Abstract
With the advent of better molecular characterization of Spitz melanocytic neoplasms, there has been increasing effort to better understand and describe the relationships between specific driver fusion and/or mutations with the clinical and histomorphological characteristics of the lesions. Structural rearrangements in MAPK genes have recently been noted to be important in Spitz neoplasms. Only very few reports however have described in detail melanocytic tumors with in frame deletions in MAP2K1. Cases in the literature with this aberration have been described as having a diagnosis of Spitz, DPN or PEM. In this study, we describe a cohort of 6 cases with MAP2K1 activating in frame deletions. The morphologic spectrum of the cases was broad. Common features of these cases include Spitzoid cytomorphology (5/6) cases, prominent melanin pigmentation (4/6) cases and DPN-like plexiform architecture (3/6) cases. The diagnoses at the time of clinical care of these cases included, nevus of Reed (1/6), desmoplastic Spitz tumor (1/6), Bapoma (1/6), deep penetrating melanocytic nevus (2/6) and melanoma (1/6). Clinical follow up was available in 3 of the 6 cases. None of the patients had a tumor recurrence. This builds on the growing literature to help expand the spectrum of changes associated with spitzoid melanocytic neoplasms.
Keywords: MAP2K1, Spitz, Melanoma, Genomics, Next Generation Sequencing
INTRODUCTION
The most common initiating event driving the formation of Spitz neoplasms are genomic rearrangements resulting in either chimeric fusion proteins or constitutively activated truncated versions of an oncogene.1,2. Multiple studies have demonstrated that some of these genomic rearrangements can produce specific and recognizable morphologies depending on the involved oncogene.2–10 Chimeric fusions or truncations involving MAP3K8 have been shown to have some characteristic morphologic features including a tendency for epithelioid cytomorphology, often with high grade nuclear atypia, and more frequent pigmentation.3 In frame deletions resulting in a truncated and constitutively activated MAP2K1 proto-oncogene have also been noted to have Spitz cytomorphology in some cases, features of pigmented epithelioid melanocytoma (PEM) in some cases or to be a an initial activating event in deep penetrating nevi (DPN) in other cases.11 In fact, in the initial report describing the genetic basis of DPNs, Yeh et all describe a two-step process for the development of DPN. As compared to blue nevi, they lack GNAQ and GNA11 mutations but instead typically arise from BRAF or in 1/3 of cases from MAP2K1 activating mutations in combination with subsequent activating mutations in beta-catenin (CTNNB1).11
In this study, we report the clinical, histologic and molecular findings in 6 MAP2K1 deletion melanocytic neoplasms which did not harbor detectable activating mutations in beta-catenin and despite that displayed a distinct Spitz or DPN-like morphology. We also provide a systematic analysis comparing many of the morphologic features of the MAP2K1 cases in this study to a larger cohort of Spitz neoplasms of other fusion types to determine which morphologic features are statistically more likely in MAP2K1 rearranged melanocytic neoplasms. Further, we review the literature on what has been reported to date regarding the clinical, morphologic and molecular findings in MAP2K1 rearranged melanocytic neoplasms.
METHODS
Case Selection and Genomic Sequencing
Study approval and waiver of consent for use of archived tissue were obtained through the Northwestern Institutional Review Board. A retrospective review of our dermatopathology database was conducted to identify melanocytic neoplasms with in frame deletions involving MAP2K1. We identified 6 cases matching the inclusion criteria. One of these cases has been partially described in a prior report3; the other 5 cases are unreported. A control set of 105 Spitz neoplasms with known genomic rearrangement were used as a control set for comparison of morphologic features with the group of MAP2K1 cases. “Spitzoid” morphology was identified according to the World Health Organization Pathology and Genetics of Tumors of the Skin (4th edition) and other relevant literature. Each case was then sequenced using the Tempus xO platform and variant-calling pipelines for a targeted 1711 DNA gene panel and whole transcriptome mRNA and RNA data was used for fusion validation and differential expression.12,13
Tumor Classification and Clinicopathologic Features
The clinical features including age, sex and location of the tumors were summarized from the medical record. Morphologic features were assessed by a board certified dermatopathologist experienced in the assessment of melanocytic tumors who was blinded to the diagnosis and fusion classification. The following morphologic features were evaluated: silhouette (plaque, wedge or nodular), cytology (epithelioid, spindled or both), nuclear atypia (mild, moderate or severe), pigmentation (absent, focal, or extensive), host inflammatory reaction (absent, non-brisk, or brisk), cell size (small, intermediate, large), mitotic figures per mm2, and for the absence or presence of Kamino bodies, maturation, ulceration, epidermal hyperplasia, plexiform growth, epithelioid sheets, pagetosis (upward scatter of single intraepidermal melanocytes), and nesting in the adnexa. Additionally, for the MAP2K1 in frame deletion cases, the presence or absence of convergence of nests around the skin adnexa and neurovascular bundle and the presence or absence of Spitz cytomorphology were assessed (Table 2).
Table 2:
Histologic Info of Cases with MAP2K1 deletions
| Histologic | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Epidermal o r Dermal | Dermal | Compound | Compound primarily dermal | Compound primarily dermal | Compound primarily dermal | Dermal |
|
Convergence of Nests Around the Adnexa and Neurovascular Bundle
Absent Present |
Present | Absent | Present | Present | Present | Absent |
|
Spitzoid cytomorphology
Absent Present |
Present | Present | Absent | Present | Present | Present |
|
Silhouette
Plaque Wedge Nodular Exophytic |
Nodular | Plaque | Nodular | Nodular | Nodular | Nodular |
|
Cytology
Epithelioid Spindled Both |
Both | Spindled | Both | Both | Both | Epithelioid |
|
Nuclear Atypia
Mild Moderate Severe |
Severe | Moderate | Severe | Moderate | Severe | Moderate to Severe |
|
Kamino body
Absent Present |
Absent | Absent | Absent | Absent | Absent | Absent |
|
Pigmentation
Absent Focal Extensive |
Extensive | Extensive | Extensive | Focal | Extensive | Focal |
|
Maturation
Absent Partial Present |
Absent | Absent | Absent | Present | Absent | Partial |
|
Ulceration
Absent Present |
Absent | Absent | Absent | Absent | Absent | Absent |
|
Inflammatory Reaction
Absent Non-Brisk Brisk |
Brisk | Brisk | Brisk | Brisk | Brisk | Non-Brisk |
|
Epidermal Hyperplasia
Absent Present |
Present | Present | Absent | Absent | Absent | Absent |
|
Plexiform
Absent Present |
Present | Absent | Present | Absent | Present | Absent |
|
Epithelioid Sheet
Absent Present |
Absent | Absent | Absent | Absent | Absent | Absent |
|
Pagetosis
Absent Present |
Absent | Absent | Absent | Present | Absent | Absent |
|
Cell Size
Small Intermediate Large |
Large | Medium | Large | Medium | Large | Large |
|
Nesting Adnexa
Absent Present |
Absent | Absent | Absent | Absent | Absent | Absent |
|
Mitotic index (per mm2)
Mean Range |
1/mm2 | Absent | 2/mm2 | 2/mm2 | 1/mm2 | <1/mm2 |
|
Lobulated Nests
Absent Present |
Absent | Absent | Absent | Absent | Absent | Absent |
Mild nuclear atypia was defined as a slightly larger nucleus than conventional nevomelanocytes. Moderate atypia was defined as a nuclear size similar to the size of keratinocytes with a hyperchromatic nuclear membrane, visible nucleolus, and variable chromatin quality. Severe nuclear atypia was defined as a nuclear size larger than keratinocytes with a hyperchromatic nuclear membrane, prominent and/or multiple nucleoli, and coarse chromatin. For host inflammatory reaction, a brisk response was defined as a diffuse infiltration of lymphocytes across the entire base of the tumor; a non-brisk response was defined as a focal infiltration of lymphocytes that does not cover the entire base.14 For cell size, the size of melanocytes was compared to the basal keratinocytes. Cells about the size of basal keratinocytes were considered small, those moderately larger than basal keratinocytes were intermediate in size and cells nearly twice the size of basal keratinocytes were considered large. Clinical information including age, gender and site of tumor was also included for analysis.
Statistical Analysis
All statistical analyses were performed in R Studio v1.2.5001 to compare morphologic features across the groups of Spitz neoplasms. Fisher’s exact test or Chi square test was used to compare associations in categorical variables. Student’s t-test was used to compare mean values. A p value of < 0.05 was considered statistically significant. All tests were two-sided.
RESULTS
Clinical Findings in MAP2K1 in frame deletion Melanocytic Neoplasms
Among 6 MAP2K1 deletions, 1 was diagnosed as a pigmented spindle cell nevus of Reed, 1 as atypical Spitz tumor, 1 as a BAP1 Melanocytic Tumor, 2 as atypical deep penetrating nevi, and 1 as a melanoma (Breslow depth 2.5mm, non-ulcerated) with deep penetrating nevus-like features. The average patient age was 28 with a range from 10 – 40 years old (Table 1). There were 2 males and 4 females. The lesions were mostly on the extremities (4/6). One lesion was on the scrotum (1/6) and one was on the clavicle (1/6). The clinical differential diagnoses were heterogenous and ranged from rule out Spitz (1/6), rule out dysplastic nevus or irritated nevus (2/6), to rule out melanoma in situ or melanoma (3/6).
Table 1:
Clinical Information on Cases with MAP2K1 deletion
| Case | Age | Gender | Location | Gross | Ddx | Clinical Followup |
|---|---|---|---|---|---|---|
| 1 | 34 | F | L calf | 7×7 mm smooth two-toned papule | r/o melanoma | WLE and SLNBx which was negative, no recurrence to date |
| 2 | 30 | M | L great toe | Not available | r/o melanoma | Consult case, re-excision recommended |
| 3 | 10 | F | L arm | Not available | r/o Spitz | Consult case |
| 4 | 27 | F | L elbow | 6 mm slightly keratotic flat topped papule | r/o SK vs MIS | Re-excised, no sequela |
| 5 | 40 | F | R superior clavicle | Not available | Irritated nevus | Consult case |
| 6 | 29 | M | Anterior scrotum | Not available | r/o dysplastic | Consult case |
Follow up was available for 3 of 6 patients. One patient was diagnosed with melanoma (Case 1, Figure 1) and treated with wide local excision and sentinel lymph node biopsy. She has had continued follow up and 5 years post operatively she has had no recurrence or distant spread. The other patients were treated only with simple re-excision without recurrence. There were no metastases, recurrences or fatal outcomes.
Figure 1:
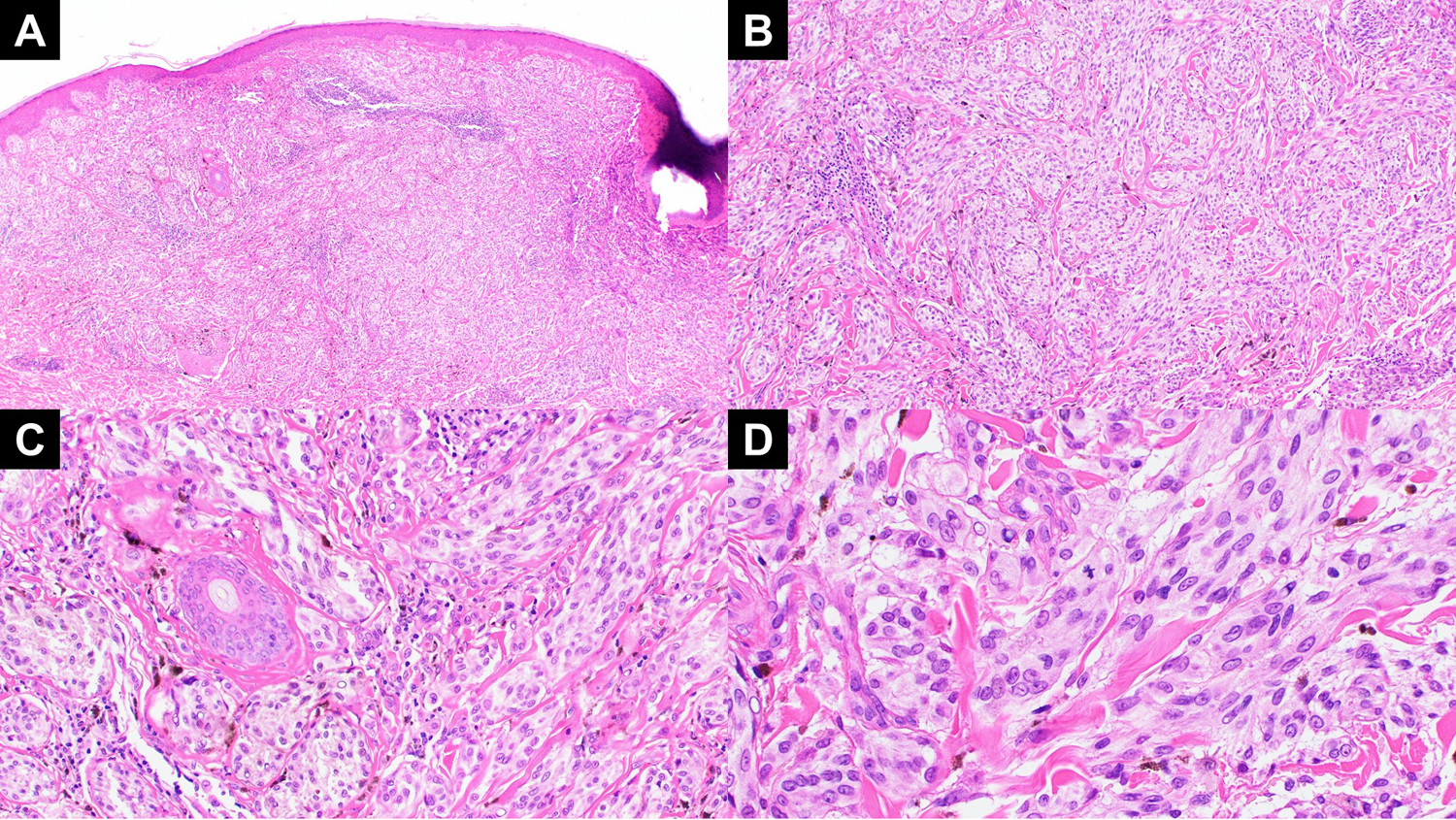
(A) A low power view of case 1 showing a nodular melanocytic lesion with a plexiform pattern. This tumor had a BRAF and tert promoter mutation in addition to the MAP2K1 in frame deletion. (B) The dermal component shows expansile growth and lack of maturation with descent with pigment extending to the base. (C) On higher power, clustering around the adnexa is notable. (D) Higher magnification view demonstrates epithelioid but not particularly Spitzoid cytomorphology of the melanocytes and the high degree of atypia with mitotic activity.
Morphologic Findings in MAP2K1 in frame deletion Melanocytic Neoplasms
Although there was significant variability in the morphologic features, there were some common findings. Among the 6 cases, 5 had Spitzoid cytomorphologic features with large cells with vesicular nuclei and abundant cytoplasm. The only exception was the case that had a coexisting BRAF and TERT promoter mutations which lacked Spitzoid cytomorphology and was diagnosed as a melanoma with deep penetrating nevus-like features because of the architecture (Figure 1). Three cases had a plexiform arrangement typical of DPN with convergence of nests around the adnexa and neurovascular bundle including the 1 case diagnosed as a malignant melanoma (Figures 1–3, Table 2). Prominent melanin pigmentation was also a common feature, with 4 cases being heavily pigmented. One case had a prominent spindle cell component and prominent desmoplasia with a nested pattern superficially and dispersion to single Spitzoid melanocytes in a sclerotic stroma at the base, typical of a desmoplastic Spitz lesion (Figure 4). In summary, three cases were DPN-like, one case was a desmoplastic Spitz tumor, one case was a spindle cell nevus of Reed (Figure 5a,b) and one was a BAPoma-like large epithelioid melanocytic tumor without loss of BAP-1 nuclear staining by immunohistochemistry (Figure 5c,d).
Figure 3:
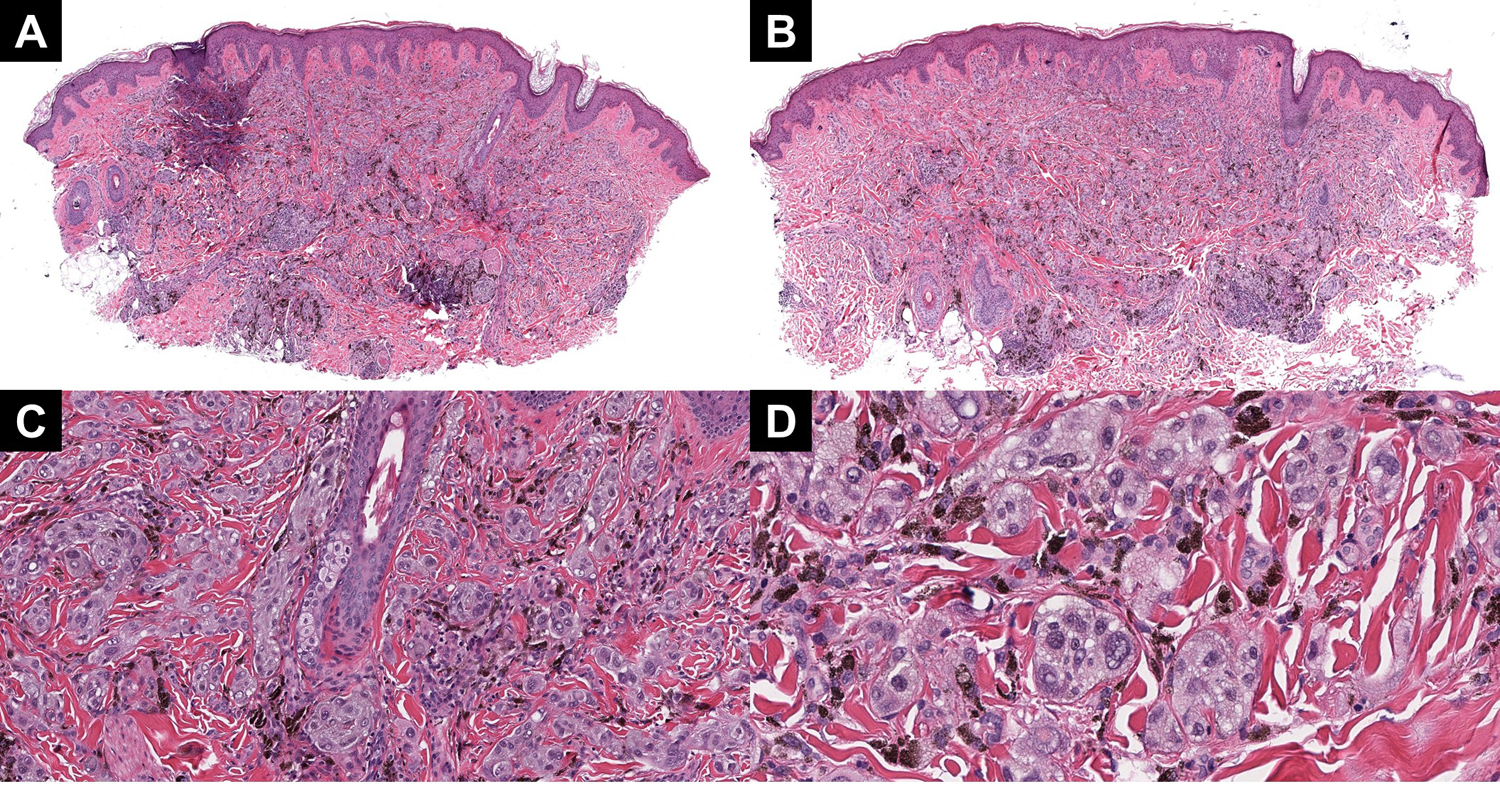
(A and B) Two profiles of case 3, highlighting the plexiform growth pattern and extensive pigmentation throughout the lesion. (C) On higher power, clustering around the adnexa and neurovascular bundle is notable. (D) The cytology is epithelioid and definitively Spitzoid, with multiply vacuolated nuclei and high-grade atypia.
Figure 4:
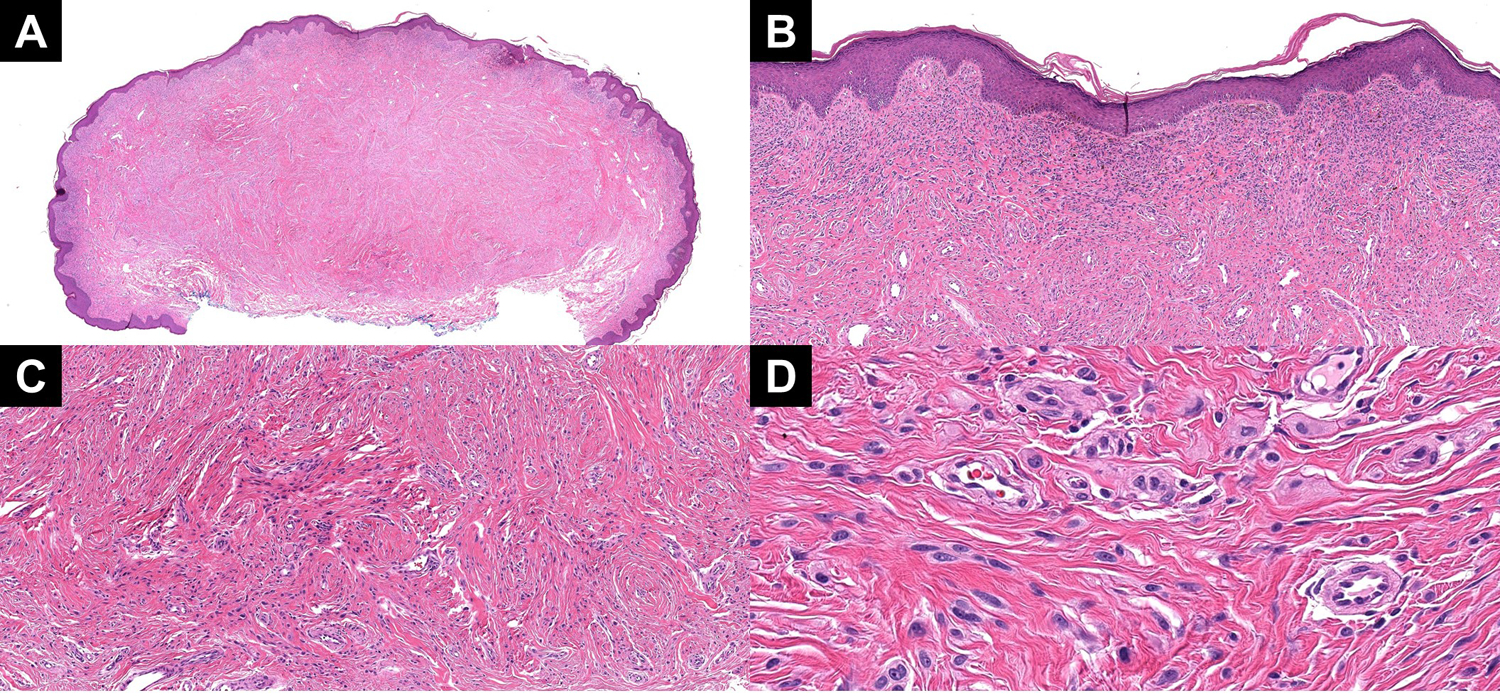
(A) A low power image showing a melanocytic tumor in a highly sclerotic stroma. (B) Superficially, the tumor showed a more nested profile. (C) With descent, the tumor developed into smaller nests and single units of Spitzoid melanocytes in a sclerotic stroma. (D) A high power view of the dispersion to single units and small nests of Spitzoid melanocytes near the base.
Figure 5:
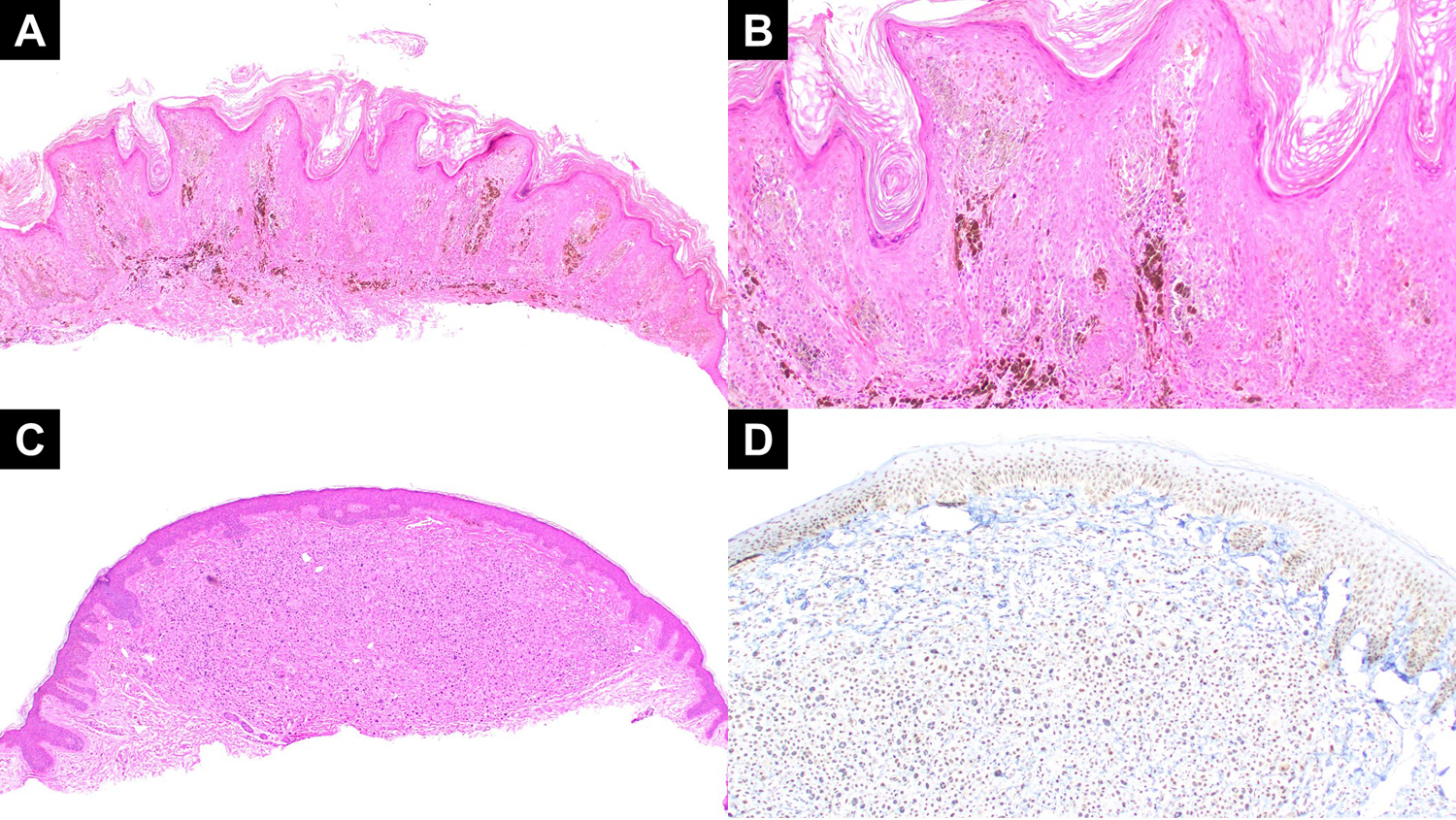
(A, B) Low power and medium power views of case 4 reveals a compound primarily epidermal melanocytic lesion with epidermal hyperplasia, spindled heavily pigmented melanocytes, and extensive melanophages. (C) A low power view of case 5 revealing a nodular proliferation of large epithelioid melanocytes with Spitzoid cytomorphology. The junction was also notable for overlying smaller more conventional appearing melanocytes. (D) A BAP-1 immunostain for case 5 showing preserved BAP-1 expression in the nuclei of the large epithelioid melanocytes in the dermis.
In comparison to a control cohort of 105 Spitz Melanocytic neoplasms with known genomic rearrangements not involving MAP2K1, the MAP2K1 cases showed a nodular silhouette more frequently (p=0.04), less epidermal hyperplasia (p=0.001), were more pigmented (p=0.006), and showed less maturation with descent (p=0.01) (Table 3).
Table 3.
Comparison of Clinical and Morphologic Findings with/without MAP2K1 deletion
| All (n = 111) | Non-MAP2K1 vs MAP2K1 | |||
|---|---|---|---|---|
|
| ||||
| Clinical | Non-MAP2K1 (n=105) | MAP2K1 (n = 6) | P | |
|
| ||||
| Age, years | 0.92 | |||
| Mean | 21.2 | 20.7 | 20.3 | |
| Range | 1–65 | 1–65 | 3–58 | |
|
| ||||
| Gender | 1.00 | |||
| Female | 63 | 54 | 9 | |
| Male | 48 | 45 | 7 | |
|
| ||||
| Location | 0.77 | |||
| Head/Neck | 19 | 19 | 4 | |
| Upper Extremity | 33 | 30 | 3 | |
| Trunk | 13 | 12 | 2 | |
| Lower Extremity | 45 | 38 | 7 | |
| Genitalia | 1 | |||
|
| ||||
| Histologic | ||||
|
| ||||
| Silhouette | ||||
| Plaque | 49 | 48 | 1 | 0.04 |
| Wedge | 30 | 30 | 0 | |
| Nodular | 31 | 26 | 5 | |
| Polypoid | 1 | 1 | 0 | |
|
| ||||
| Cytology | 0.89 | |||
| Epithelioid | 25 | 24 | 1 | |
| Spindled | 29 | 28 | 1 | |
| Both | 57 | 53 | 4 | |
|
| ||||
| Nuclear Atypia | 0.25 | |||
| Mild | 10 | 10 | 0 | |
| Moderate | 66 | 64 | 2 | |
| Severe | 35 | 31 | 4 | |
|
| ||||
| Kamino body | 0.60 | |||
| Absent | 89 | 83 | 6 | |
| Present | 22 | 22 | 0 | |
|
| ||||
| Pigmentation | 0.006 | |||
| Absent | 57 | 57 | 0 | |
| Focal | 30 | 28 | 2 | |
| Extensive | 24 | 20 | 4 | |
|
| ||||
| Maturation | 0.01 | |||
| Absent | 22 | 18 | 4 | |
| Partial | 19 | 18 | 1 | |
| Present | 70 | 69 | 1 | |
|
| ||||
| Ulceration | >0.99 | |||
| Absent | 104 | 98 | 6 | |
| Present | 7 | 7 | 0 | |
|
| ||||
| Inflammatory Reaction | 0.11 | |||
| Absent | 4 | 4 | 0 | |
| Non-Brisk | 63 | 62 | 1 | |
| Brisk | 44 | 39 | 5 | |
|
| ||||
| Epidermal Hyperplasia | 0.01 | |||
| Absent | 22 | 17 | 4 | |
| Present | 89 | 88 | 2 | |
|
| ||||
| Plexiform | 0.67 | |||
| Absent | 69 | 68 | 3 | |
| Present | 42 | 37 | 3 | |
|
| ||||
| Epithelioid Sheet | 0.60 | |||
| Absent | 89 | 83 | 6 | |
| Present | 22 | 22 | 0 | |
|
| ||||
| Pagetosis | >0.99 | |||
| Absent | 88 | 83 | 5 | |
| Present | 23 | 22 | 1 | |
|
| ||||
| Cell Size | 0.20 | |||
| Small | 21 | 21 | 0 | |
| Intermediate | 56 | 54 | 2 | |
| Large | 34 | 30 | 4 | |
|
| ||||
| Nesting Adnexa | 0.59 | |||
| Absent | 95 | 89 | 6 | |
| Present | 16 | 16 | 0 | |
|
| ||||
| Lobulated Nests | 0.59 | |||
| Absent | 90 | 84 | 6 | |
| Present | 21 | 21 | 0 | |
|
| ||||
| Mitotic index (per mm2) | 0.02 | |||
| Mean | 2.2 | 2.2 | 1.0 | |
| Range | 0–20 | 0–20 | 0–2 | |
Genomic Findings in MAP2K1 In Frame Deletion Melanocytic Neoplasms
An in-frame deletion in MAP2K1 was identified (Figure 6, Table 4) in all 6 cases. All of these were predicted to result in gain-of-function mutations. MAP2K1 p.I103_K104del was recurrent in 4 cases which was also among the most common in-frame deletion mutations in melanoma according to COSMIC. MAP2K1 p.E102_I103del and p.E41_F53del were identified in the other 2 cases. Both p.I103_K104del and p.E102_I103del affect the kinase domain of MAP2K1 and p.E41_F53del affect the NRR (negative regulatory region) domain. Though no previous study has reported p.E41_F53del from skin tumors to our knowledge, the mutation clusters around the NRR which is a common mutation hotspot in lung cancer.15
Figure 6:
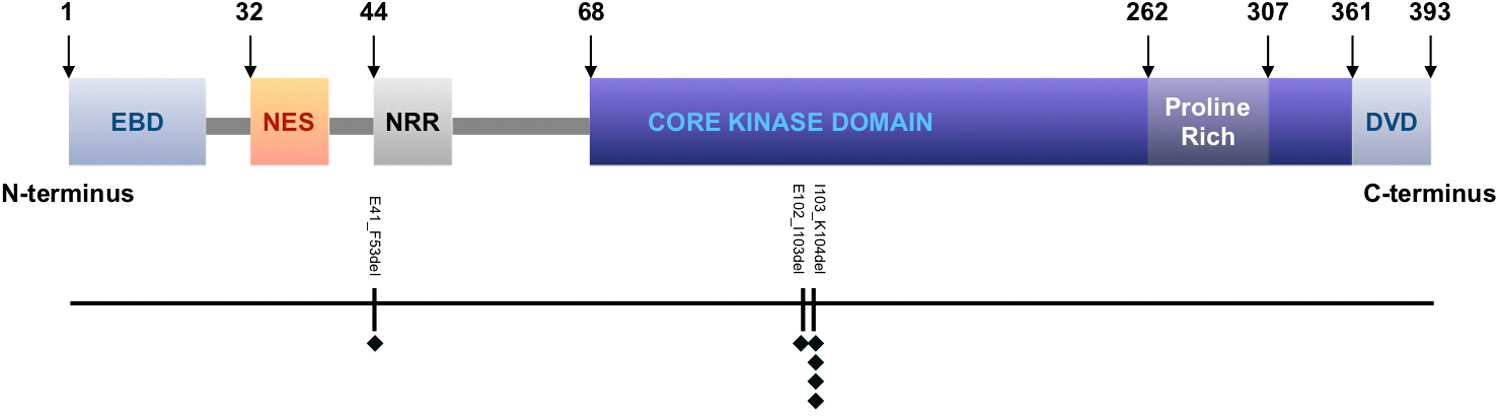
A linear model of human MAP2K1 gene showing key domains and identified mutations in this study. MAP2K1 consists of 393 amino acids. Key domains include an ERK1/2 binding domain (EBD) at the N-terminus, a nuclear export sequence (NES) domain, a negative regulatory region (NRR) domain, a kinase domain, and a MAP3K docking domain (DVD) at the C-terminus. Mutations were labeled below the model. Each black diamond represents a case.
Table 4:
Genetics of Cases with MAP2K1 deletion
| Cases | Somatic Variants / Fusion Events |
|---|---|
| 1 | MAP2K1 (p.E102_I103del); TERT (c.−146C>T); BRAF (p.V600E); IDH1 (p.R132C), LEF1 (p.M1?); CKS1B (copy number gain) |
| 2 | MAP2K1 (p.I103_K104del) |
| 3 | MAP2K1 (p.I103_K104del) |
| 4 | MAP2K1 (p.I103_K104del); RAD51B (c.854–2A>G), VUS in NF1 (p.H781L) |
| 5 | MAP2K1 (p.I103_K104del); RB1 (c.1333–12_13333–8 delGTTTGinsTTTTT); ETV6:PITX3 fusion |
| 6 | MAP2K1 (p.E41_F53del); BAP1 p.H169Y |
Other point mutations that were identified via the targeted DNA and mRNA sequencing from each case are listed in Table 3. Case 1 which was the one case diagnosed as melanoma also showed pathogenic mutations in the TERT promoter as well as in BRAF, IDH1, and LEF1. A splice region mutation at RAD51B c.854–2A>G associated with a loss of function mutation in RAD51B was found in case 4. RAD51B is the member of RAD51 family and essential for DNA repair processes. Familial RAD51B mutations at splice regions have been associated with breast cancer.16 Case 5 had two additional mutations: RB1 c.1333–12_13333–8 delGTTTGinsTTTTT and ETV6-PITX3. In this case, when the promoter of ETV6 fused with PITX3, a transcription factor, it resulted in a stop codon in PITX3 that means that this rearrangement may not produce a functional protein. As a result, while typically a fusion event acts as an initiation event, it is unclear if the ETV6-PITX3 fusion represents the initiation event in Case 5. BAP1 p.H169Y was identified in Case 6 in addition to the MAP2K1 in frame deletion. This variant falls in the ubiquitin carboxyl hydrolase (UCH) domain of the BAP1 protein which is a hotspot of missense mutations, and is predicted to cause loss of function mutation in BAP1.17 This case did not have an copy number alterations and unusually despite the typical BAP1 morphology did not show loss of nuclear staining with BAP1.
DISCUSSION
Quan et al recently reported that structural alterations in MAPK genes other than BRAF constitute approximately 8% of Spitz neoplasms.2 Genomic rearrangements in MAP3K8 typically give epithelioid Spitz with increased pigment and high-grade nuclear atypia.2 Most of the previous studies on MAPK genomic rearrangements have focused on MAP3K8. In this study we present 6 cases with MAP2K1 in-frame deletions resulting in a spectrum of morphologic changes. Common findings include Spitz like cytomorphology (5/6 cases) with large vesicular nuclei and abundant cytoplasm, a DPN-like architecture (3/6 cases), and heavy melanization (4/6 cases).
Given the limited number of cases in this series with MAP2K1 in frame deletions, it is challenging to draw strong conclusions on morphologic correlates. One potential explanation for the heterogeneous presentation seen with MAP2K1 in frame deleted melanocytic neoplasms may be that this is a class of melanocytic neoplasm in which the morphology is very influenced by secondary hits. For example, in 1/3 of cases, DPN arise from activating mutations or in frame deletions in MAP2K1 in combination with subsequent activating mutations in beta-catenin.11 Additionally in this study, the one case that lacked Spitzoid cytomorphology also carried a BRAF and TERT promoter mutation though it still had DPN like architecture. Our panel covered all exons of CTNNB1, however, no mutations were identified in this case. Also of interest is the case which morphologically showed a combined melanocytic tumor with a population of large epithelioid melanocytes with Spitzoid features typical of BAP1 melanocytic tumors. A BAP1 tumor was suspected on initial morphologic review and a BAP1 IHC was performed. Surprisingly there was no loss of nuclear staining of BAP1. However, the sequencing did show a single pathogenic mutation in BAP1. It is unclear if an alteration in the other allele was present and undetected, or if there was an epigenetic change resulting in the BAP1 morphology. This case also importantly demonstrates the limitation of IHC in detecting some BAP1 melanocytic tumors. Lastly, one case had both epithelioid and prominent spindle cytomorphology and a desmoplastic stroma best described as a desmoplastic Spitz tumor (Fig 4) and this case interestingly had a mutation in NF1. This specific NF1 mutation (H781) was reported as a variant of unknown significance (VUS) and has not been previously reported in desmoplastic melanoma. However, NF1 mutations in desmoplastic melanoma can be seen throughout most of the gene and this specific variant has been noted as relevant in other cancers such as bladder cancer.18,19
Since there are only few cases in the literature described with MAP2K1 in frame deletions with clinical follow up, there is little known about the inherent stability of this aberration and the likelihood of these neoplasms for progression to melanoma. More cases with this specific activating mutation and clinical follow up are needed to better characterize the biologic behavior of these tumors. At this time, we report cases not meeting criteria for melanoma descriptively as melanocytic tumors with activating in frame deletions of MAP2K1, document the presence or absence of other relevant mutations such as CTNNB1, BAP1, and indicate whether the morphologic features are typical of Spitz, DPN or PEM. Assessment for the presence of multiple chromosomal copy number aberrations, homozygous deletions of 9p21, and/or TERT-promoter mutations are then utilized to assess for the risk of more aggressive biologic behavior. Although there is only scant literature on this to date, current data suggests that those cases without multiple chromosomal copy number aberrations, homozygous deletions in 9p21 and/or TERT-promoter will likely have indolent behavior.
MAP2K1 in-frame deletions and mutations resulting in gain-of-function have also been described in various cancers including melanoma.20,21 The p.E102_I103 deletion and other deletions in the core kinase domain of MAP2K1 have been shown to be an alternative to a BRAF V600E mutation in Langerhans cell histiocytosis.22 MAP2K1 mutations have also been described in lung adenocarcinoma,15,23,24 histiocytic sarcoma,25 serous ovarian cancer,26 and BRAF V600E-negative hairy cell leukemia.27–29 Trametinib, a MEK inhibitor, has shown some promise in treating MAP2K1 related metastatic cancers.25,27 Germline MAP2K1 mutations result in cardiofaciocutaneous syndrome30 and mosaic expression of MAP2K1 mutations have been demonstrated in arteriovenous malformations.31 Recently, mosaic expression of a point mutation in the core kinase domain resulting in a gain-of-function mutation of MAP2K1 has recently been shown to result in increased numbers of nevi on the affected limb with secondary development of multiple melanomas within the area.32
In summary, thus far melanocytic neoplasms with MAP2K1 in the literature have been referred to as Spitz neoplasm, deep penetrating nevus, pigmented epithelioid melanocytoma and melanoma. In our study a relatively consistent feature was the presence of vesicular nuclei with abundant cytoplasm typical of Spitz neoplasms. Another common characteristic is heavy melanization (4/6) and deep penetrating nevus-like architecture with a plexiform pattern (3/6) cases. An interesting finding in this study was that two cases were diagnosed as deep penetrating nevus with typical features despite lack of CTNNB1 mutations. However, it does seem that the morphologic features of these cases can be highly influenced by secondary hits as noted in several examples from this series. Another reason to be aware of this subset of melanocytic neoplasms is that while rare we identified a single malignant case with a MAP2K1 in frame deletion that was malignant. In the instance of a malignant variant of these lesions, it is useful to be aware of the potential for treatment with MEK inhibitors in metastatic cases.
Figure 2:
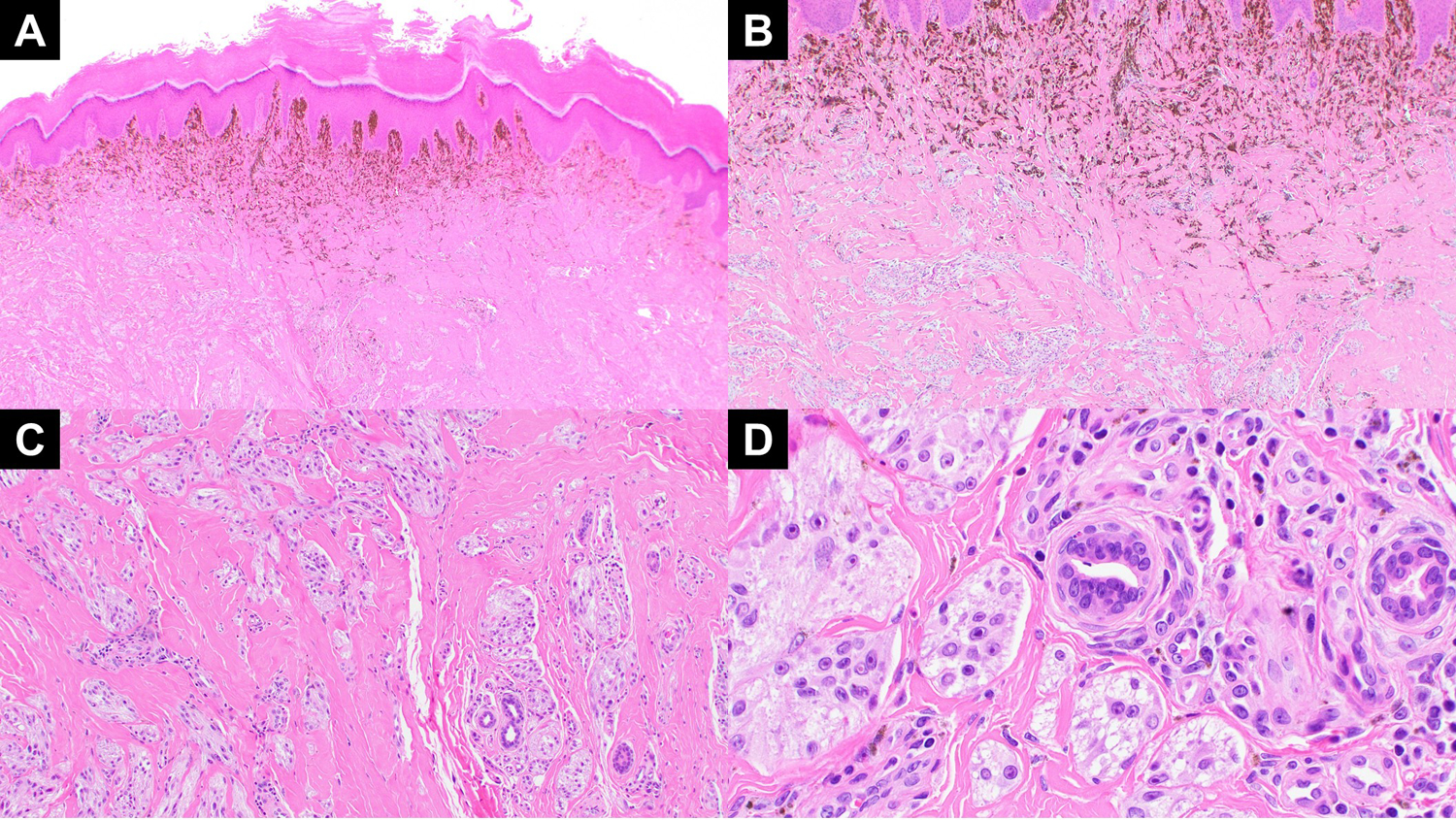
(A) A low power view of case 2 showing nodular melanocytic lesion with prominent aggregation of melanophages in the subepithelial region. (B) Highlights the top heavy melanization and plexiform arrangement of the dermal melanocytes in a sclerotic stroma. (C) The dermal melanocytes also show aggregation around the neurovascular bundles in the dermis. (D) Higher magnification view of variably dermal nests of melanocytes with moderate atypia and Spitzoid and epithelioid cytomorphology.
Acknowledgments
Fund sources: This work was supported by the IDP Foundation.
Footnotes
Conflicts of Interest: Dr. Gerami has served as a consultant for Myriad Genomics, DermTech Int., Merck and Castle Biosciences and has received honoraria for this. Dr. Busam’s research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. All other authors report no relevant conflicts of interest. This work is original and has not been previously published.
References
- 1.Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quan VL, Zhang B, Zhang Y, et al. Integrating Next-Generation Sequencing with Morphology Improves Prognostic and Biologic Classification of Spitz Neoplasms. J Invest Dermatol. 2020. [DOI] [PubMed]
- 3.Quan VL, Zhang B, Mohan LS, et al. Activating Structural Alterations in MAPK Genes Are Distinct Genetic Drivers in a Unique Subgroup Of Spitzoid Neoplasms. Am J Surg Pathol. 2019;43(4):538–548. [DOI] [PubMed] [Google Scholar]
- 4.Busam KJ, Kutzner H, Cerroni L, Wiesner T. Clinical and pathologic findings of Spitz nevi and atypical Spitz tumors with ALK fusions. Am J Surg Pathol. 2014;38(7):925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh I, de la Fouchardiere A, Pissaloux D, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol. 2015;39(5):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh I, Busam KJ, McCalmont TH, et al. Filigree-like Rete Ridges, Lobulated Nests, Rosette-like Structures, and Exaggerated Maturation Characterize Spitz Tumors With NTRK1 Fusion. Am J Surg Pathol. 2019;43(6):737–746. [DOI] [PubMed] [Google Scholar]
- 7.Yeh I, Tee MK, Botton T, et al. NTRK3 kinase fusions in Spitz tumours. J Pathol. 2016;240(3):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan VL, Panah E, Zhang B, Shi K, Mohan LS, Gerami P. The role of gene fusions in melanocytic neoplasms. J Cutan Pathol. 2019;46(11):878–887. [DOI] [PubMed] [Google Scholar]
- 9.Houlier A, Pissaloux D, Masse I, et al. Melanocytic tumors with MAP3K8 fusions: report of 33 cases with morphological-genetic correlations. Mod Pathol. 2019. [DOI] [PubMed]
- 10.Donati M, Kastnerova L, Martinek P, et al. Spitz Tumors With ROS1 Fusions: A Clinicopathological Study of 6 Cases, Including FISH for Chromosomal Copy Number Alterations and Mutation Analysis Using Next-Generation Sequencing. Am J Dermatopathol. 2020;42(2):92–102. [DOI] [PubMed] [Google Scholar]
- 11.Yeh I, Lang UE, Durieux E, et al. Combined activation of MAP kinase pathway and beta-catenin signaling cause deep penetrating nevi. Nat Commun. 2017;8(1):644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaubier N, Tell R, Huether R, et al. Clinical validation of the Tempus xO assay. Oncotarget. 2018;9(40):25826–25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaubier N, Tell R, Lau D, et al. Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget. 2019;10(24):2384–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busam KJ, Antonescu CR, Marghoob AA, et al. Histologic classification of tumor-infiltrating lymphocytes in primary cutaneous malignant melanoma. A study of interobserver agreement. Am J Clin Pathol. 2001;115(6):856–860. [DOI] [PubMed] [Google Scholar]
- 15.Arcila ME, Drilon A, Sylvester BE, et al. MAP2K1 (MEK1) Mutations Define a Distinct Subset of Lung Adenocarcinoma Associated with Smoking. Clin Cancer Res. 2015;21(8):1935–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golmard L, Caux-Moncoutier V, Davy G, et al. Germline mutation in the RAD51B gene confers predisposition to breast cancer. BMC Cancer. 2013;13:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Biswas A, Liu H, et al. Mutational Landscape of the BAP1 Locus Reveals an Intrinsic Control to Regulate the miRNA Network and the Binding of Protein Complexes in Uveal Melanoma. Cancers (Basel). 2019;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiesner T, Kiuru M, Scott SN, et al. NF1 Mutations Are Common in Desmoplastic Melanoma. Am J Surg Pathol. 2015;39(10):1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2018;174(4):1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolaev SI, Rimoldi D, Iseli C, et al. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat Genet. 2011;44(2):133–139. [DOI] [PubMed] [Google Scholar]
- 21.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown NA, Furtado LV, Betz BL, et al. High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood. 2014;124(10):1655–1658. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki H, Hikosaka Y, Okuda K, et al. MEK1 gene mutation in Japanese lung adenocarcinoma patients. Mol Med Rep. 2009;2(2):153–155. [DOI] [PubMed] [Google Scholar]
- 24.Marks JL, Gong Y, Chitale D, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008;68(14):5524–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gounder MM, Solit DB, Tap WD. Trametinib in Histiocytic Sarcoma with an Activating MAP2K1 (MEK1) Mutation. N Engl J Med. 2018;378(20):1945–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grisham RN, Sylvester BE, Won H, et al. Extreme Outlier Analysis Identifies Occult Mitogen-Activated Protein Kinase Pathway Mutations in Patients With Low-Grade Serous Ovarian Cancer. J Clin Oncol. 2015;33(34):4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andritsos LA, Grieselhuber NR, Anghelina M, et al. Trametinib for the treatment of IGHV4–34, MAP2K1-mutant variant hairy cell leukemia. Leuk Lymphoma. 2018;59(4):1008–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason EF, Brown RD, Szeto DP, et al. Detection of activating MAP2K1 mutations in atypical hairy cell leukemia and hairy cell leukemia variant. Leuk Lymphoma. 2017;58(1):233–236. [DOI] [PubMed] [Google Scholar]
- 29.Shin SY, Lee ST, Kim HJ, et al. BRAF V600E and MAP2K1 mutations in hairy cell leukemia and splenic marginal zone lymphoma cases. Ann Lab Med. 2015;35(2):257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel DH, McKenzie J, Frieden IJ, Rauen KA. Dermatological findings in 61 mutation-positive individuals with cardiofaciocutaneous syndrome. Br J Dermatol. 2011;164(3):521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Olabi L, Polubothu S, Dowsett K, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest. 2018;128(11):5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthiah S, Polubothu S, Husain A, Oliphant T, Kinsler VA, Rajan N. A mosaic variant in MAP2K1 is associated with giant naevus-spilus type congenital melanocytic naevus and melanoma development. Br J Dermatol. 2020. [DOI] [PubMed]


