Our laboratory study tests the effects of long-term, naturalistic temperature variation under current and warmer climate simulations using hellbender salamanders. We found that hellbenders are resilient to large, naturalistic temperature changes. Animals under the warmer simulations fared better in winter and more poorly in summer than current climate simulations.
Keywords: thermal physiology, salamander, immune function, growth, climate change, Amphibian
Abstract
Cold-adapted hellbender salamanders that inhabit cool mountain streams are expected to fare poorly under warmer projected climate scenarios. This study investigated the physiological consequences of long-term, naturalistic temperature variation on juvenile hellbenders under simulated current and warmer (+1.6 C) climates vs. controlled steady temperatures. Mean temperature and temperature variability were both important predictors of growth as indicated by monthly body mass change (%), stress as indicated by neutrophil:lymphocyte (N:L) ratio and bacteria-killing ability of blood. Cold exposure in hellbenders was associated with weight loss, increased N:L ratios and reduced Escherichia coli killing ability of blood, and these effects were less pronounced under a warmer climate scenario. These observations suggest that cold periods may be more stressful for hellbenders than previously understood. Growth rates peaked in late spring and late fall around 14–17°C. Hellbenders experiencing warmer simulated climates retained body condition better in winter, but this was counter-balanced by a prolonged lack of growth in the 3-month summer period leading up to the fall breeding season where warmer simulated conditions resulted in an average loss of −0.6% body mass/month, compared to a gain +1.5% body mass/month under current climate scenario. Hellbenders can physiologically tolerate projected warmer temperatures and temperature fluctuations, but warmer summers may cause animals to enter the fall breeding season with a caloric deficit that may have population-level consequences.
Introduction
The Appalachian region is a biodiversity hotspot for salamanders and harbours deep evolutionary diversity from distantly related taxa (Kozak and Wiens, 2010). The climatic niches have been consistent over long evolutionary periods, and distributional models predict rapid losses in terrestrial species’ ranges due to climate change (Kozak and Wiens, 2010; Milanovich et al., 2010). These predictions have been borne out by real-world observations of altitudinal shifts in hybrid zones between terrestrial species linked to increasing temperatures (Walls, 2009), and declines in aquatic species have been observed in association with changing precipitation and streamflow patterns (Lowe, 2012). Other studies, however, documented physiological and behavioural plasticity in making animals more resilient to climate-change declines, and call for more detailed knowledge of animal physiology to incorporate into climate models (Gvoždík, 2012; Riddell et al., 2018). The number of optimal growth days for animals may shift with changing temperatures (Westhoff and Paukert, 2014), and increasing the number of optimum growth days is one factor that may be connected to minor increases in redback salamander body size observed in warming climates (McCarthy et al., 2017). A better understanding of the physiological mechanisms that drive species’ responses to climate change will help inform predictive models, identify at-risk populations and develop mitigation strategies (Bozinovic et al., 2013; Rohr et al., 2013).
The hellbender (Cryptobranchus alleganiensis Daudin, 1803) is a fully aquatic salamander species that occurs in cold, fast-flowing Appalachian streams and is the largest salamander species in the USA. Cryptobranchids rely entirely on cutaneous respiration and have lateral skin folds that increase their body surface area, thereby facilitating oxygen uptake (Guimond and Hutchison, 1973). This respiration mode confines hellbenders to highly oxygenated aquatic environments and are considered sensitive to warmer temperatures, as dissolved oxygen levels decrease with increasing temperatures (Guimond and Hutchison, 1973; U.S. Fish and Wildlife Service, 2018). Over the past several decades, the species has experienced enigmatic declines over most of its range; it is believed that 225 populations are extirpated, 219 are declining and just 126 are stable or increasing (U.S. Fish and Wildlife Service, 2018). Lack of juvenile recruitment has been observed in many other hellbender populations across the species’ range, and this factor, rather than adult mortality, seems to be a major factor in the species’ ongoing, broadscale decline (Wheeler et al., 2002; Humphries and Pauley, 2005; Unger et al., 2013; Bodinof Jachowski and Hopkins, 2018). Siltation (due to loss of riparian buffer) and pollution are thought to be an underlying causes of these declines (Nickerson and Briggler, 2007; Keitzer et al., 2013; Pitt et al., 2017; Bodinof Jachowski and Hopkins, 2018; U.S. Fish and Wildlife Service, 2018). Furthermore, hellbenders, particularly the Ozark subspecies (Cryptobranchus alleganiensis bishopi), are susceptible to foot lesions with unknown aetiology or population consequences that can progress to severe tissue necrosis and loss of the entire foot (Nickerson et al., 2011; Hardman et al., 2020). While the role of climate change in these observed declines are uncertain, future climate predictions for Appalachia include fewer frost days, warmer air temperatures, more frequent heat waves and increased periods of drought punctuated by intense rainstorms (Karl et al., 2009; U.S. Fish and Wildlife Service, 2018). Simulated models predicting stream temperature changes under warming climate scenarios predict a reduction in optimal growth days for the Ozark hellbender subspecies (Westhoff and Paukert, 2014). Although hellbenders are adapted to cold habitats, there is evidence of plasticity in the thermal maximums. Animals that have been acclimated to higher temperatures demonstrate higher critical thermal maximum temperatures (Hutchison et al., 1973), but may also lead to reduced fecundity and survival (Cavieres et al., 2020). Thermal acclimation is well studied among amphibians reviewed in (Angilletta, 2009), but this research generally focuses on acclimation to constant temperatures. Studies of increasing temperature variability were associated with increased susceptibility to diseases, pointing to another mechanism of how climate changes may exacerbate amphibian declines (Raffel et al., 2006; Rohr and Raffel, 2010). Given that most ectotherms live in thermally dynamic habitats, there is a need to better understand how both mean temperature and short-term temperature variation influences both physiology, stress, acclimation and ultimately the health of individuals (Folguera et al., 2011).
Using the same laboratory population of eastern hellbenders studied here, we conducted a previous experiment investigating the effects of realistic patterns of short-term (hourly and daily) changes in water temperature on hellbender growth and immune function. We found that hellbenders were resilient (in terms of immune function, body growth and behavioural activity patterns) to relatively large, but incremental, temperature changes (Δ10°C over 7 days) that were well within the species’ thermal limits. Unexpectedly, short-term temperature variation stimulated the hellbender innate immune system, measured as an increase in plasma bacteria killing ability (Terrell et al., 2013). This study builds upon the previous study to investigate the effects of longer-term temperature variation (still using hourly temperature data) and overall warmer temperatures (simulating projected climate change) on hellbender physiology. We maintained hellbenders at temperatures representative of either current in-stream conditions, or warmer conditions impacted by climate change (+1.6C). Over nearly a year, we evaluated monthly changes in body growth rates, innate immune function, and cellular indicators of physiological stress. We tested the hypothesis that hellbenders would demonstrate reduced growth, decreased innate immune function, and increased stress during periods of high temperature and periods of temperature change. We predicted that these effects would be observed more frequently among hellbenders maintained at warmer overall temperatures (+1.6°C) representing future climate conditions, and that these responses would not be observed in control animals held at a constant optimum temperature.
Materials and methods
Animal husbandry
Eighteen juvenile C. alleganiensis alleganiensis (age, 3 years; sex, unknown) were collected from the wild as an egg mass and reared in captivity at the Buffalo Zoo. These animals were transferred to the Appalachian Salamander Lab at Smithsonian’s National Zoological Park (NZP, Washington, DC, USA) where they were maintained in groups of three among six independent 40-gal aquaria (Fig. 1). Each aquarium was connected to a 30-gal sump tank on a closed-loop pumping system, as described previously (Terrell et al., 2013). We maintained oxygen levels at or near saturation using water pumps to produce a stream of high-flow water from an inlet in the tank headspace. Individuals within each aquarium were isolated by egg crate dividers. Hellbenders were fed (5 g per tank section) twice a week in the afternoons with a mixed, commercially produced diet of live crayfish, ghost shrimp and chopped earthworms and frozen/thawed krill. Food was usually entirely consumed, unless hellbenders were experiencing very cold or warm temperatures, uneaten food and faeces were removed each morning in daily spot checks. Aquaria were cleaned once a week, partially (30%) emptied and refilled with filtered municipal water of the same temperature (±2°C). Water temperatures in each aquarium were recorded hourly using HOBO Water Temperature Pro v2 data loggers (U22-001, Onset Computer Corporation, Bourne, MA, USA). Using an astronomical timer, lighting was set to match the daylength corresponding to water temperature data at the latitude where eggs were collected (described below). All animal procedures were approved by the Smithsonian’s NZP Institutional Animal Care and Use Committee (IACUC #11–19).
Figure 1.

Appalachian salamander laboratory exhibit with experimental setups. Juvenile hellbenders C. alleganiensis (a) were held in groups of three per tank with temperatures controlled by automated chiller system to replicate simulated climate treatments as part of a public exhibition on climate change research on salamanders at the Smithsonian National Zoological Park’s Reptile Discovery Center (b).
Temperature experiment
Prior to the experiment, water temperatures in the hellbenders’ stream of origin (Allegheny River watershed) were recorded every 30 minutes for nearly 1 year (343 days; 4 September 2011 to 11 August 2012; range, −0.1 to 29.4°C; mean, 11.6°C) using a HOBO Water Temperature Pro v2 data logger that we placed under the nest rock of origin (U22-001, Onset Computer Corporation, Bourne, MA, USA). To understand the physiological effects of seasonal temperature variation and climate warming, these data were used to experimentally changing temperatures on an hourly basis to recreate in situ temperature patterns (Fig. 2a) using a custom-engineered system and Siemens Insight Workstation (Terrell et al., 2013). These were compared to control animals held at a constant temperature. Eighteen hellbenders with a mean weight of 145 g (SD ±15 g) were randomly assigned to three groups of six animals. The first group was exposed to this in situ pattern of temperature variation as closely as possible (i.e. ‘current climate group’; mean, 14.6°C; range, 3.7–28.2°C). A second treatment was exposed to a similar pattern of temperature change, but with values increased by an average of ~1.6°C (i.e. ‘warmer climate group’; mean, 16.2°C; range, 5.8–29.6°C). A control group (n = 6 hellbenders) was maintained at relatively constant temperatures (mean, 14.6°C; range, 14.1–16.0°C). The continuous 259-day ex situ experiment was carried out between 1 November 2012 and 17 July 2013, reproducing temperature data recorded in situ from 4 September 2011 to 11 August 2012. We omitted an 84-day period representing mid and late winter (3 December 2011–24 February 2012) from our simulated data because these temperatures (mean, 2.3 ± 1.5°C) were near/below the lower operating limit of the chiller system (2°C).
Figure 2.
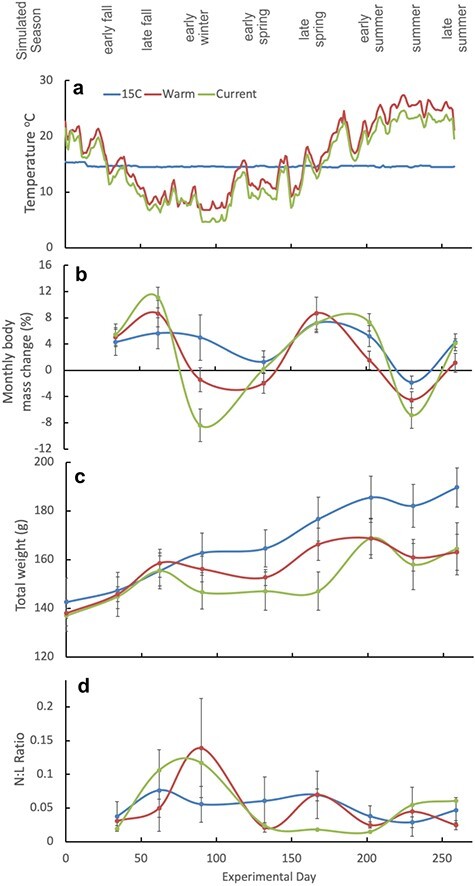
Simulated climatic variations in temperature in relation to changing body mass and N:L ratios. (a) Experimental seasonal conditions applied to the three treatment groups (simulated current season, simulated warming seasons and 15°C control no seasonal variation). Lower panels show means of each treatment group (n = 6 animals) ±standard error for growth as measured by the following: (b) monthly body mass change (%), (c) total weight and (d) N:L ratio.
Blood collection and weight measurement
Blood samples (≤0.4 ml) were collected by a veterinarian via the caudal tail vein from all hellbenders within a 3-hour period once every 28–43 days throughout the temperature experiment, depending on NZP veterinarians’ schedules. Hellbenders were captured using a hand net and restrained using a wet towel, and blood was collected within 5 minutes of initiating animal capture, using a 22-gauge needle and 1-ml syringe. Nitrile gloves were worn for all animal handling procedures, and towels and gloves were changed between successive animals. Immediately after collection, a small volume (~5 μl) of blood was smeared onto a glass microscope slide in duplicate for leukocyte counts. After drying, the slides were fixed in 100% methanol for 5 minutes, stained with DipQuick (MWI Veterinary Supply, Boise, ID, USA) and examined at 1000× magnification using a standard light microscope. For each smear, 100 leukocytes were counted and identified as neutrophils, lymphocytes, eosinophils or monocytes (Heatley and Johnson, 2009), then used to calculate the neutrophil:lymphocyte (N:L) ratio. Higher N:L ratios are an important indicator of stress in amphibians and are a more reliable indicator of longer-term stress than corticosterone, which increases very rapidly in response to handling (Davis and Maerz, 2008; Davis and Maney, 2018; Litmer et al., 2020). The remainder of each blood sample was immediately transferred to a heparinized tube and placed in an ~4°C cooler. Chilled blood samples were centrifuged (15 minutes; 3200 × g) within 2 hours to isolate plasma for bacterial killing assays. Plasma samples were frozen at −80°C until analysis.
Each animal was weighed immediately after blood collection using a sterile, empty plastic container. The animal was returned to its tank immediately after weighing, and the weight of the container then was recorded to account for the container itself and any tank water carried over with the hellbender. Monthly weight change was calculated for each hellbender as the percent changed in weight compared to the previous weight measurement, divided by the number of days since the last measurement (ranging from 28 to 43 days) then multiplied by 30 days.
Bacteria-killing assay
Bacteria-killing ability assays are a way to measure of protein complement activity in the blood and are considered an indicator of innate immune function. The bacteria-killing ability of C. alleganiensis plasma was tested separately against Escherichia coli (ATCC8739), Pseudomonas aeruginosa (ATCC 9027) and Stenotrophomonas maltophilia (ATCC 49130; Fisher Scientific, Waltham, MA, USA), as described previously (Liebl and Martin, 2009; Terrell et al., 2013). Briefly, an isolated colony of each bacterial species (Fisher Scientific, Waltham, MA, USA) was used to prepare a stock solution, the concentration of which was determined by plating serial dilutions (10−4, 10−5, 10−6, 10−7) and counting colony forming units (CFUs). Thawed plasma samples (5 μl) were combined with bacteria (20 μl diluted to 106 CFUs/ml) and amphibian phosphate-buffered saline (PBS) (75 μl) in duplicate in a 96-well plate. A blank (plasma and PBS only) was included for each sample. Positive (plasma-free) and negative (bacteria-free) controls were included in each plate in triplicate. To control for inter-assay variation, all treatment groups from a given date were included in the same plate. Plates were shaken and subsequently incubated (21°C, 1 hour) to allow bacteria killing to occur. Tryptic soy broth (200 μl) was then added to each well (except for blanks), and plates were incubated (30°C) for 12 hours (E. coli) or 16 hours (P. aeruginosa and S. maltophilia) to allow bacterial growth to occur. Length of incubation corresponded to the amount of time needed for the bacterium to reach its exponential growth phase, which we previously determined by measuring changes in sample absorbance over time. Absorbance was read at 405 nm immediately after incubation. Bacteria-killing ability was calculated as follows:
Bacteria killing ability = (1 – ((Asample – Aplasma blank)/(Apositive control – ATSB blank))) × 100, where A = absorbance at 405 nm.
Statistical analyses
All analyses were conducted using R software (R Development Core Team, 2021). Temperature variables (mean, minimum, maximum) were calculated from the HOBO data logger in each tank for the 7–28 days prior to each blood draw. Temperatures from 0 to 7 days before the blood draw were not included because our previous study found that hellbender immune responses to temperature change take at least a week to manifest (Terrell et al., 2013). Temperature difference was calculated as the difference between the minimum and maximum temperature values within each 7–28 day period. To evaluate the effect of mean temperature and absolute temperature change on response variables (i.e. monthly body mass change, bacteria-killing ability and N:L), we used a Generalized Linear Mixed Model Penalized Quasi-Likelihood (GLMMPQL) analysis in the MASS package in R (Ripley et al., 2019). We selected this approach to accommodate nested random variables (tank and individual) in a non-linear model with an unbalanced design (some missing data because blood sampling attempts were occasionally unsuccessful), with fewer assumptions than GLMs (Venables and Ripley, 2002). Models were tested for variance inflation resulting from multicollinearity of predictors using caret in R (Kuhn et al., 2021). Model variables were only retained if, after accounting for multiple comparisons, the generalized inflation factor was lower than 5 (James et al., 2013). Selected significant (P < 0.05) associations with temperature were plotted using the stat_smooth function in the ggplot2 package of R to better visualize thermal optima using predicted relationship with 95% confidence interval.
Results
Growth
Treatment group, experimental day, mean temperature, temperature change and the interaction between mean temperature and temperature change were all significant predictors of body mass change (Table 1; Fig. 2b; main effects plots in supplementary materials S1 and experimental data S2). Total body mass of the control animals increased over the course of the experiment and they attained heavier weights by the conclusion of the experiment (Fig. 2c), while the two simulated climate treatments with changing temperatures had more halting growth patterns (Fig. 2c). The total body mass of animals in both simulated climate treatment groups started and ended at similar points (Fig. 2c), but the seasonal patterns of change varied in a way that may have conservation implications. Hellbenders in warmer simulated temperatures retained body mass better in winter than current climate simulations, but this was counter-balanced by a prolonged lack of growth in the 3-month summer period. In the summer period that precedes the fall breeding season, warmer simulated conditions resulted in an average loss of −0.6% monthly body mass change, compared to a gain +1.5% monthly body mass change under current climate simulations (Fig. 2b,c). We had expected the control animals to have a constant body mass change over the course of the experiment, but we observed similar, but less pronounced, fluctuations in body mass change to simulated climate treatments (Fig. 2b). Overall, optimum predicted growth occurred between 15°C and 17°C, and hellbenders lost body mass at temperatures below 9°C and above 23°C (Fig. 3a). We found no evidence that large temperature changes themselves negatively affected growth; rather, hellbenders appeared most susceptible to declining body mass when temperatures were cold and relatively constant, or very warm (Fig. 3a). This interaction is visible in Fig. 3a with more constant temperatures (bluer dots) falling below the curve and more variable temperatures (yellower dots) falling above the predicted curve.
Table 1.
Main effects of a GLMMPQL analysis examining the effects of treatment, experimental day, mean temperature and temperature change; the variances due to tank (n = 6) and individual (ID) (n = 18) were accounted for in the model as nested random factors
| Body mass change ~ treatment + day + mean temp * temp change, random = list(~1|Tank,~1|ID) | |||||
|---|---|---|---|---|---|
| Value | Std error | DF | t | P | |
| (Intercept) | −14.82 | 6.37 | 122 | −232 | 0.021* |
| Current climate treatment | −10.45 | 2.38 | 3 | −4.38 | 0.021* |
| Warmer climate treatment | −10.93 | 2.57 | 3 | −4.24 | 0.024* |
| Day | −0.32 | 0.007 | 122 | −4.35 | >0.001*** |
| Mean temp | 1.54 | 0.43 | 122 | 3.54 | 0.006** |
| Temp change | 3.28 | 0.78 | 122 | 4.21 | >0.001*** |
| Mean temp: temp change | −0.153 | 0.049 | 122 | −3.11 | >0.002** |
| N:L ~ treatment + day + mean temp + temp change, random = list(~1|Tank,~1|ID) | |||||
| Value | Std error | DF | t | P | |
| Intercept | 0.081 | 0.015 | 99 | 5.10 | >0.001*** |
| Current climate treatment | 0.038 | 0.02 | 3 | 1.79 | 0.171 |
| Warmer climate treatment | 0.055 | 0.02 | 3 | 2.45 | 0.091 |
| Day | 0.000007 | 0.00005 | 99 | 0.13 | 0.89 |
| Mean temp | −0.002 | 0.0009 | 99 | −2.09 | 0.038* |
| Temp change | −0.005 | 0.0018 | 99 | −3.09 | 0.003** |
| PKA ~ treatment + day + mean temp + temp change, random = list(~1|Tank,~1|ID) | |||||
| Value | Std error | DF | t | P | |
| Intercept | 26.38 | 2.87 | 86 | 9.18 | >0.001*** |
| Current climate treatment | −10.29 | 3.73 | 3 | −2.75 | 0.07 |
| Warmer climate treatment | −7.31 | 4.05 | 3 | −1.80 | 0.16 |
| Day | 0.09 | 0.012 | 86 | 7.29 | >0.001*** |
| Mean temp | −0.002 | 0.0009 | 86 | −2.09 | 0.038* |
| Temp change | 0.86 | 0.38 | 86 | 2.26 | 0.026* |
| SKA ~ treatment + day + mean temp + temp change, random = list(~1|Tank,~1|ID) | |||||
| Value | Std error | DF | t | P | |
| Intercept | 28.61 | 3.47 | 94 | 8.23 | >0.001*** |
| Current climate treatment | −6.93 | 4.65 | 3 | −1.48 | 0.23 |
| Warmer climate treatment | −1.51 | 4.99 | 3 | −0.30 | 0.78 |
| Day | 0.03 | 0.013 | 94 | 2.12 | >0.03* |
| Mean temp | −0.14 | 0.21 | 94 | −0.70 | 0.48 |
| Temp change | 0.06 | 0.43 | 94 | 0.13 | 0.89 |
| EKA ~ treatment + day + mean temp + temp change, random = list(~1|Tank,~1|ID) | |||||
| Value | Std error | DF | t | P | |
| Intercept | 44.64 | 12.48 | 86 | 3.57 | >0.006** |
| Current climate treatment | −52.53 | 16.48 | 3 | −3.18 | 0.049* |
| Warmer climate treatment | −64.62 | 17.68 | 3 | −3.65 | 0.035* |
| Day | −0.05 | 0.05 | 86 | −1.08 | 0.28 |
| Mean temp | 1.62 | 0.73 | 86 | 2.21 | 0.029* |
| Temp change | 3.80 | 1.54 | 86 | 2.46 | 0.015* |
Figure 3.
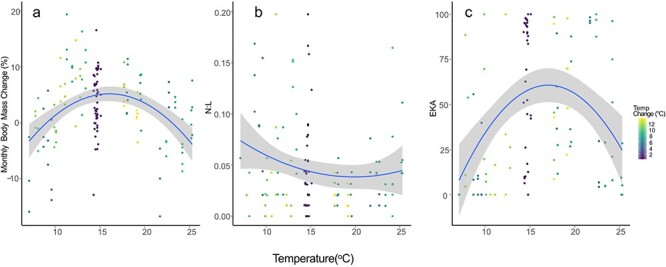
The effects of temperature on growth and immune function. (a) Monthly body mass change, (b) N:L ratios and (c) ability of hellbender plasma to kill E. coli bacteria (EKA) relative to the mean water temperature (x-axis) and temperature change (colour of dots) in the 7–28 day period preceding blood collection. The blue trendline illustrates a polynomial regression with a 95% confidence interval (grey shading).
Immune function
N:L ratios (a potential indicator of stress) were significantly influenced by mean temperature and temperature change, but not climate treatment or experimental day (Table 1; Fig. 2d). While control animals held relatively constant N:L ratios throughout the experiment, peak N:L ratios in the simulated climate treatments occurred in winter (Fig. 2d). Overall, the lowest predicted N:L ratios occurred at temperatures between 16°C and 23°C (Fig. 3b), and while warm temperatures were associated with slightly elevated N:L ratios, the effect of cold temperatures was much more pronounced. Similar to monthly body mass change data, we found no evidence that large temperature changes induced stress; rather, hellbenders appeared most susceptible to high N:L ratios when temperatures were cold and relatively constant (Fig. 3b).
Among all animals, including controls, P. aeruginosa killing ability (PKA) approximately doubled between experimental Day 100 and 150, but was relatively stable outside this period (Fig. 4b). There were no known changes to animal husbandry or the experimental protocol that could explain this PKA pattern. The only potential explanation that we identified was the influence of day length, which, during the course of the experiment, precisely followed a naturalistic pattern corresponding to the source habitat of this experimental population, as described in the methods. Between experimental Day 100 and 150, daylength increased from ≤11.5 hours to ≥13 hours, coincident with the increase in PKA (Fig. 4b). A follow-up statistical analysis, described in the methods, revealed a highly significant association between PKA and experimental day (P > 0.001; Supplementary data S2). The same model indicated that PKA was significantly associated with mean temperature (P = 0.038) and temperature change (P = 0.026; Supplementary data S2), but not climate treatment group (Table 1; Fig. 4b).
Figure 4.
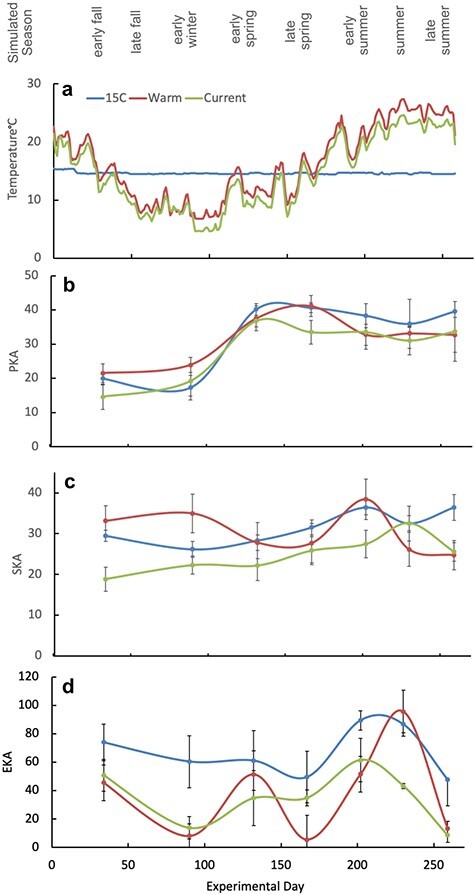
Simulated climatic variations in temperature in relation to bacteria-killing ability of blood. (a) Experimental seasonal conditions applied to the three treatment groups (simulated current season, simulated warming seasons and 15°C control no seasonal variation). Lower panels show bacteria-killing ability of blood means of each treatment group (n = 6 animals) ±standard error. (b) PKA, (c) SKA, (d) EKA.
Stenotrophomonas maltophilia killing ability (SKA) changed significantly (P = 0.03) with experimental day, but temperature (mean or absolute change) did not significantly affect the ability of hellbender plasma to kill S. maltophilia (Table 1. Fig. 4c). Rather, plasma samples consistently killed 10–40% of the bacteria, regardless of temperature at which hellbenders were maintained (Fig. 4c).
Escherichia coli killing ability (EKA) varied significantly in relation to climate treatment, mean temperature (P = 0.029*) and temperature change (0.015*) (Table 1; Fig. 4d). Maximum EKA occurred when hellbenders were maintained at temperatures between 14°C and 21°C (Fig. 3c).
Discussion
Our study yielded several key findings. First, our data suggest that the optimal temperature range for hellbender growth is 15–17°C and that optimum body temperature (i.e. environmental temperature) for some components of hellbender immune function (i.e. bacteria killing) is 14–23°C. Second, hellbenders are susceptible to reduced growth and decreased immune function at both warm (>22°C) and cool (<14°C) temperatures that are well within the species’ normal thermal environment. Third, hellbenders are resilient to relatively large (but naturalistic) temperature changes and these changes may even stimulate growth and immune function. Fourth, it is essential to consider both aspects of temperature—absolute values and variability—to understand hellbender thermal physiology. Finally, we observed that photoperiod may also influence hellbender growth and immune function, but we did not experimentally evaluate daylength as an independent factor.
Our finding that hellbender growth is optimal between 14°C and 17°C is consistent with the natural history of this species, which is well known to rely on cool, well-oxygenated streams (Nickerson and Mays, 1973; Mayasich et al., 2003), and these data may help to inform optimum growth days in future climate simulation models (Westhoff and Paukert, 2014). Similarly, our finding that hellbender growth and immune function declined at warmer temperatures, particularly above 22°C, is consistent with the well-established characterization of hellbenders as being susceptible to negative effects of warmer temperatures (Hutchison and Hill, 1976; Mayasich et al., 2003; Westhoff and Paukert, 2014). However, our study also reveals negative consequences of prolonged cold exposure in hellbenders in terms of weight loss, elevated N:L ratios and EKA of blood. These indicators have all been correlated with stress in other studies and are thought to be important factors when considering the potential effects of climate change on amphibian populations (Rollins-Smith, 2017). This is an important point, and one that may be overlooked in species conservation efforts, even cold-adapted species may be susceptible to cold stress (Angilletta, 2009). For example, prolonged exposure to cold temperatures is known to inhibit antimicrobial peptide synthesis in the wood frog (Lithobates sylvatica), a species that is uniquely adapted to harsh winter environments (Matutte et al., 2000). While our current study focused on measures of innate immune function (i.e. N:L ratios and protein complement activity, as measured by bacteria-killing ability), it is likely that the hellbender adaptive immune system is similarly inhibited by cold temperatures, given the strong dependence of the entire immune system on temperature in ectothermic species (Angilletta, 2009). Indeed, in the northern leopard frog, prolonged cold exposure reduces both protein complement activity (innate immunity) and T-lymphocyte proliferative ability (adaptive immunity) (Green and Cohen, 1977; Maniero and Carey, 1997). It is worth noting, however, that the stress from cold exposure can also have beneficial immune effects after the cold period has been relieved. For example, cold exposure of frogs prior to disease or parasite exposure trials resulted in elevated N:L ratios at the start of the experiment, but these animals maintained lower disease and parasite loads than frogs not subject to cold treatment (Greenspan et al., 2017; Wersebe et al., 2019). In late spring, we observed a reduction in N:L ratios and increase in EKA in the treatment group that had experienced more winter-cold stress, pointing to the possible benefits of cold exposure periods in hellbenders. Given that all climate change scenarios predict lower minimum temperatures and fewer frost days (Easterling et al., 2000) it is essential to further consider the potential immune consequences of changes in cold exposure when evaluating the effects of climate change on hellbenders.
Our finding that hellbenders were resilient to relatively large temperature changes (i.e. ≥10°C over 14 days) is consistent with our previous research, which demonstrated that naturalistic, short-term patterns of thermal variation (i.e. fluctuating between 10°C and 26°C over 7 days) did not inhibit growth of juvenile hellbenders (Terrell et al., 2013).
Amphibian research involving bacteria-killing assays typically utilize E. coli as the sole microbial species, particularly when small blood volumes preclude multiple assays (e.g. Savage et al., 2016), and others have emphasized that these assays are not easily generalizable and we need to develop immune assays that are best suited to the host species (Liebl and Martin, 2009), which is why we selected these three assays. We found that temperature and thermal variation both predicted PKA and EKA of hellbender plasma similar to findings of an earlier experiment on these animals (Terrell et al., 2013), while SKA was fairly consistent across treatment groups and was not a particularly useful indicator of the effects of temperature on immune function. While there was no effect of experimental day on EKA, PKA was most strongly influenced by experimental day, even for control animals and so we relied more heavily on the EKA model to interpret the effects of temperature on immune function in this experiment.
Experimental day most strongly predicted PKA and body mass change, and to a lesser extent, SKA. However, it was cross-correlated with daylength and so we suspect that daylength, or some other systematic experimental factor, was responsible. The seasonal growth and PKA changes were observed in all treatment groups, including control animals. Daylength affects the pineal gland activity and melatonin production that is connected to growth, behaviour, immune function and development that physiologically prepare an animal to anticipate seasonal conditions (Walton et al., 2011; Andreazzoli and Angeloni, 2017; Ruchin, 2019; de Vlaming and Olcese, 2020). Photoperiod is therefore the most likely factor causing the seasonal changes we observed in the control animals and should be accounted for in future investigations.
The climatic changes associated with temperature variability could have synergistic or antagonistic effects on populations (Estay et al., 2014). Our study suggests that cold stress is negative for hellbenders in terms of growth, but may have a positive effect on immune function after the cold stress has been relieved, so the population-level effects of this tradeoff remain unknown. Hellbender breeding is thought to be connected to temperature, food availability, water flow and daylength, but these variables are confounded with seasonal changes so it is uncertain which are the primary cues (Browne et al., 2013). Nonetheless, for hellbenders summer is a period of high stream productivity and an optimal feeding time to establish a positive energy balance needed for breeding season when females lay eggs and males guard nests from conspecifics that may cannibalize the eggs (Browne et al., 2013). We observed that hellbenders were surprisingly tolerant of warmer temperatures, but stopped feeding at both higher and lower temperatures. The net annual effects on growth were similar for both current and warmer climate treatments, but the net negative energy balance in the warmer scenario occurs mostly in summer immediately preceding a breeding season and could therefore be more detrimental to population growth. If hellbender breeding season is cued by photoperiod, it is possible that this is yet another example of well-documented climate-change driven mismatches to stable photo periods that jeopardize animal fitness (Walker et al., 2019). If this hypothesis is correct it could also account for localized patterns of reduced recruitment observed in catchments where riparian canopy has been lost (Bodinof Jachowski and Hopkins, 2018) and stream temperatures are warmer (Caissie, 2006). Several avenues of research remain unexplored, including the effect of temperature on hellbender adaptive immune function, as well as the relative roles of temperature versus photoperiod as a cue for seasonal events (e.g. spermatogenesis, oogenesis and breeding behaviour). Ultimately, a better understanding of hellbender physiology will benefit this imperilled, fascinating species by informing in situ conservation efforts and improving the captive husbandry programs that support these efforts.
Funding
This work was supported by the David H. Smith Fellowship Program, which is funded by the Cedar Tree Foundation and administered by the Society for Conservation Biology.
Acknowledgements
We thank the Buffalo Zoo in New York for providing the captive-reared animals used in this research. We also thank the animal care staff at the National Zoo’s Reptile Discovery Center for assistance with this research.
References
- Andreazzoli M, Angeloni D (2017) The amphibian clock system. In Biological Timekeeping: Clocks, Rhythms and Behaviour, Springer, New Delhi, pp. 211–222. [Google Scholar]
- Angilletta MJ (2009) Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford University Press, Oxford. [Google Scholar]
- Bodinof Jachowski CM, Hopkins WA (2018) Loss of catchment-wide riparian forest cover is associated with reduced recruitment in a long-lived amphibian. Biol Conserv 220: 215–227. [Google Scholar]
- Bozinovic F, Catalan TP, Estay SA, Sabat P (2013) Acclimation to daily thermal variability drives the metabolic performance curve. Evol Ecol Res 15: 579–587. [Google Scholar]
- Browne R, Li H, Wang Z, Okada S, Hime P, McMillan A, Wu M, Diaz R, McGinnity D, Briggler J (2013) The giant salamanders (Cryptobranchidae): Part B. Biogeography, ecology and reproduction. Amphib Reptile Conserv 5: 30–50. [Google Scholar]
- Caissie D (2006) The thermal regime of rivers: a review. Freshw Biol 51: 1389–1406. [Google Scholar]
- Cavieres G, Rezende EL, Clavijo-Baquet S, Alruiz JM, Rivera-Rebella C, Boher F, Bozinovic F (2020) Rapid within- and transgenerational changes in thermal tolerance and fitness in variable thermal landscapes. Ecol Evol 10: 8105–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Maerz JC (2008) Comparison of hematological stress indicators in recently captured and captive paedomorphic mole salamanders, Ambystoma talpoideum. Copeia 2008: 613–617. [Google Scholar]
- Davis AK, Maney DL (2018) The use of glucocorticoid hormones or leucocyte profiles to measure stress in vertebrates: What’s the difference? Methods Ecol Evol 9: 1556–1568. [Google Scholar]
- Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2000) Climate extremes: observations, modeling, and impacts. Science (80-) 289: 2068–2074. [DOI] [PubMed] [Google Scholar]
- Estay SA, Lima M, Bozinovic F (2014) The role of temperature variability on insect performance and population dynamics in a warming world. Oikos 123: 131–140. [Google Scholar]
- Folguera G, Bastías DA, Caers J, Rojas JM, Piulachs MD, Bellés X, Bozinovic F (2011) An experimental test of the role of environmental temperature variability on ectotherm molecular, physiological and life-history traits: Implications for global warming. Comp Biochem Physiol A Mol Integr Physiol 159: 242–246. [DOI] [PubMed] [Google Scholar]
- Green N, Cohen N (1977) Effect of temperature on serum complement levels in the leopard frog, Rana pipiens. Dev Comp Immunol 1: 59–64. [DOI] [PubMed] [Google Scholar]
- Greenspan SE, Bower DS, Webb RJ, Berger L, Rudd D, Schwarzkopf L, Alford RA (2017) White blood cell profiles in amphibians help to explain disease susceptibility following temperature shifts. Dev Comp Immunol 77: 280–286. [DOI] [PubMed] [Google Scholar]
- Guimond RW, Hutchison VH (1973) Aquatic respiration: an unusual strategy in the hellbender Cryptobranchus alleganiensis alleganiensis (Daudin). Science (80- ) 182: 1263–1265. [DOI] [PubMed] [Google Scholar]
- Gvoždík L (2012) Plasticity of preferred body temperatures as means of coping with climate change? Biol Lett 8: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman RH, Irwin KJ, Sutton WB, Miller DL (2020) Evaluation of severity and factors contributing to foot lesions in endangered Ozark hellbenders, Cryptobranchus alleganiensis bishopi. Front Vet Sci 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatley JJ, Johnson M (2009) Clinical technique: amphibian hematology: a practitioner’s guide. J Exot Pet Med 18: 14–19. [Google Scholar]
- Humphries WJ, Pauley TK (2005) Life history of the hellbender, Cryptobranchus alleganiensis, in a West Virginia Stream. Am Midl Nat 154: 135–142. [Google Scholar]
- Hutchison VH, Engbretson G, Turney D (1973) Thermal acclimation and tolerance in the hellbender, Cryptobranchus alleganiensis. Copeia 1973: 805. [Google Scholar]
- Hutchison VH, Hill LG (1976) Thermal selection in the hellbender, Cryptobranchus alleganiensis, and the mudpuppy, Necturus maculosus. Herpetologica 32: 327–331. [Google Scholar]
- James G, Witten D, Hastie T, Tibshirani R (2013) An Introduction to Statistical Learning. Current Medicinal Chemistry, Springer, New York. [Google Scholar]
- Karl T, Melillo J, Peterson T, Hassol S (2009) Global Climate Change Impacts in the United States. Cambridge University Press, Cambridge. [Google Scholar]
- Keitzer SC, Pauley TK, Burcher CL (2013) Stream characteristics associated with site occupancy by the eastern Hellbender, Cryptobranchus alleganiensis alleganiensis, in Southern West Virginia. Northeast Nat 20: 666–677. [Google Scholar]
- Kozak KH, Wiens JJ (2010) Niche conservatism drives elevational diversity patterns in Appalachian salamanders. Am Nat 176: 40–54. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Wing J, Weston S, Williams A, Keefer C, Engelhardt A, Cooper T, Mayer Z, Kenkel B, Benesty M, et al. (2021) caret: Classification and Regression Training. R package version 6.0-86.
- Liebl AL, Martin LB (2009) Simple quantification of blood and plasma antimicrobial capacity using spectrophotometry. Funct Ecol 23: 1091–1096. [Google Scholar]
- Litmer AR, Freake M, Murray CM (2020) Neutrophil: lymphocyte ratios as a measure of chronic stress in populations of the hellbender (Cryptobranchus alleganiensis) across a habitat quality gradient. Copeia 108: 403–415. [Google Scholar]
- Lowe WH (2012) Climate change is linked to long-term decline in a stream salamander. Biol Conserv 145: 48–53. [Google Scholar]
- Maniero GD, Carey C (1997) Changes in selected aspects of immune function in the leopard frog, Rana pipiens, associated with exposure to cold. J Comp Physiol B Biochem Syst Environ Physiol 167: 256–263. [DOI] [PubMed] [Google Scholar]
- Matutte B, Storey KB, Knoop FC, Conlon JM (2000) Induction of synthesis of an antimicrobial peptide in the skin of the freeze-tolerant frog, Rana sylvatica, in response to environmental stimuli. FEBS Lett 483: 135–138. [DOI] [PubMed] [Google Scholar]
- Mayasich J, Grandmaison D, Phillips C (2003) Eastern Hellbender. Eastern Hellbender Status Assessment Report.
- McCarthy T, Masson P, Thieme A, Leimgruber P, Gratwicke B (2017) The relationship between climate and adult body size in redback salamanders (Plethodon cinereus). Geo Geogr Environ 4: 1–9. [Google Scholar]
- Milanovich JR, Peterman WE, Nibbelink NP, Maerz JC (2010) Projected loss of a salamander diversity hotspot as a consequence of projected global climate change. PLoS One 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson CA, Ott CM, Castro SL, Garcia VM, Molina TC, Briggler JT, Pitt AL, Tavano JJ, Byram JK, Barrila J et al. (2011) Evaluation of microorganisms cultured from injured and repressed tissue regeneration sites in endangered giant aquatic Ozark hellbender salamanders. PLoS One 6: 1–10 10.1371/journal.pone.0028906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson MA, Briggler JT (2007) Harvesting as a factor in population decline of a long-lived salamander; the Ozark hellbender, Cryptobranchus alleganiensis bishopi Grobman. Appl Herpetol 4: 207–216. [Google Scholar]
- Nickerson MA, Mays CE (1973) The Hellbenders: North American “Giant Salamanders”. Milwaukee Public Museum Press, Milwaukee. [Google Scholar]
- Pitt AL, Shinskie JL, Tavano JJ, Hartzell SM, Delahunty T, Spear SF (2017) Decline of a giant salamander assessed with historical records, environmental DNA and multi-scale habitat data. Freshw Biol 62: 967–976. [Google Scholar]
- R Development Core Team (2021) R: A language and environment for statistical computing.
- Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ (2006) Negative effects of changing temperature on amphibian immunity under field conditions. Funct Ecol 20: 819–828. [Google Scholar]
- Riddell EA, Odom JP, Damm JD, Sears MW (2018) Plasticity reveals hidden resistance to extinction under climate change in the global hotspot of salamander diversity. Sci Adv 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D (2019) MASS 7.3-51.5 Support Functions and Datasets for Venables and Ripley’s MASS “Modern Applied Statistics with S” (4th edition, 2002) Package for R.
- Rohr JR, Raffel TR (2010) Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci U S A 107: 8269–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Blaustein AR, Johnson PTJ, Paull SH, Young S (2013) Using physiology to understand climate-driven changes in disease and their implications for conservation. Conserv Physiol 1: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA (2017) Amphibian immunity–stress, disease, and climate change. Dev Comp Immunol 66: 111–119. [DOI] [PubMed] [Google Scholar]
- Ruchin AB (2019) The effect of the photoperiod on the larval development and growth of two amphibian species (Amphibia: anura). Biol Rhythm Res 52: 1492–1500 https://doi.org/101080/0929101620191631025. [Google Scholar]
- Savage AE, Terrell KA, Gratwicke B, Mattheus NM, Augustine L, Fleischer RC (2016) Reduced immune function predicts disease susceptibility in frogs infected with a deadly fungal pathogen. Conserv Physiol 4: cow011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell KA, Quintero RP, Murray S, Kleopfer JD, Murphy JB, Evans MJ, Nissen BD, Gratwicke B (2013) Cryptic impacts of temperature variability on amphibian immune function. J Exp Biol 216: 4204–4211. [DOI] [PubMed] [Google Scholar]
- U.S. Fish and Wildlife Service (2018) Species status assessment report for the eastern hellbender (Cryptobranchus alleganiensis alleganiensis).
- Unger SD, Sutton TM, Williams RN (2013) Projected population persistence of eastern hellbenders (Cryptobranchus alleganiensis alleganiensis) using a stage-structured life-history model and population viability analysis. J Nat Conserv 21: 423–432. [Google Scholar]
- Venables W, Ripley B (2002) Modern Applied Statistics with S. Springer Science+Business Media, New York. [Google Scholar]
- de Vlaming V, Olcese J (2020) The pineal and reproduction in fish, amphibians, and reptiles. In The Pineal Gland, CRC press, Boca Raton, pp. 1–29. [Google Scholar]
- Walker WH, Meléndez-Fernández OH, Nelson RJ, Reiter RJ (2019) Global climate change and invariable photoperiods: a mismatch that jeopardizes animal fitness. Ecol Evol 9: 10044–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls SC (2009) The role of climate in the dynamics of a hybrid zone in Appalachian salamanders. Glob Chang Biol 15: 1903–1910. [Google Scholar]
- Walton JC, Weil ZM, Nelson RJ (2011) Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol 32: 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersebe M, Blackwood P, Guo YT, Jaeger J, May D, Meindl G, Ryan SN, Wong V, Hua J (2019) The effects of different cold-temperature regimes on development, growth, and susceptibility to an abiotic and biotic stressor. Ecol Evol 9: 3355–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff JT, Paukert CP (2014) Climate change simulations predict altered biotic response in a thermally heterogeneous stream system. PLoS One 9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler BA, Prosen E, Mathis A, Wilkinson RF (2002) Population declines of a long-lived salamander: A 20+-year study of hellbenders, Cryptobranchus alleganiensis. Biol Conserv 109: 151–156. [Google Scholar]


