Abstract
Advances in public health and medical care have enabled better pregnancy and birth outcomes. The rates of perinatal health indicators such as maternal mortality and morbidity; fetal, neonatal, and infant mortality; low birthweight; and preterm birth have reduced over time. However, they are still a public health concern, and considerable disparities exist within and between countries. For perinatal researchers who are engaged in unraveling the tangled web of causation for maternal and child health outcomes and for clinicians involved in the care of pregnant women and infants, artificial intelligence offers novel approaches to prediction modeling, diagnosis, early detection, and monitoring in perinatal health. Machine learning, a commonly used artificial intelligence method, has been used to predict preterm birth, birthweight, preeclampsia, mortality, hypertensive disorders, and postpartum depression. Real-time electronic health recording and predictive modeling using artificial intelligence have found early success in fetal monitoring and monitoring of women with gestational diabetes especially in low-resource settings. Artificial intelligence–based methodologies have the potential to improve prenatal diagnosis of birth defects and outcomes in assisted reproductive technology too. In this scenario, we envision artificial intelligence for perinatal research to be based on three goals: (1) availability of population-representative, routine clinical data (rich multimodal data of large sample size) for perinatal research; (2) modification and application of current state-of-the-art artificial intelligence for prediction and classification in health care research to the field of perinatal health; and (3) development of methods for explaining the decision-making processes of artificial intelligence models for perinatal health indicators. Achieving these three goals via a multidisciplinary approach to the development of artificial intelligence tools will enable trust in these tools and advance research, clinical practice, and policies to ensure optimal perinatal health.
Keywords: artificial intelligence, low birthweight, machine learning, perinatal health, preterm birth, severe maternal morbidity
Introduction
The perinatal period represents a vital period for maternal and infant health. Progress in perinatal health has been made in the past decades worldwide, represented by the decreased maternal and neonatal mortality, low birthweight (LBW), and preterm birth (PTB) rates. Recent studies have shown that artificial intelligence (AI) has the potential to accelerate this progress. This review aims to present an overview of perinatal health indicators, summarize evidence on the current state of evidence for AI application in perinatal health, and discuss future directions for this field.
Perinatal health indicators
Maternal mortality and morbidity; fetal, neonatal, and infant mortality; LBW (defined as birthweight <2500 g);1 and PTB (defined as gestational age at birth <37 weeks)1 are common indicators of perinatal health that are of public health concern and have significant implications for both the mother and child.
Some of the perinatal health indicators are included in the Sustainable Development Goals (SDGs) of the World Health Organization. SDG 3 that relates to good health and well-being includes goals to reduce maternal and neonatal mortality. One of the targets of SDG 3 is to reduce maternal mortality ratio to less than 70 per 100,000 births by 2030, with no country having a maternal mortality ratio of more than twice the global average. Another target is to reduce neonatal mortality in all countries to at least 12 per 1000 live births. Besides this, a target of SDG 3 is to increase health financing and training of the health workforce, which relates to the use of current methods that are relatively cheap but efficient to improve perinatal health and well-being. This indicates that adequate antenatal care, skilled care at birth, and postnatal maternal and infant care are important for perinatal health and well-being.2 This has been illustrated by a study that found a midwife-led continuity of care to reduce PTBs by nearly 24%.3 The American College of Obstetrics and Gynecology recommends provision of postpartum care as a continuum rather than a single encounter;4 therefore, one needs to focus on the woman not only during pregnancy but throughout the life course5 and incorporate improved health care system as a component of maternal and child care.
Maternal indicators
There is considerable difference in maternal mortality and morbidity rates between high-income countries (HICs) and low-income countries (LICs). In 2017, the maternal mortality ratio was estimated to be 11 per 100,000 live births in HIC, whereas in LIC it was 462 per 100,000 live births with the sub-Saharan Africa having the highest estimate at 534 per 100,000 live births.6 Notably, about 90% maternal deaths occur in LICs and lower-middle-income countries.7 Most maternal deaths occur due to obstetric hemorrhage, infections, hypertensive disorders during pregnancy, preexisting chronic health conditions, complications from delivery, and unsafe abortion.8,9
Although maternal mortality has reduced in most HICs mainly due to skilled attendant care, severe maternal morbidity (SMM) is still a concern in most countries. In LIC, in addition to obstetric causes such as hemorrhage, hypertensive disorders during pregnancy, and sepsis due to obstetric infections, non-obstetric cause such as sepsis is a major contributor of maternal mortality.10 There are long-term implications of SMM—for example, preeclampsia is associated with an increased risk for cardiovascular disease for the mother.11 For the infants, SMM is associated with increased risk for fetal death, PTB, and LBW.1
Irrespective of regions (HIC or LIC), SMM has been mainly associated with components of quality of maternal care.10,12 In LIC, these are related to poverty, physical access to care, lack of information, substandard care, and cultural beliefs and practices, and in some regions limited use of evidence approach to obstetric practice.10 Some of these factors are common to HICs, whereas factors such as provider misdiagnosis, lack of coordination between providers, and racial/ethnic disparities in access to care are important additional risk factors for SMM in HICs.1 In addition to disparities between HIC and LIC, there are disparities in maternal mortality and SMM within countries with the rates being higher among non-White women and women living in rural areas.13
It is important to note that most maternal deaths are preventable,12 and there is no standardized definition of SMM.
Child health indicators
Like maternal mortality, neonatal and infant mortality rates have reduced worldwide, with significant differences between HIC and LIC. In HIC, the neonatal mortality rate1 is 3.0 per 1000 live births, and the infant mortality rate1 is 4.0 per 1000 live births. In contrast, in LIC, the corresponding rates are 27 per 1000 live births and 48 per 1000 live births, respectively.14 Neonatal mortality constitutes one-third of deaths under 5 years of age, and like maternal morbidity it is related to lack of quality or skilled care at birth and treatment postpartum.3 The leading causes of neonatal/infant mortality are PTB; LBW; congenital anomalies; pregnancy complications such as infections, diabetes, or hypertensive disorders during pregnancy; intrapartum-related complications;3 and sudden and unexpected infant deaths.15
Globally, PTB rates range from 5% to 18%16 with a higher burden among LICs and middle-income countries with 60% of the burden borne by countries in sub-Saharan Africa and South Asia alone.17 The prevalence of PTB has reduced in some HICs such as the United Kingdom and the Netherlands, but it has increased in countries such as Chile and Belgium (2000–2015).18 It accounts for about 75% of perinatal mortality and more than 50% of long-term morbidity such as neurodevelopmental impairments and respiratory and gastrointestinal complications.19 About 40% to 45% of PTBs are due to spontaneous labor with intact membranes, 30% to 35% are indicated (vaginal or caesarean), and the remaining 25% to 30% are due to premature rupture of membranes. The increase in PTBs in singletons can be mainly attributed to an increase in indicated PTBs.19 The pathogenesis and mechanisms of PTB are not well known, but most of the risk factors for its occurrence are associated with increased systemic inflammation.19 Important risk factors for PTB include preeclampsia, congenital anomalies, multiple gestation, infection, maternal smoking, being African American, and stressful life events.19–21 There is a 15% to 50% risk of PTB in subsequent pregnancies due to recurrent intrauterine infections and preexisting chronic health conditions. Furthermore, multiple pregnancies are at higher risk for spontaneous PTB, whereas preeclampsia, eclampsia, and intrauterine growth restriction are important risk factors for indicated PTBs.19 But an individual patient data analysis from four countries with high human development index concluded that 21 known risk factors for PTB can explain only 35% of the total risk for PTB.21
LBW prevalence has reduced in most countries, but in sub-Saharan Africa, it has continued to rise mainly because of the high prevalence of maternal infection. Although the rates have decreased over time in Southern Asia, nearly half of the world’s total LBW live births occur in this region with the primary cause being maternal undernutrition.22 Another major risk factor for LBW is prematurity. LBW accounts for about 60% of neonatal mortality. Both LBW and PTB, if not fatal, are associated with an increased risk for mortality and morbidity in childhood and cardiovascular disease,23,24 diabetes,24,25 and obesity in adulthood.25
Other indicators of perinatal health
Other indicators of perinatal health include congenital anomalies, which are related to perinatal health indicators such as PTB and neonatal and infant mortality.26 Globally, congenital anomalies including chromosomal (e.g. aneuploidy), single gene defects, and non-chromosomal anomalies (e.g. congenital heart defects) are the second leading cause of neonatal death after birth asphyxia and birth trauma,27 whereas in the United States it is the leading cause of infant mortality.28 Besides the long-term physical, cognitive, and social impact on the child29 and parental quality of life,30 it is associated with substantial economic burden; in the United States, the cost of birth defect–associated hospitalizations was estimated to be US$22.9 billion in 2013.31 Besides this, it is a significant contributor to PTB (more than 2.5 times higher risk) and LBW (more than 3.5 times higher risk).32 Common risk factors for congenital anomalies include chromosomal (most common being aneuploidy) or genetic, smoking, alcohol, substance abuse, diabetes mellitus, multiple gestation,33 advanced maternal age, environmental teratogens, infections such as Zika and rubella,27 and increased body mass index.34
It is being recognized that to achieve better perinatal health, one needs to have a comprehensive definition of perinatal health indicators. To this end, the Euro-Peristat project has strived to identify indicators that span across the entire health care continuum in Europe with quality of care included as an important indicator.35
AI in perinatal health
AI is a field of science that simulates human intelligence and behavior to perform a specific task. It can aid in decision-making process and improve medical care. In health care, AI can be used for prediction modeling, diagnosis, early detection, and monitoring. Machine learning (ML), either supervised, semi-supervised, unsupervised, or reinforcement, is a subset of AI that involves the use of algorithms and computer models to achieve a certain goal. Furthermore, deep learning is an ML technique that uses neural networks akin to the neurons in the human brain where given input, multiple levels of representation of data can be extracted to solve a problem.36
ML can be used to learn from existing data and make predictions from new data. Unlike parametric models in statistics, fewer assumptions are required in modeling via ML.37 ML models have the potential to perform better than traditional statistical models because of their ability to deal with nonlinear complex data, multiple interactions between variables, and handle multiple predictors and chain of events simultaneously.38 In addition, in health care research, it is helpful to know the spectrum of phenotypes for a specific outcome for better predictions, which is possible through deep learning that enables better phenotyping using multimodal data such as imaging, laboratory tests, clinical diagnosis, and genetics.39
In a scoping review of AI in pregnancy, Oprescu et al. found that ML has been used in more than two-thirds of the studies about predictive modeling followed by computational intelligence.40 They found that the most common models used for supervised learning were classification or regression models, whereas for unsupervised learning, clustering and dimensionality reduction were predominantly used, and deep learning was used in about 7% of the studies only. The commonly used ML algorithms are decision trees followed by support vector machines, logistic regression, and artificial neural networks.40
ML methods have been used to predict PTB,41 birthweight, and postpartum depression using existing data, whereas predictions about preeclampsia, mortality, hypertensive disorders during pregnancy, labor, and delivery have been made using both real-time and existing data.40 Furthermore, Mboya et al. found that ML may enable better prediction of perinatal mortality depending on the method used.37 However, using data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Global Network Maternal Newborn Health Registry study, Shukla et al. found that if one used the same list of variables for predictive modeling of stillbirth and neonatal morality, then the Area Under the Receiver Operating Characteristic (AUROC)s of logistic regression and top-performing ML models did not differ substantially.38 They also found that prenatal or pre-delivery variables had lower predictive accuracy than immediate post-delivery variables in the prediction of neonatal mortality. Furthermore, this study revealed birthweight to be most important predictor of neonatal mortality, whereas the contribution of other variables was not found to be substantial.38 In women with gestational diabetes, ML has been used both for predictions and monitoring.40 Furthermore, ML can be used to make real-time diagnostic predictions such as the need for caesarean delivery or labor induction.40
One of the areas that ML has great potential for is in birth defects research. It has been used for aneuploidy detection using unsupervised learning methods such as artificial neural networks42 and hidden Markov model combined with supervised learning methods such as decision tree and support vector machine.43 Methods such as Fetal Intelligent Navigation Echocardiography (FINE) that use ML have been utilized in the prenatal diagnosis of congenital birth defects especially congenital heart defects.39
Perinatal researchers have also used ML methods to analyze large, multidimensional omics data. Stelzer et al.44 integrated maternal metabolome, proteome, and immunome data using a stacked generalization algorithm to predict the timing of spontaneous labor. Jehan et al.45 found higher predictive accuracy for PTB when transcriptomics, metabolomics, and proteomics data from five low and middle-income countries (LIMCs) were integrated into a single model instead of analyzing them separately suggesting the role of multiple biological systems in the etiology of PTB.
In real-time settings, cardiotocography using AI has the potential to reduce observer bias and increase better interpretation of fetal heart rate and uterine contractions; however, there is limited evidence for its superior performance to predict adverse pregnancy outcomes.46 Real-time fetal electrocardiogram recordings can be used by patients and clinicians to monitor fetal state. AI-based sensors can be used to monitor blood glucose and blood pressure, which are especially useful in low-resource settings. This has become all the more important since the COVID-19 pandemic where health care professionals are burdened by the huge surge of patients because of which patients with preexisting conditions are not being able to seek timely help and monitoring.40 This is also related to one of the most promising area of AI applications, that is, mobile health (mHealth). mHealth is extremely useful for prenatal care especially in low-resource settings, where community health workers can facilitate care delivery, monitor health, and enable patient self-management.39,47 Even in high-resource settings, mHealth enables personalized monitoring to provide support to pregnant women.40
Future directions and role of AI in perinatal health
AI for perinatal health is in its nascent stages. If developed methodically and in collaboration with perinatal health specialists, AI research can benefit clinical aspects of perinatal health and even research into applied AI at large. With more efficient AI tools serving perinatal health care researchers and clinicians that better predict conditions more precisely and sooner than current methods, AI can have stronger clinical impact. However, even more important than the need to develop robust AI is the need to develop explainable AI or AI that is transparent and able to be understood by humans.48 In matters of health, development and validation of AI models should bring with it trust about how the model is actually processing and functioning.49 Much more subtly and crucially, this AI should be understandable by clinicians since clinicians are tasked with diagnosis and treatment. For these reasons, a vision of AI for health and perinatal health care especially hinges on efficiency and “explainability.”
Thus, AI for perinatal health should have three goals for future research. First, AI research should aim to amass population-representative, routine clinical data for health research at large and specifically perinatal research. AI is a powerful tool that can be carefully built to include multimodal data but often requires large sample size to demonstrate strong performance. Thus, routinely collected, administrative primary and secondary care electronic health record (EHR) data that are representative of the population and collected systematically offer a solid foundation to begin AI research.50 However, it is worth noting the inherent limitations of EHR data such as imprecise measurement, informed presence bias,51 and missing data.
Second, current state-of-the-art AI for prediction and classification in health care research should be modified and applied to the field of perinatal health. Research in AI for health has been growing in popularity. There has been much work in prediction of events and conditions in neurology, cardiology, and other medical focus areas.52 As an exemplar, in neurology, multimodal data—high-dimensional brain imaging and genetics—can be used in a deep-learning model for improved prediction of epilepsy.53 Another example is its use in anesthesiology where it can be used to improve the depth of anesthesia monitoring, pain management, and prediction of operative, postoperative, and critical care–related events.54 Furthermore, in cardiovascular medicine, it can be used in the management of heart failure, including identification of patients at risk, development of risk assessment tools, and the use of multimodal EHR data for clinical decision support in these patients.55 Another use in cardiology is in electrocardiography AI research where convolutional neural networks can be used to identify subclinical disease or prognostic factors of disease.55 Prediction of PTB and LBW would be an important research contribution in perinatal health. A 2013 study found only an estimated 5% relative reduction in PTBs for years 2010 to 2015 resulting in US$3 billion savings in total economic cost if evidence-based interventions were implemented in countries with very high human development index.56 Also, two-thirds of the variation in total risk for PTB is still unaccounted for despite decades of research. This highlights the potential for research into factors that can lead to greater reduction in PTB and economic burden too. In addition, multi-task learning is an exciting area in ML research that involves simultaneous learning of multiple tasks by a shared model.57 Pregnancy is a unique and finite period that involves shared maternal–fetal exposures resulting in pregnancy and infant outcomes. In this context, multi-task learning can be applied to study exposures common or unique to both the mother and fetus, interactions between them, and fetal outcomes such as live birth, stillbirth, congenital anomalies, LBW, and maternal outcomes such as obstetric hemorrhage, intrapartum infections using a single model.
Furthermore, real-time electronic health recording and predictive modeling have found initial success in the field of pregnancy health40 and can especially be applied to health care systems in LIC. In HIC, 81% of the population is estimated to be living in urban areas, whereas in LIC this proportion is 33%.14 Women in rural areas, irrespective of development status, have poor access to medical care and resources.58 In addition, in HIC, the number of beds per 1000 people is 5.3, whereas in LIC it is 0.8 and in more than 55% of the countries the rate of nursing and midwifery is 10 per 10,000 population.7 In these settings, mHealth can help pregnant women with conditions such as preeclampsia to identify signs and symptoms and seek timely help. In low-resource settings where health care professionals mostly rely on last menstrual period to estimate gestational age, AI-based tools can enable a cost-effective and more accurate estimation of gestational age. AI can facilitate screening process for SMM in HICs as well as LICs for faster review based on a list of criteria.
Finally, AI for perinatal health requires methods for explaining the decision-making processes of its models. Recently, with an explosion of AI methods such as machine, deep, and reinforcement learning–based models for prediction tasks, the need to ask the question: “What is AI actually learning?” remains ever important. Answering this question requires the development of tools for responsibly gauging and vetting AI models. In the past decade, some tools to explain the importance of input variables in image and static data such as the Shapley Additive Explanations (SHAP)59 and even explainers for longitudinal data60 have allowed subject specialists to query what the model is attributing as signal. These methods have even been used in the field of perinatal health to understand input features strongly associated to the condition of autism spectrum disorder.61 When these tools illuminate the importance of a particular feature, specialists can sort these explanations into four categories as shown in Figure 1. While features in squares 1 and 4 demonstrate agreement and little controversy between the subject area specialists and AI explainers, features sorted into the remaining two squares suggest further analyses to understand the actual importance of those features. AI understanding and clinical understanding might not always be in line with one another, and many such explanations may reside in squares 2 and 3; the resolution of these disagreements and progress in AI-driven perinatal health care require clinicians and AI specialists working side by side.
Figure 1.
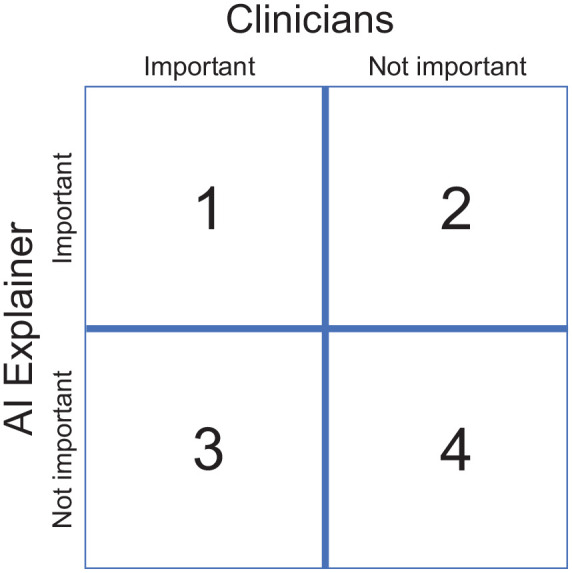
Explanations found important/non-important by clinicians (horizontal) and artificial intelligence (AI) explainers (vertical).
With these three goals, collaboration between perinatal health specialists and AI experts can yield robust AI tools along with trust in the tools. Both are necessary for progress and more importantly, clinical acceptance in areas such as perinatal health care.
Conclusion
In this review, we have summarized and highlighted the potential of AI-based algorithms and methods for the development of novel prediction models, better diagnosis, early identification, and monitoring of women during pregnancy, labor, and postpartum to advance research, clinical practice, and policies, and ensure optimal perinatal health.
Although this is a comprehensive and broad review of perinatal health indicators using AI where we have proposed a threefold approach to the use of AI in perinatal health research, it is not a systematic review that is a structured synthesis of evidence based on a priori protocol. Therefore, it is subject to biases such as publication bias and reviewer bias regarding choice of studies included and biases due to non-assessment of quality of studies included.
While our three goals for adaptation of AI for perinatal health care pave the way for novel findings for the field, we note that AI without societal acceptance and integration is insufficient for impacting clinical care. While robust performing AI and explainable AI can provide explanations for these black-box tools, integrating AI tools into the clinical setting and building community trust require engagement from every faction of society: from health care professionals to governmental workers and policy makers. Therefore, a multidisciplinary approach involving health care professionals, epidemiologists, statisticians, AI scientists, community health workers, organizations and institutions (local, national, and global), engineers, local governments, policy makers, and most importantly the mother herself is required to improve health of mothers and children in developed and developing nations alike.
Footnotes
Author contributions: All authors were involved in conceptualization, writing-review, and editing; read and approved the final article; and take responsibility for the integrity of the work as a whole.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Rema Ramakrishnan  https://orcid.org/0000-0002-6784-8319
https://orcid.org/0000-0002-6784-8319
References
- 1.World Health Organization. International statistical classification of diseases and related health problems. 10th revision, Vol. 2. 5th ed.2016, https://icd.who.int/browse10/Content/statichtml/ICD10Volume2_en_2016.pdf?ua=1
- 2.World Health Organization. Sustainable Development Goals (SDGs), https://www.who.int/health-topics/sustainable-development-goals#tab=tab_2 (2020, accessed 06 September 2021).
- 3.World Health Organization. Newborns: improving survival and well-being, https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality (2020, accessed 22 May 2021).
- 4.ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol 2018; 131(5): e140–e150. [DOI] [PubMed] [Google Scholar]
- 5.Collier A-RY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. NeoReviews 2019; 20(10): e561–e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization, 2019. [Google Scholar]
- 7.United Nations. Sustainable Development Goals: Goal 3: ensure healthy lives and promote well-being for all at all ages, https://www.un.org/sustainabledevelopment/health/ (2021, accessed 21 May 2021).
- 8.World Health Organization. Maternal mortality, https://www.who.int/news-room/fact-sheets/detail/maternal-mortality (2019, accessed 9 June 2021).
- 9.Centers for Disease Control and Prevention. Pregnancy mortality surveillance system, https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm (2020, accessed 9 June 2021).
- 10.Geller SE, Koch AR, Garland CE, et al. A global view of severe maternal morbidity: moving beyond maternal mortality. Reprod Health 2018; 15(1): 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amaral LM, Cunningham MW, Jr, Cornelius DC, et al. Preeclampsia: long-term consequences for vascular health. Vasc Health Risk Manag 2015; 11: 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Maternal mortality: key facts, https://www.who.int/news-room/fact-sheets/detail/maternal-mortality#:~:text=TheMMRinlowincome,birthsinhighincomecountries (accessed 21 May 2021).
- 13.Rivara FP, Fihn SD.Severe maternal morbidity and mortality: JAMA Network Open call for papers. JAMA Netw Open 2020; 3(1): e200045. [Google Scholar]
- 14.The World Bank. Data, https://data.worldbank.org/indicator/SH.DYN.NMRT (2021, accessed 21 May 2021).
- 15.Division of Reproductive Health National Center for Chronic Disease Prevention and Health Promotion. Infant mortality. Centers for Disease Control and Prevention, https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm (2020, accessed 22 May 2021).
- 16.World Health Organization. Preterm birth, https://www.who.int/news-room/fact-sheets/detail/preterm-birth (2018, accessed 22 May 2021).
- 17.Vogel JP, Chawanpaiboon S, Moller A-B, et al. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol 2018; 52: 3–12. [DOI] [PubMed] [Google Scholar]
- 18.Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019; 7(1): e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008; 371(9606): 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behrman RE, Butler AS. (eds). Preterm birth: causes, consequences, and prevention. Washington, DC: The National Academies Press, 2007. [PubMed] [Google Scholar]
- 21.Ferrero DM, Larson J, Jacobsson B, et al. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLoS ONE 2016; 11(9): e0162506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blencowe H, Krasevec J, de Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2019; 7(7): e849–e860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CJ, Ryckman KK, Barnabei VM, et al. The impact of birth weight on cardiovascular disease risk in the Women’s Health Initiative. Nutr Metab Cardiovasc Dis 2016; 26(3): 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markopoulou P, Papanikolaou E, Analytis A, et al. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. J Pediatr 2019; 210: 69–80.e5. [DOI] [PubMed] [Google Scholar]
- 25.Jornayvaz FR, Vollenweider P, Bochud M, et al. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: the CoLaus study. Cardiovasc Diabetol 2016; 15: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wildman K, Blondel B, Nijhuis J, et al. European indicators of health care during pregnancy, delivery and the postpartum period. Eur J Obstet Gynecol Reprod Biol 2003; 111: S53–S65. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Congenital anomalies: key facts, https://www.who.int/news-room/fact-sheets/detail/congenital-anomalies (2020, accessed 1 June 2021).
- 28.Centers for Disease Control and Prevention. Infant mortality, https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm (2020, accessed 1 June 2021).
- 29.Centers for Disease Control and Prevention. About CDC’s work on birth defects, https://www.cdc.gov/ncbddd/birthdefects/aboutus.html#:~:text=Birthdefectsaffect1in,%2Ccognitive%2Candsocialchallenges (2020, accessed 1 June 2021).
- 30.Gregory MR, Prouhet PM, Russell CL, et al. Quality of life for parents of children with congenital heart defect: a systematic review. J Cardiovasc Nurs 2018; 33(4): 363–371. [DOI] [PubMed] [Google Scholar]
- 31.Arth AC, Tinker SC, Simeone RM, et al. Inpatient hospitalization costs associated with birth defects among persons of all ages—United States, 2013. MMWR Morb Mortal Wkly Rep 2017; 66(2): 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolan SM, Gross SJ, Merkatz IR, et al. The contribution of birth defects to preterm birth and low birth weight. Obstet Gynecol 2007; 110(2 Pt 1): 318–324. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Birth defects research and tracking, https://www.cdc.gov/ncbddd/birthdefects/research.html (2020, accessed 1 June 2021).
- 34.Persson M, Cnattingius S, Villamor E, et al. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ 2017; 357: j2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.European Perinatal Health Report. Core indicators of the health and care of pregnant women and babies in Europe in 2015, 2015, https://www.europeristat.com/images/EPHR2015_Euro-Peristat.pdf [Google Scholar]
- 36.Bengio Y.Deep learning of representations for unsupervised and transfer learning. PMLR 2012; 27: 17–36. [Google Scholar]
- 37.Mboya IB, Mahande MJ, Mohammed M, et al. Prediction of perinatal death using machine learning models: a birth registry-based cohort study in northern Tanzania. BMJ Open 2020; 10(10): e040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla VV, Eggleston B, Ambalavanan N, et al. Predictive modeling for perinatal mortality in resource-limited settings. JAMA Netw Open 2020; 3(11): e2026750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson L, Boland MR.Towards deep phenotyping pregnancy: a systematic review on artificial intelligence and machine learning methods to improve pregnancy outcomes. Brief Bioinform. Epub ahead of print 6 January 2021. DOI: 10.1093/bib/bbaa369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oprescu AM, Miró-amarante G, García-Díaz L, et al. Artificial intelligence in pregnancy: a scoping review. IEEE Access 2020; 8: 181450–181484. [Google Scholar]
- 41.Koivu A, Sairanen M.Predicting risk of stillbirth and preterm pregnancies with machine learning. Health Inf Sci Syst 2020; 8(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neocleous AC, Nicolaides KH, Schizas CN.Intelligent noninvasive diagnosis of aneuploidy: raw values and highly imbalanced dataset. IEEE J Biomed Health Inform 2017; 21(5): 1271–1279. [DOI] [PubMed] [Google Scholar]
- 43.Teder H, Paluoja P, Rekker K, et al. Computational framework for targeted high-coverage sequencing based NIPT. PLoS ONE 2019; 14(7): e0209139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stelzer IA, Ghaemi MS, Han X, et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci Transl Med 2021; 13(592): eabd9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jehan F, Sazawal S, Baqui AH, et al. Multiomics characterization of preterm birth in low- and middle-income countries. JAMA Netw Open 2020; 3(12): e2029655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutomski JE, Meaney S, Greene RA, et al. Expert systems for fetal assessment in labour. Cochrane Database Syst Rev 2015; 4: CD010708. [Google Scholar]
- 47.White A, Thomas DSK, Ezeanochie N, et al. Health worker mHealth utilization: a systematic review. Comput Inform Nurs 2016; 34(5): 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagras H.Toward human-understandable, explainable AI. Computer 2018; 51: 28–36. [Google Scholar]
- 49.Dosilovic FK, Brcic M, Hlupic N.Explainable artificial intelligence: a survey. In: Proceedings of the 2018 41st international convention on information and communication technology, electronics and microelectronics, MIPRO 2018, Opatija, 21–25 May 2018. [Google Scholar]
- 50.Gavrielov-Yusim N, Friger M.Use of administrative medical databases in population-based research. J Epidemiol Community Health 2014; 68: 283–287. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein BA, Bhavsar NA, Phelan M, et al. Controlling for informed presence bias due to the number of health encounters in an electronic health record. Am J Epidemiol 2016; 184(11): 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Rao S, Solares JRA, et al. BEHRT: transformer for electronic health records. Sci Rep 2020; 10(1): 7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pedersen M, Verspoor K, Jenkinson M, et al. Artificial intelligence for clinical decision support in neurology. Brain Commun 2020; 2(2): fcaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto DA, Witkowski E, Gao L, et al. Artificial intelligence in anesthesiology: current techniques, clinical applications, and limitations. Anesthesiology 2020; 132(2): 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Jimenez F, Attia Z, Arruda-Olson AM, et al. Artificial intelligence in cardiology: present and future. Mayo Clin Proc 2020; 95(5): 1015–1039. [DOI] [PubMed] [Google Scholar]
- 56.Chang HH, Larson J, Blencowe H, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 2013; 381(9862): 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crawshaw M.Multi-task learning with deep neural networks: a survey. arXiv 2009.09796, https://arxiv.org/abs/2009.09796
- 58.Baird AG, Wright N.Poor access to care: rural health deprivation. Br J Gen Pract 2006; 56(529): 567–568. [PMC free article] [PubMed] [Google Scholar]
- 59.Lundberg SM, Lee SI.A unified approach to interpreting model predictions. In: Advances in neural information processing systems, Long Beach, CA, 4–9 December 2017. [Google Scholar]
- 60.Rao S, Li Y, Ramakrishnan R, et al. BEHRT-HF: an interpretable Transformer-based, deep learning model for prediction of incident heart failure. In: European society of cardiology congress 2020: the digital experience, Virtual meeting, 29 August–1 September 2020. [Google Scholar]
- 61.Caly H, Rabiei H, Coste-Mazeau P, et al. Machine learning analysis of pregnancy data enables early identification of a subpopulation of newborns with ASD. Sci Rep 2021; 11: 6877. [DOI] [PMC free article] [PubMed] [Google Scholar]


