Abstract
Infectious keratitis is a sight-threatening microbial infection. The prevalence of antimicrobial resistance in cases of infectious keratitis has increased the demand for fortified compounded antimicrobial drops. Even with proper medical management, severe cases of infectious keratitis can further evolve into corneal perforation, requiring surgical intervention in the form of keratoplasty to control the infectious process. Due to the invasive nature of the procedure and the shortage of available donor tissue around the world, alternative treatments are needed for the management of progressive infectious keratitis. In ophthalmology, photodynamic therapy (PDT) has been used for numerous applications. PDT with Rose Bengal as a photosensitizer combined with green light optical irradiation (RB-PDAT) is a novel treatment with dual purpose: to arrest the infection from progressing and strengthen the collagen of the cornea. RB-PDAT may be considered as an adjunct therapy in severe cases of infectious keratitis to minimize the need for a therapeutic keratoplasty.
Keywords: rose bengal photodynamic antimicrobial therapy, cornea, ocular surface, infectious keratitis, epithelial defect
Introduction
Prevalence and Current Standard of Care
The incidence of infectious keratitis (IK) is on the rise. In a rural region in North America, Nevitt et al. demonstrated a notable increase from 2.5 cases per 100,000 people in the 1950s to 11 cases per 100,000 people in 1980.(1) In California, Jeng et al. observed an even greater incidence of IK at a rate of 27.6 cases per 100,000 people annually. IK occurred at a higher prevalence among contact lens wearers, where Staphylococcus aureus was identified as the most frequently recovered organism.(2) The predominant type of microorganism may vary based on the geographic area of clinical practice. In India, the most common etiology of IK is fungal keratitis.(3)
Recently, the increased prevalence of antimicrobial resistance requires the use of fortified antimicrobial drops sourced from compounding pharmacy services. However, this method of treatment subjects the ocular surface to significant toxicity.(4) Even with proper medical therapy, some cases of IK will evolve into corneal perforation and require a therapeutic penetrating keratoplasty (TPK). Corneal transplantation on an infected or inflamed ocular surface is associated with many complications and has a guarded prognosis. (5) Therefore, alternative treatments are needed for the management of advanced and severe cases of infectious keratitis.
Collagen Cross-Linking
A recent addition to the armamentarium against IK is the use corneal collagen cross-linking (CXL), a procedure based on combining a photosensitizer chemical (riboflavin) and an ultraviolet light source with a specific wavelength to excite the chemical. Collagen cross-linking results in the formation of stronger chemical bonds between adjacent fibrils, with the added benefit of the cornea becoming more resistant to enzymatic digestion by collagenases.(6) In the 1960s, Tsugita et al. reported that the application of riboflavin and ultraviolet A (UV-A) light led to inactivation of the tobacco mosaic virus.(7) Since then, this knowledge has been used to eradicate microorganisms in many applications, including the sterilization of water, surfaces, and blood products. The mechanism of action is described as the release of reactive oxygen species upon riboflavin activation by UVA light, which compromises the pathogen DNA and RNA.
CXL with riboflavin and ultraviolet (UV) light was introduced as a first-line treatment to prevent the progression of keratoconus and/or corneal ectasias as early as 2003.(8,9) The first publication describing the use of CXL in the context of human corneal infections was by Iseli et al in 2008. Their group reported CXL treatment in five patients with IK that did not respond to standard medical therapy. Post-CXL, they documented that the progression of corneal melting was halted and infiltrate size was effectively reduced in four out of five patients who received the therapy.(10) Since then, several case reports, case series, and prospective non-randomized studies on the use of CXL for corneal infections and the term Photo-Activated Chromophore for Keratitis Corneal Cross-Linking (PACK-CXL) have been used in many recent publications. (11-16)
The first randomized controlled clinical studies comparing PACK-CXL as an adjunct procedure to conventional medical protocol by Said et al and Kasetsuwan et al, did not show a significant difference in corneal healing and final visual outcome compared to standard antibiotic eye drop therapy in patients with severe IK.(17, 18) Nevertheless, PACK-CXL reduced late-onset complications of IK, such as corneal perforation. Bamdad et al demonstrated a faster recovery of epithelial defects and infiltrates when PACK-CXL was added to the treatment regimen.(19) Summaries of clinical trials are shown in Table 1.
Table 1.
Summary of all prospective or randomized clinical trials for cross-linking in IK over the last 10 years
| Author year | Methods | Participants Setting |
Groups Intervention |
Outcomes | Time Fu |
|---|---|---|---|---|---|
| Price 2012 | Pilot study Prospective Non - randomized |
40 patients USA |
Organisms Bacterial 24 eyes (Staph spp, Moraxella, Pseudomonas, Serratia, Enterococcus) Fungal 7 eyes (Penicillium, Fusarium, Aspergillus), Acanthamoeba 2 eyes, Herpes 1 eye. All had Riboflavin CXL treatment ( 4 patients 30 minutes) 36 patients randomized to different UVA light treatment time from 15 to 45 minutes) + standard medical care treatment Infiltrate diameter range: 1 to 12 mm2 |
The success rate was higher for bacterial infections than fungal infections The two cases of Acanthamoeba did not appear to be significantly influenced |
|
| Makdoumi 2012 | Pilot study Non-randomized |
16 patients Sweden |
Organisms Bacterial (Staph spp, Corynebacterium, Micrococcus, Propionibacterium) All received Riboflavin CXL Dresden protocol alone Only 2 patients received additional antibiotic. Corneal ulcer size 0.1 to 2.5 mm |
All eyes responded to the photochemical treatment with improvement in symptoms and signs of reduced inflammation. Epithelial healing was achieved in all cases. Antibiotic administration was necessary in two cases | |
| Said 2014 | Randomized Clinical trial |
21 eyes Egypt |
Organisms Bacterial, Fungi or Acanthamoeba Group 1 PACK-CXL Dresden protocol + Standard medical treatment Group 2 Standard medical treatment Infiltrate diameter range in both groups : 2 to 10 mm |
Three patients in the control group had corneal perforation, whereas patients treated with PACK-CXL did not experience this complication. | - |
| Uddaraju 2015 | Randomized controlled trial |
13 eyes India |
Organisms Aspergillus, Fusarium, Unidentified fungal corneal ulcers. Group 1 Standard medical treatment Group 2 Standard medical treatment + Riboflavin CXL Dresden protocol Infiltrate diameter range in both groups : 5 to 7.48 mm |
CXL used as adjuvant therapy for recalcitrant deep stromal fungal keratitis did not improve outcomes. The trial was stopped before full enrollment because of a marked difference in the rate of perforation between the 2 groups. |
6 weeks |
| Bamdad 2015 | Randomized clinical trial | 22 patients Iran |
Organisms Bacterial not species not specified Group 1 Standard medical treatment Group 2 Standard medical treatment + Riboflavin CXL Dresden protocol Infiltrate diameter 19.25 mm2 |
Beneficial effect of CXL in patients with moderate bacterial keratitis. In addition to accelerating epithelialization, this method shortens the course of treatment and may minimize or remove the need for surgery or other serious sequelae, such as corneal perforation. | - |
| Venkatesh Prajna 2020 | Randomized clinical trial | 403 patients India |
Organisms: Filaments Group 1: natamycin alone, Group 2: natamycin plus CXL, Group 3: amphotericin alone, and Group 4: amphotericin plus CXL. Riboflavin CXL Dresden protocol Moderate size corneal ulcers |
Unable to find a difference in 24-hour culture positivity between those randomized to amphotericin and those randomized to natamycin when evaluated as a group regardless of whether or not they received CXL (coefficient 1.10; 95% CI, 0.47e2.54; P 1/4 0.84). No difference in infiltrate or scar size, percentage of epithelialized or adverse events when comparing CXL with no CXL or the 2 topical medications. |
3 months |
PACK-CXL: Photo Activated Chromophore for Keratitis-Corneal Cross-linking; Spp: species; CXL: cross-linking; BCVA: Best-corrected visual acuity
CXL for the Treatment of Fungal Ulcers
A randomized clinical trial reported by Prajna et al showed no evidence in favor of using adjunct PACK-CXL in the primary treatment of filamentous fungal ulcers (Table 1). The use of PACK-CXL in fungal keratitis remains a topic of debate. Li et al reported a successful use of PACK-CXL for eight cases of fungal keratitis.(20) However, Uddaraju et al in a prospective randomized clinical trial, found that PACK-CXL was not helpful for cases of deep stromal fungal keratitis, with five eyes in the PACK-CXL group and three eyes in the non-CXL group having experienced treatment failure by 6 weeks.(21) Interestingly, modifying parameters of the Dresden protocol such as increasing the UV fluence may lead to better outcomes. Richoz et al reported an accelerated protocol using 18mW/cm2 for 5 minutes and 36 mW/cm2 for 2.5 minutes maintaining high bacterial killing for Staphylococcus aureus and Pseudomonas aeruginosa in-vitro.(22) Recently Knyazer et al described the use of accelerated PACK-CXL (30mW/cm2 for 3 minutes, total dose of 5.4 J/cm2) as additional treatment for resistant IK, with no associated complications.(22, 23) Also, another interesting finding by Hafezi et al, is that the application of fluorescein staining prior to the procedure competes with riboflavin for the absorption of UV-A during PACK-CXL, which reduces the antimicrobial effect of the procedure.(24). Our group reported a case series of three patients with infectious keratitis unresponsive to standard medical treatment (Pseudomonas aeruginosa, Mycobacterium chenolae, and Curvularia spp) treated with PACK-CXL as an adjunct treatment with good long term follow-up and results.(25)
Management of Infectious Keratitis at Bascom Palmer
In our institution, an estimated number of 900 cases of presumed infectious keratitis are treated annually. With the support of our microbiology and pathology laboratory, about 55% of our keratitis cases are confirmed culture-positive for microbiology. Our eye hospital is the largest university eye hospital in the region, with a dedicated ophthalmic microbiology laboratory for clinical and research work. We are able to identify many cases of infectious keratitis given these resources and our geographic area of practice in a tropical climate with easy access to the ocean, swimming pools, and agricultural activities with secondary organic trauma, bring a significant number of patients with presumed infectious keratitis to our emergency room and our clinics. Even with access to standard medical care and the best diagnostic tools available, out institution performs around 70 therapeutic corneal transplants a year. TPK may be curative in many patients, however, the long-term visual prognosis is guarded in most of these eyes. Severe complications such as corneal neovascularization, corneal graft rejection, secondary glaucoma, and recurrence of infection can occur. (26) In an effort to improve clinical outcomes in patients with progressive infectious keratitis, our group decided to incorporate the use of PACK-CXL in our armamentarium against IK. Our preliminary work in the laboratory showed that the use of PACK-CXL was not effective in-vitro for the 3 most common fungal organism that we see associate with IK. As a result, we decided to investigate the possibility of using a different photosensitizing agent activated by a specific light source.
Rose Bengal Background
Rose Bengal (RB) is a dye routinely used in ophthalmology clinics to stain for corneal or conjunctival epithelial defects and degeneration.27 The main constituents of RB are iodine derivates of di- and tetrachloro fluorescein. It is commonly used in solution to stain damaged corneal and conjunctival cells. RB neither penetrates nor dyes mucins, and therefore presents better adherence to epithelial cells whose external surface is not covered by an intact coat of mucins.(28) In consideration of the utility of PACK-CXL and as an alternative therapeutic tool , research by our group have focused on the in vitro and in vivo antimicrobial efficacy of photodynamic antimicrobial therapy (PDAT). By using Rose Bengal as a photosensitizer activated by green light, the RB-PDAT method has proven to kill organisms in the cornea and strengthen the collagen fibers.
Mechanism of Photodynamic Therapy
Photodynamic therapy involves the activation of a photosensitizing agent by light ranging from ultraviolet-A (UV-A) to about 750nm. The photosensitizer reaches an excited state that undergoes a reaction with ambient oxygen to create singlet oxygen (SO) and reactive oxygen species (ROS), singlet oxygen being the most efficacious element produced by rose bengal while riboflavin only produces ROS. These SO and ROS then react with intracellular components and produces cell inactivation and death.29 In the field of ophthalmology, Photodynamic therapy has been used for numerous applications including the treatment of corneal neovascularization, choroidal neovascularization in age-related macular degeneration and experimentally, for tumor treatment and Acanthamoeba keratitis.30,31 A custom-built LED source was designed and fabricated by the University of Miami Ophthalmic Biophysics laboratory. The irradiation head was assembled using an array of twenty- four light emitting diodes (LEDs). A single green LED (L1-0-G5TH45-1, LED supply, Randolph, VT, USA) has a 518 nm peak irradiance (I40%: 500-541 nm) and produces 2.2mW/cm2 over a surface of 28.3 cm2. As recently as 2020, Peterson et al established development of a-singlet oxygen dosimeter to optimize RB-PDAT (32). Using our RB-PDT system we compared the in vitro efficacy of rose bengal versus riboflavin as photosensitizing agents for photodynamic therapy (PDT) on different microorganisms. Our team demonstrated in vitro antimicrobial effects on a variety of microbes, including multiple species of bacteria and fungi.(27,33,34) Our laboratory reported an in vitro study using MRSA isolates from patients with keratitis, which resulted in a complete growth inhibition using Rose Bengal PDAT compared to minimal inhibition when riboflavin PDAT was used. Others have demonstrated a possible role for RB-PDAT in treating Acanthamoeba infections. Atalay et al demonstrated in vivo and in vitro that RB-PDAT plays a fundamental role and is effective in decreasing the parasitic load and clinical severity of Acanthamoeba castellanii keratitis(35,36)
Safety and Toxicity Studies
Many studies have validated the safety and efficacy of RB-PDAT in vivo. (37). Moreover, our team demonstrated that performing RB-PDAT does not cause toxicity to the corneal endothelium and did not affect the viability of the limbal stem cell in vivo (38). Given the potential for RB-PDAT for the use of treatment for progressive infectious keratitis, we were able to establish the safety of this procedure. Resistance to treatment has not been observed with RB-PDAT.(39) The promising in vitro and in vivo results from our institution were used for clinical guidance toward the use for RB-PDAT to treat patients with progressive IK that have been unresponsive to standard medical therapy.
Bascom Palmer RB-PDAT Protocol
As it is a novel application, our group established the following standard protocol: A lid speculum is placed under topical anesthesia (sterile lidocaine 1% and proparacaine 0.5%), followed by an injection of 2 mL of 2% lidocaine with epinephrine 1:10,000 into the bulbar subconjunctival space at 12 o’clock and 6 o’clock with a 30 gauge needle. If the ulcer has a small epithelial defect, the area surrounding it is debrided to obtain an 8-mm de-epithelialized area to increase Rose Bengal absorption. An 8 mm corneal sponge (Beaver Visitec International, Waltham, Massachusetts, USA) soaked in RB is placed over the cornea. Followed by 3 to 5 drops every 3 minutes over the following 30 minutes to maintain corneal saturation. The sponge is then removed and a custom-made disposable shield measuring 10 mm x 15 mm with a central 9-mm opening was placed to protect the corneoscleral limbus from the green light irradiation. The anterior corneal surface is irradiated with a custom-made 6 mW/cm2 green LED light source for 15 minutes for a total energy density exposure of 5.4 J/cm2. The anterior corneal surface is irrigated with balanced saline solution (Alcon Laboratories, Fort Worth, Texas, USA) every 3 minutes throughout the light exposure to prevent corneal dehydration. Finally, a bandage contact lens (Airoptix AQUA; Alcon, Fort Worth, Texas, USA) is placed to protect the ocular surface.
The first successful reported case of RB-PDAT was in a patient with Fusarium keratoplasticum keratitis. In this case a therapeutic graft was avoided, and we were able to quiet the inflammation and later perform an optic graft with excellent clinical and visual outcomes. This case demonstrated proof-of-principle of the potential of PDAT in the management of resistant infectious keratitis. (40, 41). Our group published a retrospective pilot study using RB-PDAT as an adjunct treatment for severe IK, with a total of 18 patients. Seventeen had positive microbial cultures results, with cases divided between Acanthamoeba (n=10), Fusarium spp. (n=4), Pseudomonas aeruginosa (n=2), and Curvularia spp. (n=1). In addition, one patient’s cultures showed the presence of multiple microorganisms. All patients had resolution of the infection within 38 days of the first RB-PDAT treatment. The current standard therapy for infectious keratitis unresponsive to treatment is a therapeutic penetrating keratoplasty, which requires surgical intervention and the placement of a donor graft. Kirkness et al. demonstrated that in 116 therapeutic grafts, 43 failed (a rate of 51%) due to rejection or recurrence of infection within 5-years post-operatively.26 In contrast, the prognosis of a corneal transplant in eyes with inactive infection was much-improved, with 90% of grafts surviving for 5-years. Our pilot study defined RB-PDAT treatment as successful if therapeutic penetrating keratoplasty was avoided. This was achieved in 72% of the patients treated with RB- PDAT.(42) Interestingly, in a second analysis, we have noted that patients with resistant and severe forms of IK that required therapeutic keratoplasty who had previous RB-PDAT treatment demonstrated successful therapeutic graft adherence, these grafts remaining clear for a long time without reinfections, rejection, or failure. (25) We believe that RB-PDAT not only has an antimicrobial effect, also may have a beneficial effect on corneal grafts by regressing blood and lymphatics vessels by induction of apoptosis in vascular endothelial cells. (41). Moreover, the benefit of using CXL in donor corneas to increase graft survival has been published. CXL with riboflavin may prevent the recurrence of infection in cases of infectious keratitis after TPK, increasing the survival rate of corneal grafts.(43-45). Titiyal et al published a prospective interventional study which evaluated the outcomes of therapeutic keratoplasty using riboflavin CXL-treated and non-treated donor corneas in fungal keratitis. Eighty percent of patients in the CXL-treated donor group showed clear corneal grafts at 6 months compared to 22% of patients in the non-treated group. Interestingly, none of the patients in the CXL-treated donor group had recurrence of infections compared to 22% in the non-treated CXL group.(43) This is preliminary data and an exciting area of research that may bring new tools to prolong longevity of high-risk corneal grafts. On the other hand, another benefit of using RB-PDAT is an increased resistance to collagenase digestion (46,47). If antimicrobial eradication is not achieved with RB-PDAT, its protective effect in preventing or delaying corneal melt, can then allow for the use of conventional medical antimicrobial therapy to eradicate the infection.
Conclusion
Although our laboratory and clinical results of using RB-PDT as a potential tool in the management of IK are encouraging, the treatment still has limitations. We understand how deep RB can reach in normal stromal tissue, however the data on RB penetration of corneas with active infection and inflammation remains to be seen. Our clinical work has focused mostly on patients with advanced disease. The use of a light activated photosensitizer agent for Infectious Keratitis continues to be a challenge, but an area of opportunity for developing therapeutics. Novel potential treatments such as PACK-CXL and RB-PDAT have shown success but have yet to be evaluated in prospective clinical trials. New improvements in technology as well as better understanding of the mechanism of action will help improve our patient outcomes. Currently, RB-PDAT may thus be considered as an adjunct therapy in severe cases of infectious keratitis in an attempt to avoid TPK and optimize future visual potential. Currently, additional in vitro, in vivo, and human clinical trials are underway at different institutions worldwide. Our group hopes that in the future Rose Bengal photodynamic therapy can be considered as a primary treatment for infections and to help mitigate the prevention of blindness from corneal infections.
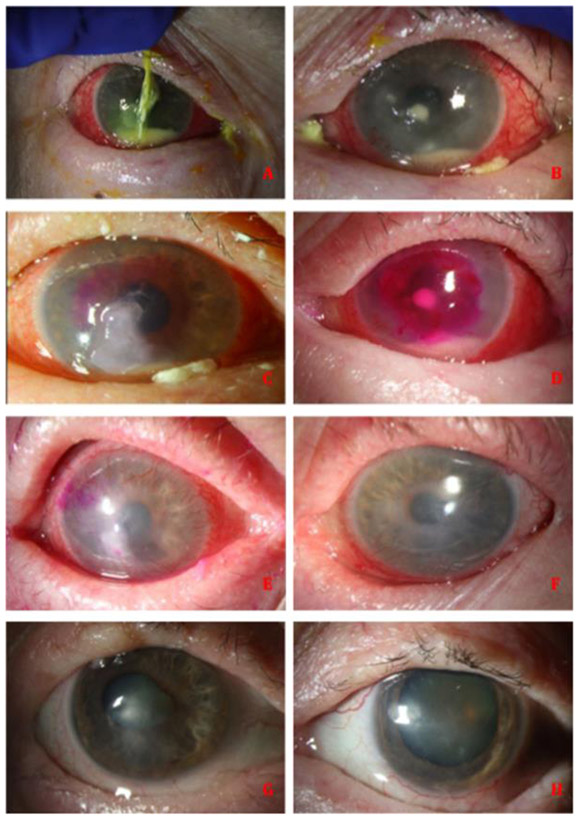
Figure 1.
A case of a 68-year-old female patient, with a history of Ocular Cicatricial Pemphigoid and a confirmed diagnosis of bilateral Curvularia keratitis. (A, B) Clinical appearance at presentation with bilateral deep corneal infiltrates, paracentral melting, thinning and hypopyon. (C, D) RB-PDAT was performed due to lack of response to standard medical treatment. (E, F) Appearance of the ocular response to RB-PDAT procedure where corneal melt and thinning have stopped, a decreased in conjunctival inflammation with less infiltrate, and grossly significant improvement. The corneal epithelium has healed and steroid drops were started. (G, H) Patient with a bilateral clinical resolution of the infection.
Footnotes
Conflict of Interest
Jean Marie Parel and Guillermo Amescua are authors of a University of Miami invention disclosure on the instrument described herein.
Diego Altamirano, Jaime Martinez, and Katherine D Leviste each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993;111(12):1665–71. [DOI] [PubMed] [Google Scholar]
- 2.Jeng BH, Gritz DC, Kumar AB, Holsclaw DS, Porco TC, Smith SD, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128(8):1022–8. [DOI] [PubMed] [Google Scholar]
- 3.Ranjini CY, Waddepally VV. Microbial Profile of Corneal Ulcers in a Tertiary Care Hospital in South India. J Ophthalmic Vis Res. 2016;11(4):363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107(8):1497–502. [DOI] [PubMed] [Google Scholar]
- 5.Hossain P, Tourkmani AK, Kazakos D, Jones M, Anderson D, Blood NHS, et al. Emergency corneal grafting in the UK: a 6-year analysis of the UK Transplant Registry. Br J Ophthalmol. 2018;102(1):26–30. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29(9):1780–5. [DOI] [PubMed] [Google Scholar]
- 7.Tsugita A, Okada Y, Uehara K. Photosensitized inactivation of ribonucleic acids in the presence of riboflavin. Biochim Biophys Acta. 1965;103(2):360–3. [DOI] [PubMed] [Google Scholar]
- 8.Ashwin PT, McDonnell PJ. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol. 2010;94(8):965–70. [DOI] [PubMed] [Google Scholar]
- 9.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–7. [DOI] [PubMed] [Google Scholar]
- 10.Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008;27(5):590–4. [DOI] [PubMed] [Google Scholar]
- 11.Makdoumi K, Mortensen J, Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea. 2010;29(12):1353–8. [DOI] [PubMed] [Google Scholar]
- 12.Panda A, Krishna SN, Kumar S. Photo-activated riboflavin therapy of refractory corneal ulcers. Cornea. 2012;31(10):1210–3. [DOI] [PubMed] [Google Scholar]
- 13.Skaat A, Zadok D, Goldich Y, Varssano D, Berger Y, Ezra-Nimni O, et al. Riboflavin/UVA photochemical therapy for severe infectious keratitis. Eur J Ophthalmol. 2014;24(1):21–8. [DOI] [PubMed] [Google Scholar]
- 14.Sorkhabi R, Sedgipoor M, Mahdavifard A. Collagen cross-linking for resistant corneal ulcer. Int Ophthalmol. 2013;33(1):61–6. [DOI] [PubMed] [Google Scholar]
- 15.Price MO, Tenkman LR, Schrier A, Fairchild KM, Trokel SL, Price FW Jr. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg. 2012;28(10):706–13. [DOI] [PubMed] [Google Scholar]
- 16.Makdoumi K, Mortensen J, Sorkhabi O, Malmvall BE, Crafoord S. UVA-riboflavin photochemical therapy of bacterial keratitis: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):95–102. [DOI] [PubMed] [Google Scholar]
- 17.Kasetsuwan N, Reinprayoon U, Satitpitakul V. Photoactivated Chromophore for Moderate to Severe Infectious Keratitis as an Adjunct Therapy: A Randomized Controlled Trial. Am J Ophthalmol. 2016;165:94–9. [DOI] [PubMed] [Google Scholar]
- 18.Said DG, Elalfy MS, Gatzioufas Z, El-Zakzouk ES, Hassan MA, Saif MY, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377–82. [DOI] [PubMed] [Google Scholar]
- 19.Bamdad S, Malekhosseini H, Khosravi A. Ultraviolet A/riboflavin collagen cross-linking for treatment of moderate bacterial corneal ulcers. Cornea. 2015;34(4):402–6. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Jhanji V, Tao X, Yu H, Chen W, Mu G. Riboflavin/ultravoilet light-mediated crosslinking for fungal keratitis. Br J Ophthalmol. 2013;97(5):669–71. [DOI] [PubMed] [Google Scholar]
- 21.Uddaraju M, Mascarenhas J, Das MR, Radhakrishnan N, Keenan JD, Prajna L, et al. Corneal Cross-linking as an Adjuvant Therapy in the Management of Recalcitrant Deep Stromal Fungal Keratitis: A Randomized Trial. Am J Ophthalmol. 2015;160(1):131–4 e5. [DOI] [PubMed] [Google Scholar]
- 22.Richoz O, Kling S, Hoogewoud F, Hammer A, Tabibian D, Francois P, et al. Antibacterial efficacy of accelerated photoactivated chromophore for keratitis-corneal collagen cross-linking (PACK-CXL). J Refract Surg. 2014;30(12):850–4. [DOI] [PubMed] [Google Scholar]
- 23.Knyazer B, Krakauer Y, Baumfeld Y, Lifshitz T, Kling S, Hafezi F. Accelerated Corneal Cross-Linking With Photoactivated Chromophore for Moderate Therapy-Resistant Infectious Keratitis. Cornea. 2018;37(4):528–31. [DOI] [PubMed] [Google Scholar]
- 24.Richoz O, Gatzioufas Z, Francois P, Schrenzel J, Hafezi F. Impact of fluorescein on the antimicrobial efficacy of photoactivated riboflavin in corneal collagen cross-linking. J Refract Surg. 2013;29(12):842–5. [DOI] [PubMed] [Google Scholar]
- 25.Martinez JD, Arboleda A, Naranjo A, Aguilar MC, Durkee H, Monsalve P, et al. Long-term outcomes of riboflavin photodynamic antimicrobial therapy as a treatment for infectious keratitis. Am J Ophthalmol Case Rep. 2019;15:100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.-.Kirkness CM, Ficker LA, Steele ADM, et al. The role of penetrating keratoplasty in the management of microbial keratitis. Eye. 1991; 5:425–431. [PubMed: 1743358] [DOI] [PubMed] [Google Scholar]
- 27.Arboleda A, Miller D, Cabot F, Taneja M, Aguilar MC, Alawa K, et al. Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014;158(1):64–70 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murube J Rose bengal: the second most commonly used surfocular vital stain. Ocul Surf. enerode 2014;12(1):14–22. [DOI] [PubMed] [Google Scholar]
- 29.-.Dai T, Fuchs BB, Coleman JJ, et al. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front Microbiol. 2012; 3:120. [PubMed: 22514547] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.-.van der Bergh H. Photodynamic therapy of age-related mcular degeneration: history and principles. Sem Ophthalmol. 2001; 16(4):181–200. [DOI] [PubMed] [Google Scholar]
- 31.Sheppard JD, Epstein RJ, Lattanzio FA, Marcantonio D, Williams PB. Argon Laser Photodynamic Therapy of Human Corneal Neovascularization After Intravenous Administration of Dihematoporphyrin Ether. Am J Ophthalmol. 2006; 141(3):524–529. [PubMed: 16490500] [DOI] [PubMed] [Google Scholar]
- 32.Peterson Jeffrey C, Silgado Juan D, Weisson Ernesto H., Meizoso Rene, Arrieta Esdras, Mintz Keenan, Ruggeri Marco, Manns Fabrice, Parel Jean-Marie A; Dosimetry for Rose Bengal Photodynamic Antimicrobial Therapy (RB-PDAT) for treatment of infectious keratitis. Invest. Ophthalmol. Vis. Sci 2020;61(7):405. [Google Scholar]
- 33.Halili F, Arboleda A, Durkee H, Taneja M, Miller D, Alawa KA, et al. Rose Bengal- and Riboflavin-Mediated Photodynamic Therapy to Inhibit Methicillin-Resistant Staphylococcus aureus Keratitis Isolates. Am J Ophthalmol. 2016;166:194–202. [DOI] [PubMed] [Google Scholar]
- 34.Durkee H, Arboleda A, Aguilar MC, Martinez JD, Alawa KA, Relhan N, et al. Rose bengal photodynamic antimicrobial therapy to inhibit Pseudomonas aeruginosa keratitis isolates. Lasers Med Sci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atalay HT, Dogruman-AI F, Sarzhanov F, Ozmen MC, Tefon AB, Aribas YK, et al. Effect of Riboflavin/Rose Bengal-Mediated PACK-CXL on Acanthamoeba Trophozoites and Cysts in Vitro. Curr Eye Res. 2018;43(11):1322–5. [DOI] [PubMed] [Google Scholar]
- 36.-.Hatice Tuba Atalay, Bet F, Sarzhanov F, Ozmen MC, Tefon AB, Aribas YK,uca, Nilüfer Yeşlırmak, Mehmet Cüneyt Özmen, Sidre Erganiş, Atike Burçin Tefon, Funda Dogruman- Al& Kamil Bilgihan (2020): Rose Bengal-Mediated Photodynamic Antimicrobial Treatment of Acanthamoeba Keratitis, Current Eye Research, DOI: 10.1080/02713683.2020.1731830 [DOI] [PubMed] [Google Scholar]
- 37.Zhu H, Alt C, Webb RH, Melki S, Kochevar IE. Corneal Crosslinking With Rose Bengal and Green Light: Efficacy and Safety Evaluation. Cornea. 2016;35(9):1234–41. [DOI] [PubMed] [Google Scholar]
- 38.Naranjo A, Pelaez D, Arrieta E, Salero-Coca E, Martinez JD, Sabater AL, et al. Cellular and molecular assessment of rose bengal photodynamic antimicrobial therapy on keratocytes, corneal endothelium and limbal stem cell niche. Exp Eye Res. 2019;188:107808. [DOI] [PubMed] [Google Scholar]
- 39.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amescua G, Arboleda A, Nikpoor N, Durkee H, Relhan N, Aguilar MC, et al. Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea. 2017;36(9):1141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez JD, Naranjo A, Amescua G, Dubovy SR, Arboleda A, Durkee H, et al. Human Corneal Changes After Rose Bengal Photodynamic Antimicrobial Therapy for Treatment of Fungal Keratitis. Cornea. 2018;37(10):e46–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •42. Naranjo A, Arboleda A, Martinez JD, Durkee H, Aguilar MC, Relhan N, et al. Rose Bengal Photodynamic Antimicrobial Therapy for Patients With Progressive Infectious Keratitis: A Pilot Clinical Study. Am J Ophthalmol. 2019;208:387–96. RB-PDAT may be considered as an adjunct therapy in severe cases of infectious keratitis to minimize the need for a therapeutic keratoplasty.
- •43. Titiyal JS, Karunakaran A, Kaur M, Rathi A, Agarwal T, Sharma N. Collagen Cross-Linked Therapeutic Grafts in Fungal Keratitis. Ophthalmology. 2018;125(9):1471–3. An exciting area of research that may bring new tools to prolong longevity of high-risk corneal grafts
- 44.Hou Y, Le VNH, Toth G, Siebelmann S, Horstmann J, Gabriel T, et al. UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high-risk corneal transplant survival. Am J Transplant. 2018;18(12):2873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou Y, Le VNH, Clahsen T, Schneider AC, Bock F, Cursiefen C. Photodynamic Therapy Leads to Time-Dependent Regression of Pathologic Corneal (Lymph) Angiogenesis and Promotes High-Risk Corneal Allograft Survival. Invest Ophthalmol Vis Sci. 2017;58(13):5862–9. [DOI] [PubMed] [Google Scholar]
- 46.Fadlallah A, Zhu H, Arafat S, Kochevar I, Melki S, Ciolino JB. Corneal resistance to keratolysis after collagen crosslinking with rose bengal and green light. Invest Ophthalmol Vis Sci 2016;57(15):6610–6614. 36. [DOI] [PubMed] [Google Scholar]
- 47.Cherfan D, Verter EE, Melki S, et al. Collagen cross-linking using rose bengal and green light to increase corneal stiffness. Invest Ophthalmol Vis Sci 2013;54(5):3426–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]