent setup for comments on determining the amount of plasmid to use. The 5 pg level suggested was determined for CMV-EGFP DI attB.
Monitoring development
-
18
Allow the injected embryos to develop at 18 °C in 0.3X MMR, 3% ficoll until stage 6 or 7, (about 6-8 hours after fertilization) and then transfer to 0.3X MMR. The rate of development is temperature dependant. Embryonic developmental stages are determined using criteria described in the “Normal Table of Xenopus laevis (Daudin)” 29. Examine at least twice daily and remove damaged embryos.
-
19
Monitor the embryos at least twice a day for expression of the GFP reporter using a fluorescent microscope set up to detect green fluorescence (see Equipment setup).
-
20
Once expression is detected, photograph the embryos using a fluorescent microscope with a digital camera. Once embryos begin to move, anesthetize them with 0.02% Tricaine to obtain clear photographs.
CRITICAL STEP: It is critical to include non-injected, mock injected and plasmid-only controls during the screening process. Xenopus embryos auto fluoresce at the same wavelengths that excite GFP (see figure 2d and 2j). This is especially evident in the gut, and gall bladder, but low levels of background fluorescence is evident throughout the embryos. Photo documentation requires that identical illumination and exposure be used for image capture. When images are refined in programs like Adobe Photoshop control images and experimental images should be processed together.
Figure 2.
Injection set-up. Single-cell embryos are aligned along a glass slide and injectd with approximately 10 nl of solution. To allow easier visualization of the needle, it was loaded with an aqueous solution of bromophenol blue. Scale bar, 1mm.
ANTICIPATED RESULTS
TROUBLESHOOTING
Confirmation of plasmid integration by Southern blotting genomic DNA
-
21
Isolate 30-50 μg of DNA from a single stage-46 tadpole using Qiagen DNeasy Tissue Kits, following the manufacturers instructions.
CRITICAL STEP: Because single embryos are analyzed it is easier to wait until at least stage 46 to isolate DNA. This allows the accumulation of enough DNA to carry out the assay.
-
22
Digest 10 μg of genomic DNA with Hind III (or any enzyme that cleaves the integrated plasmid at a single site) overnight at 37°C, according to the enzyme manufacturer’s instructions.
-
23
Run the digested DNA on a 1% agarose gel (TAE) in 1X TAE buffer. To ensure separation we use a gel that is 12 × 14 cm and run it at 90 volts for about 5 hours or until the dye front is 2-4 cm from the bottom of the gel. We routinely add ethidium bromide directly to the agarose gel to a final concentration of 0.5-1.0 μg/ml. Photograph the completed gel illuminated on a UV transilluminator.
CRITICAL STEP: Include control lanes with linearized plasmid, size standards and DNA isolated from non-injected embryos and embryos injected with integration plasmid alone.
-
24
Transfer the DNA onto a positively charged Hybond membrane (using standard Southern Blot protocols).
-
25
The plasmid used to make the transgene is also used to make a radioactive probe. We use the Rediprime II Random Prime Labeling System (Amersham) and 32P- dCTP following the manufacturer’s instructions.
CAUTION: Radioactive material should be handled with care to prevent contamination. Work area should be monitored before and after use of radioactive compounds. 32P should be shielded with approved plexiglass shields.
-
26
Rinse the filter in 5X SSC then pre-hybridize in Ambion RapidHyb at 45° C for 60 minutes with agitation.
-
27
Boil the probe for 5 minutes at 100°C and immediately place on ice. Add probe to the hybridization solution containing the filter and hybridize overnight at 45°C.
CAUTION: When boiling the probe, secure the lid with a lid guard or poke a small hole in the top of the tube to avoid accidental opening of the tube due to pressure build up.
-
28
After hybridization, wash the blot at 68°C to a stringency of 0.1X SSC, 0.1% SDS. Start with a rinse using 2X SSC with 0.1% SDS, followed by two 100 ml washes using 2X SSC with 0.1% SDS (5 minutes each), two 100 ml washes using 1X SSC with 0.1% SDS (10 minutes each), and four 100 ml washes using 0.1X SSC with 0.1% SDS (60 minutes each).
-
29
Washed blots should be sandwiched in plastic wrap. Use the blot to expose Kodak XAR film in a cassette with intensifying screens at -80°C. Develop the first exposure 12-24 hours later. If the signal is weak, an additional exposure lasting 48-120 hours may be needed. An example of the data expected can be seen in Allen and Weeks (2005)22
ANTICIPATED RESULTS
TIMELINE
Preparation for injection
Steps 1-6: Cloning; typically 1-2 weeks. Once the clone has been verified, it can be grown and the DNA extracted in 4 days.
Steps 7-9: Integrase mRNA production; 1 day (can be performed in parallel with steps 1-6)
Injection
Day 1: Step 10: Stimulation of oogenesis; Injection of female frogs with hormone takes less than an hour. Although there is some variability, frogs injected at 10 P.M. and housed at 18-20°C are normally ready to lay eggs by 8 A.M.
Day 2: Step 11-16: Harvesting Xenopus eggs; Eggs are harvested every 60 to 90 minutes until the frog no longer lays eggs. Typically 5 to 6 clutches of 500 to 1000 eggs are obtained/frog.
Step 17: Embryo injection; Injections take about 30 minutes/ group of embryos. 5 or 6 different samples are injected into 100 embryos so each group has 500-600 embryos.
Days 2- 6: Steps 18-20: Monitoring development: 5 days (up to stage 46)
Validation of transgene integration
Steps 21-29: Southern blotting; typically 4-8 days.
ANTICIPATED RESULTS
Steps 9: Bam HI cut plasmid gives a product about 1890 nucleotides long terminated by run-off, Eco RI cut plasmid gives a product about 1954 nucleotides long terminated by the T7 terminator sequence in pET11 phiC31polyA.
Step 20: We have observed survival rates of embryos injected with integrase mRNA and attB containing plasmid to be similar to embryos injected with plasmid alone.
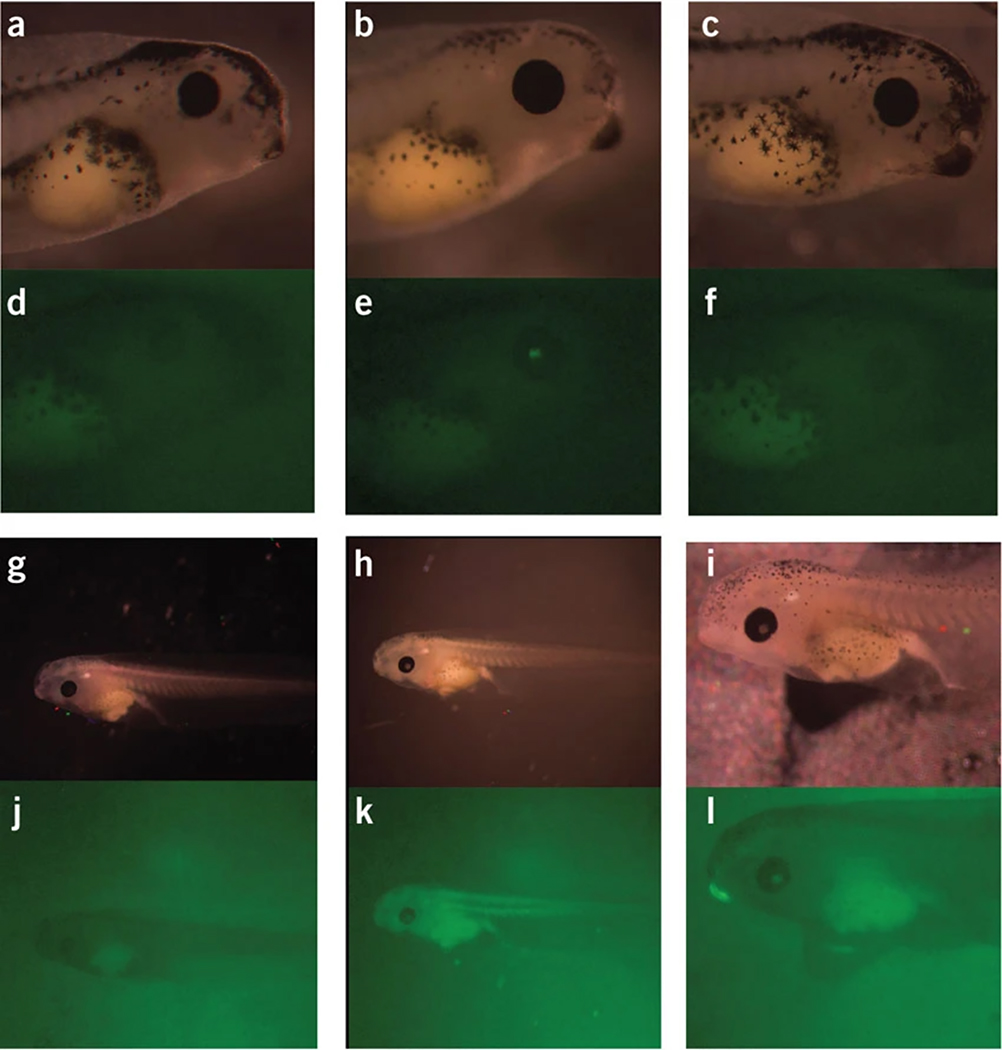
We start to detect green fluorescence with the insulated CMV-GFP construct at about stage 22 using a fluorescent Zeiss dissecting microscope (Stemi SV 11) with GFP filter set and a 100 W Atto Arc fluorescent light source. For the insulated crystallin lens plasmid we can detect glow in the eyes at about stage 34. Figure 2 shows typical examples of embryos injected with integrase mRNA and either CMV-EGFP DI attB or CL-EGFP DI attB (figure 2e and 2k). Figure 2f shows a failed integration after injection with integrase and the or CL-EGFP DI attB, figure 2l shows an embryo injected with integrase and CMV-EGFP DI attB that did not show uniform expression.
One difference between this method and some other commonly used transgenic techniques are that we tend to see single insertions. The practical result is that GFP visualization is a bit later than methods where multiple inserts are generated. Earlier or more sensitive detection can be achieved using digital cameras. We have captured color brightfield and GFP embryo pictures from our Zeiss fluorescent dissecting microscope prior to the time when GFP fluorescence was clearly visible through the ocular lenses. Even greater sensitivity was accomplished using a Zeiss Axioplan 2 microscope with a black and white Zeiss Axiocam. Images were oriented and processed with Adobe Photoshop 7.0.
Using the plasmids described, the CMV-GFP reporter, successfully integrated with insulators, expressed ubiquitously in the embryo. The crystallin lens-GFP reporter expressed only in the lens of the eye. Unpublished trials from our lab indicate that other tissue specific reporters are also appropriately expressed.
Step 20, 29: When embryos were analyzed at stage 46 between 25-35% of the embryos were transgenic as judged both by expression of GFP, and by analysis of single embryos by genomic Southern blots.
TROUBLESHOOTING:
Step 1: The promoter or sequence of interest is very large.
We have not established an upper limit for plasmids that can be integrated. So far we have been able to successfully integrate plasmids just over 20 Kbp.
Step 1 and 2: The reporter plasmids won’t grow.
The reporter plasmids are slow growers and carry Kan resistance. Plating on ampicillin plates (more commonly used by labs) will not yield transformants.
Step 4: The plasmids have unexpected restriction digestion pattern.
Recombination may have occurred between or within insulators. Pick and check additional colonies or transform into an E. coli strain less supportive of plasmid rearrangement.
Step 9: Failure to detect integrase RNA after synthesis.
This could occur because insufficient template was used or if there is RNase contamination. Repeat step 8after cleaning and checking plasmid. Contaminating RNase can be removed by proteinase K treatment, or phenol:cholorform extraction followed by ethanol precipitation, Details for these protocols can be found in methods manuals previously referenced [Sive, 2000 #36]24
Step 20: All the embryos appear to have mosaic expression.
Mosaic expression at high concentrations of plasmid is normal, but may mask the expression of integrated plasmid. Titrate levels of plasmid injected in the absence of integrase until the GFP reporter is no longer seen (see Reagent setup)
If mosaic reporter (GFP) expression is detected when the reporter plasmid is co-injected with integrase then you should check your plasmid preparation (steps 1-6) to make sure the insulators have not rearranged. It is prudent to confirm plasmid sequence/restriction digestion pattern before carrying out an injection study and to use E.coli strains for plasmid growth that are designed to reduce recombination.
Step 20: Embryos co-injected with reporter plasmid and integrase look no different than those injected with reporter plasmid alone.
If co-injection with integrase mRNA does not allow you to detect expression of the reporter then the problem may be with the quality of the integrase RNA (Step 8-9) or contamination with RNase (Step 5). A quality check on the mRNA might include examining it on a denaturing agarose gel to ensure that you have a single band of the appropriate size, or using it to prime in vitro translation of the integrase protein. You can check for RNase contamination by incubating the mRNA with the injection solution or with the reporter plasmid DNA for several hours at room temperature followed by gel analysis for RNA degradation.
Step 20: The CMV-GFP and CL-GFP reporters give the expected results, embryos injected with GFP under the control of the promoter we are interested in studying don’t glow or seem active for longer than we anticipated.
The CMV promoter is very robust, and the CL promoter expresses in an easily visualized spot- the lens of the eye. Because copy number is single or low for integrated reporters, promoters that are not too active may not generate enough GFP to see immediately or without the help of digital photographic capture. Similarly, the GFP constructs described here make protein with a fairly long half-life (perhaps as long as 80 hours). Assays that require more temporally exact output may need to switch to reporters that are less stable, or more sensitive than the GFPs used in these vectors. An alternative is to fix embryos and probe for GFP mRNA using whole mount in situ hybridization.
Step 20: Some of the embryos have the expected pattern of expression; some don’t seem to express the reporter at all, and a few look mosaic.
We typically see about 25-35% of the embryos with the expected pattern. Most of the rest have no visible reporter gene expression, but there are often a few that have aberrant expression. So far this is part of the background noise in the protocol.
Figure 3.
Photo documentation of embryos. All images were captured using a SPOT camera and a fluorescent Zeiss dissecting microscope (Stemi SV 11) with GFP filter set and a 100 W Atto Arc fluorescent light source. Panels a-f represent stage 42 embryos from an experiment testing CL-EGFP DI attB. Panels a-c are brightfield images, Panels d-f show fluorescence images. Panels a and d show a non-injected embryo. Panels b and e show an embryo positive for integration that was injected with 5 pg of CL-EGFP DI attB + 1 ng integrase mRNA. Panels c and f show an embryo negative for integration that was injected with 5 pg of CL-EGFP DI attB + 1 ng integrase.
Panels g-l are stage 46 embryos from an experiment testing CMV-EGFP DI attB. Panels g-i show brightfield images. Panels j-l show fluorescence images. Panels g and j show non-injected embryos. Panels h and k show an embryo positive for integration that was injected with 5 pg of CMV-EGFP DI attB + 1 ng integrase mRNA. Panels i and l show an embryo that is not uniformly expressing GFP that was injected with 5 pg of CMV-EGFP DI attB + 1 ng integrase mRNA.
Figure 4.
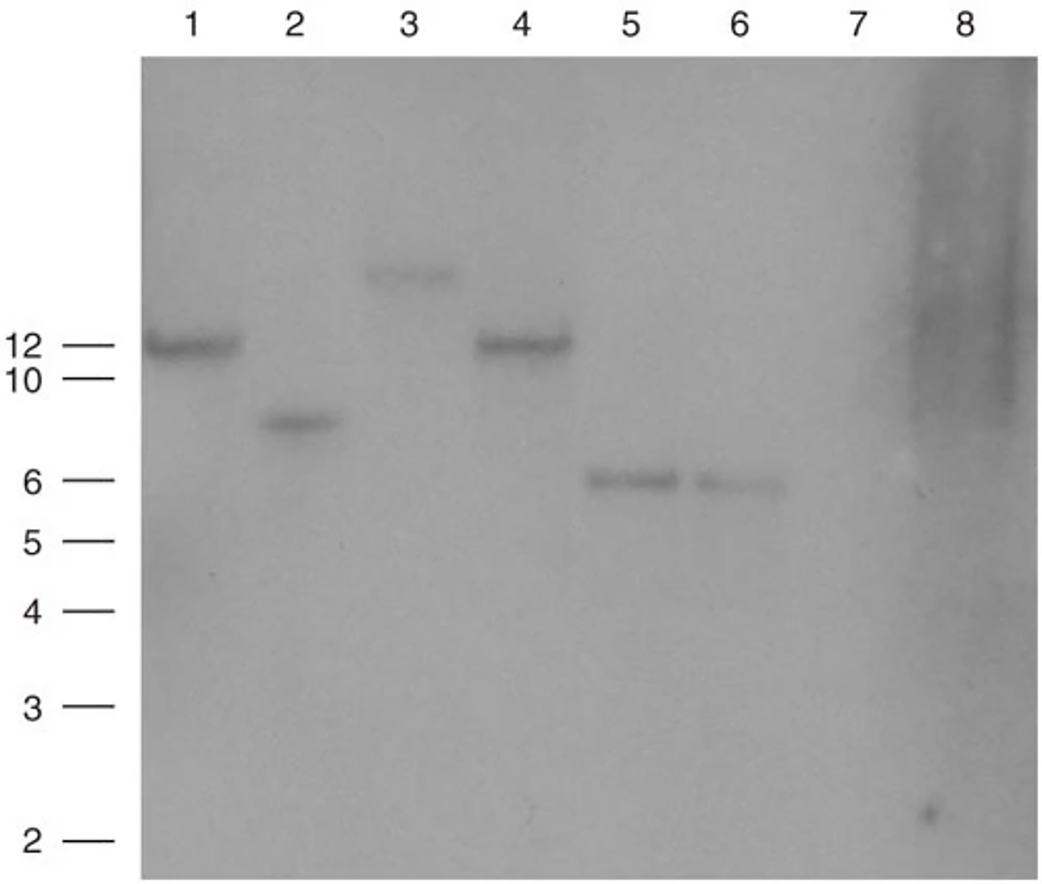
Southern blot analysis of experimental embryos indicates the insertion of CL-EGFP DI attB into the embryonic genome. Size markers in kbl are indicated to the left of the Southern blot. A total of 2.5-5 μg of genomic DNA was digested with BamHI and transferred to a positively charged nylon membrane. The membrane was probed with 32P –labeled EGFP. Lanes 1-4 contain DNA from embryos transgenic with the insulated CL-EGFP DI attB. These embryos were injected with plasmid and integrase RNA, expressed GFP in their eyes and were harvested at Stage 46. Lane 5 and 6 contain 10 pg and 1 pg of linearized CL-EGFP attB. Lane 8 contains DNA from a Stage-46 embryo injected with 5 pg of CL-EGFP DI attB alone and did not show GFP expression in its eyes.
References
- 1.Smith MC, Burns RN, Wilson SE, Gregory MA. The complete genome sequence of the Streptomyces temperate phage straight phiC31: evolutionary relationships to other viruses. Nucleic Acids Res. 1999;27:2145–55. doi: 10.1093/nar/27.10.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorpe HM, Wilson SE, Smith MC. Control of directionality in the site-specific recombination system of the Streptomyces phage phiC31. Mol Microbiol. 2000;38:232–41. doi: 10.1046/j.1365-2958.2000.02142.x. [DOI] [PubMed] [Google Scholar]
- 3.Thyagarajan B, Olivares EC, Hollis RP, Ginsburg DS, Calos MP. Site-specific genomic integration in mammalian cells mediated by phage phiC31 integrase. Mol Cell Biol. 2001;21:3926–34. doi: 10.1128/MCB.21.12.3926-3934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groth AC, Olivares EC, Thyagarajan B, Calos MP. A phage integrase directs efficient site-specific integration in human cells. Proc Natl Acad Sci U S A. 2000;97:5995–6000. doi: 10.1073/pnas.090527097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thyagarajan B, Guimaraes MJ, Groth AC, Calos MP. Mammalian genomes contain active recombinase recognition sites. Gene. 2000;244:47–54. doi: 10.1016/s0378-1119(00)00008-1. [DOI] [PubMed] [Google Scholar]
- 6.Thomason LC, Calendar R, Ow DW. Gene insertion and replacement in Schizosaccharomyces pombe mediated by the Streptomyces bacteriophage phiC31 site-specific recombination system. Mol Genet Genomics. 2001;265:1031–8. doi: 10.1007/s004380100498. [DOI] [PubMed] [Google Scholar]
- 7.Olivares EC, et al. Site-specific genomic integration produces therapeutic Factor IX levels in mice. Nat Biotechnol. 2002;20:1124–8. doi: 10.1038/nbt753. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz-Urda S, et al. Stable nonviral genetic correction of inherited human skin disease. Nat Med. 2002;8:1166–70. doi: 10.1038/nm766. [DOI] [PubMed] [Google Scholar]
- 9.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–82. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalberg TW, Genise HL, Vollrath D, Calos MP. phiC31 integrase confers genomic integration and long-term transgene expression in rat retina. Invest Ophthalmol Vis Sci. 2005;46:2140–6. doi: 10.1167/iovs.04-1252. [DOI] [PubMed] [Google Scholar]
- 11.Ginsburg DS, Calos MP. Site-specific integration with phiC31 integrase for prolonged expression of therapeutic genes. Adv Genet. 2005;54:179–87. doi: 10.1016/S0065-2660(05)54008-2. [DOI] [PubMed] [Google Scholar]
- 12.Held PK, et al. In vivo correction of murine hereditary tyrosinemia type I by phiC31 integrase-mediated gene delivery. Mol Ther. 2005;11:399–408. doi: 10.1016/j.ymthe.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Thyagarajan B, Calos MP. Site-specific integration for high-level protein production in mammalian cells. Methods Mol Biol. 2005;308:99–106. doi: 10.1385/1-59259-922-2:099. [DOI] [PubMed] [Google Scholar]
- 14.Bertoni C, et al. Enhancement of plasmid-mediated gene therapy for muscular dystrophy by directed plasmid integration. Proc Natl Acad Sci U S A. 2006;103:419–24. doi: 10.1073/pnas.0504505102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalberg TW, et al. Integration specificity of phage phiC31 integrase in the human genome. J Mol Biol. 2006;357:28–48. doi: 10.1016/j.jmb.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 16.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–83. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 17.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 18.Sparrow DB, Latinkic B, Mohun TJ. A simplified method of generating transgenic Xenopus. Nucleic Acids Res. 2000;28:E12. doi: 10.1093/nar/28.4.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan FC, Chen Y, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev Dyn. 2006;235:247–52. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- 20.Ogino H, McConnell WB, Grainger RM. Highly efficient transgenesis in Xenopus tropicalis using I-SceI meganuclease. Mech Dev. 2006;123:103–13. doi: 10.1016/j.mod.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Thermes V, et al. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev. 2002;118:91–8. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 22.Allen BG, Weeks DL. Transgenic Xenopus laevis embryos can be generated using phiC31 integrase. Nat Methods. 2005;2:975–9. doi: 10.1038/nmeth814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West AG, Gaszner M, Felsenfeld G. Insulators: many functions, many mechanisms. Genes Dev. 2002;16:271–88. doi: 10.1101/gad.954702. [DOI] [PubMed] [Google Scholar]
- 24.Kay BK, Peng HB, editors. Xenopus laevis: Practical uses in Cell and Molecular Biology. Academic Press, Inc.; San Diego, CA: 1991. [Google Scholar]
- 25.Sive HL, Grainger RM, Harland RM, editors. Early Development of Xenopus laevis:A Laboratory Manual. Cold Spring Harbor Press; Cold Spring Harbor, N.Y.: 2000. [Google Scholar]
- 26.Hollis RP, et al. Phage integrases for the construction and manipulation of transgenic mammals. Reprod Biol Endocrinol. 2003;1:79. doi: 10.1186/1477-7827-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell AC, West AG, Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–96. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- 28.Offield MF, Hirsch N, Grainger RM. The development of Xenopus tropicalis transgenic lines and their use in studying lens developmental timing in living embryos. Development. 2000;127:1789–97. doi: 10.1242/dev.127.9.1789. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) : a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland Pub.; New York: 1994. [Google Scholar]