Abstract
Objective: Lung cancer is the most common cancer and can appear as a solitary pulmonary nodule. Early detection of lung cancer in this patient population would be beneficial for the disease management. In this study, the potential application of circulating tumor cells (CTCs) on early detection of lung cancer in this population was investigated. Methods: The number of CTCs in bronchoalveolar lavage fluid and serum levels of tumor-related markers, cancer antigen 125 (CA125), carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) were measured in patients with a solitary pulmonary nodule. The association between CTCs and lung cancer was examined. The diagnosis performances of CTCs and selected tumor-related markers were compared. Results: The CTC positivity was significantly associated with lung cancer (P = .009). The sensitivity of CTCs and CA125, CEA, NSE, and CA125/CEA/NSE was 75%, 5.6%, 0%, 25%, and 33%, respectively. The sensitivity of CTCs was improved from 75% to 83% by the combination with CA125 or NSE. Conclusion: CTCs may be helpful for the early detection of lung cancer in patients with a solitary pulmonary nodule.
Keywords: circulating tumor cells, lung cancer, solitary pulmonary nodule
Introduction
According to the World Health Organization,1 lung cancer is the most common cancer (2.09 million cases) and the most common cause of cancer death (1.76 million deaths). The 5-year survival rate of patients with different stage of lung cancer (stage I, 67%; stage III, 23%)2 suggested the benefits of early diagnosis for improving the prognosis. Low-dose computed tomography (CT) for lung cancer screening has been recommended for the high-risk population in Canada, the EU, Japan, and the United States.3 In addition, a number of serum tumor-related markers have been explored for early detection of lung cancer. Cancer antigen 125 (CA125), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE) and others have been investigated for lung cancer screening in several clinical trials.4 Multiple techniques based on the analysis of exhaled breath condensate, volatile organic compounds, or specific genomic features, have also been developed for the early diagnosis of lung cancer.5
Circulating tumor cells (CTCs) are cells that detach from a primary solid tumor and enter into the bloodstream, which become an emerging target to provide pathological information for diagnosis and prognosis in a variety of cancers.6 The CELLSEARCH® Circulating Tumor Cell Kit, an in vitro diagnostic device approved by the US Food and Drug Administration (USFDA), is used to assess the prognosis of metastatic breast or metastatic colorectal cancer. However, to date no clinical data clearly support the utility of this medical device for the disease management of lung cancer. On the other hand, recent studies showed that the sensitivity and specificity of CTCs identified by the chromosome enumeration probe 8 (CEP8) are 83.3% and 98.6% in patients with lung cancer, respectively.7,8 This unique CEP8+ CTCs has been examined in a series of studies, which showed a significant association with diagnosis and/or prognosis of lung cancer with promising sensitivity and specificity.9-11 According to the potential application of the CEP8+ CTCs on lung cancer management, this technique was utilized in this study.
The primary objective of this study is to investigate the potential of CTCs as a biomarker for early detection of lung cancer. The association between the identification of CTCs and lung cancer was examined. The diagnosis performance between CTCs and selected serum tumor-related markers were analyzed from patients with a solitary pulmonary nodule in outpatients of our hospital, Zhuhai People’s Hospital (Zhuhai hospital affiliated with Jinan University), China.
Materials and Methods
Patients
Inpatients in Zhuhai People’s Hospital (Zhuhai hospital affiliated with Jinan University), China from September 2020 to February 2021 were enrolled in this prospective, evaluator-blinded study. The inclusion criterion was patients who had a solitary pulmonary nodule12 first identified by chest CT imaging. Exclusion criteria were patients who (1) received radiotherapy, chemotherapy, or immunotherapy recently; (2) had cancer history; (3) were not able/willing to receive sampling (blood, sputum, bronchoalveolar lavage fluid [BALF] or lung biopsy); (4) had no clear diagnosis. This study was approved by the institutional review board of Zhuhai People’s Hospital (IRB number: LW-[2020] no. 17) and registered (clinical trial registration number: 20200731105109530). All patients gave signed informed to allow the analyses of their medical records and samples. All samples used in this study were collected at the same timepoint after the informed consent was signed.
Detection of CTCs and Tumor-Related Markers
A CEP8 CTC detection kit (Cyttel®, Cyttel Bio), which is developed based on CD45-FISH technique,7,8 was used for identification of CTCs, and the experimental procedure was according to the user manual. Briefly, CTCs were first enriched from BALF by depletion of CD45+ cells. The remaining cells were fixed on a slide and hybridized with CEP8 probe. The identification criteria for CTCs (DAPI+/CD45−/CEP8+, the number of CEP8 signal dots ≥ 3) have been described previously.7,8
The serum levels of 3 tumor-related markers, CA125 (Siemens Healthcare Diagnostics Inc.), CEA (Siemens Healthcare Diagnostics Inc.), and NSE (Roche Diagnostics GmbH), were measured in the peripheral blood samples using electrochemiluminescence with an analyzer (Cabas6000-e601, Roche, Swiss).
The cut-off value of a CTC positive result was determined as CTC count ≥2, which has been published previously.11 The upper limits of normality were 35 U/mL for CA125, 5 ng/mL for CEA, and 16.3 ng/mL for NSE.
Statistics
The association between CTCs and lung cancer was tested by the Fisher’s exact test. A P < .05 was considered statistically significant. Statistical analyses were assessed with SPSS 25.0 statistics software (IBM Corp.). The diagnosis performance was expressed by sensitivity and specificity. Sensitivity was defined as the probability of a positive test given that the patient had lung cancer. Specificity was defined as the probability of a negative test given that the patient had no lung cancer.
Results
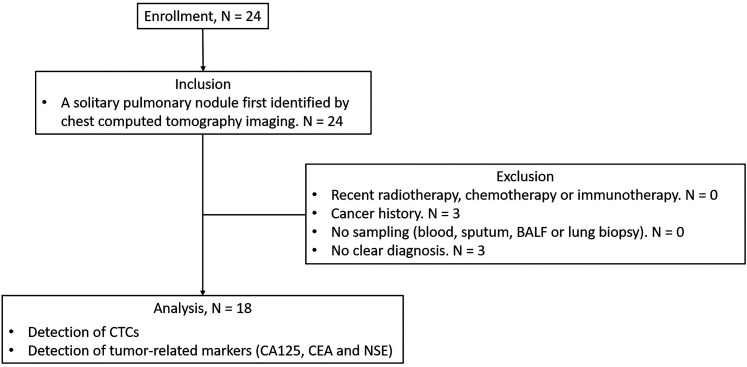
As shown in Figure 1, a total of 24 patients were enrolled and screened for the eligibility of this study. Twenty-four patients had a first identified solitary pulmonary nodule. Among them, 0 recently received radiotherapy, chemotherapy or immunotherapy; 3 had a cancer history; 0 had no sampling/biopsy; 3 had no clear diagnosis. Finally, 18 patients were analyzed in this study; 61.1% (11/18) of them were males. The mean age was 55.4 ± 11.8 years; 27.8% (5/18) of them had smoking history or were current smoking. The nodules were 11.1% (2/18) in a diameter with ≥8 to <10 mm, 38.9% (7/18) in a diameter with ≥10 to <20 mm, and 50.0% (9/18) in a diameter with ≥20 to <30 mm. The confirmed diagnosis of lung cancer, sarcoidosis, tuberculosis, and pneumonia was 66.7% (12/18), 22.2% (4/18), 5.6% (1/18), and 5.6% (1/18), respectively (Table 1). Information on the nodule size and CTC findings for each patient was shown in Table 2.
Figure 1.
Study flow diagram. Twenty-four patients were enrolled in this study. Six of them were excluded due to cancer history (n = 3) and no clear diagnosis (n = 3). Finally, data from 18 patients were analyzed in this study.
Table 1.
Baseline Characteristics.
| Characteristics | Value |
|---|---|
| Number of cases, n | 18 |
| Male, n (%) | 11 (61.1) |
| Age (years), mean ± SD | 55.4 ± 11.8 |
| < 40 years, n (%) | 2 (11.1) |
| ≥ 40 to < 50 years, n (%) | 6 (33.3) |
| ≥ 50 to < 60 years, n (%) | 2 (11.1) |
| ≥ 60 years, n (%) | 8 (44.4) |
| Smoking, n (%) | 5 (27.8) |
| Single nodule, n (%) | 18 (100) |
| Size of the nodule | |
| ≥ 8 to < 10 mm, n (%) | 2 (11.1) |
| ≥ 10 to < 20 mm, n (%) | 7 (38.9) |
| ≥ 20 to < 30 mm, n (%) | 9 (50.0) |
| Diagnosis | |
| Lung cancer, n (%) | 12 (66.7) |
| Sarcoidosis, n (%) | 4 (22.2) |
| Tuberculosis, n (%) | 1 (5.6) |
| Pneumonia, n (%) | 1 (5.6) |
Table 2.
Information on the Nodule Size and CTC Findings for Each Patient.
| Patient no. | Nodule size (cm in diameter) | CTC findings | ||
|---|---|---|---|---|
| Result | CTC count | CEP8+ signal dots in each CTC | ||
| 1 | 1.5 | Negative | 0 | Not identified |
| 2 | 2.4 | Negative | 0 | Not identified |
| 3 | 2.2 | Positive | 2 | 3; 3 |
| 4 | 2.6 | Negative | 0 | Not identified |
| 5 | 1.8 | Positive | 2 | 3; 3 |
| 6 | 2.1 | Negative | 0 | Not identified |
| 7 | 2.7 | Negative | 0 | Not identified |
| 8 | 1.0 | Negative | 0 | Not identified |
| 9 | 0.7 | Positive | 2 | 3; 3 |
| 10 | 1.8 | Positive | 2 | 3; 3 |
| 11 | 2.9 | Positive | 3 | 3; 3; 3 |
| 12 | 1.5 | Positive | 2 | 3; 3 |
| 13 | 1.9 | Negative | 0 | Not identified |
| 14 | 2.9 | Positive | 4 | 3; 3; 3; 4 |
| 15 | 0.5 | Negative | 1 | 5 |
| 16 | 2.0 | Positive | 4 | 3; 3; 3; 3 |
| 17 | 2.4 | Positive | 4 | 3; 3; 3; 4 |
| 18 | 1.1 | Negative | 0 | Not identified |
CEP8+: chromosome enumeration probe 8-positive; CTC: circulating tumor cell.
Considering the small sample size of this study, the Fisher’s exact test was utilized to examine the significance of the association between the detection of CTCs and lung cancer. As shown in Table 3, the P value was .009, which reached a statistical significance at the level of P < .05. Furthermore, the diagnostic performance for lung cancer between the CTCs and tumor-related markers, CA125, CEA, and NSE, were analyzed (Table 4). CTCs had a greater diagnostic performance with 75% of sensitivity and 100% of specificity. CA125 and CEA were positive in 5.6% and 0% of patients analyzed, respectively. Compared to CTCs, NSE and CA125/CEA/NSE combination had a relatively low sensitivity (25% and 33%, respectively). Furthermore, the combination of CTC with CA125 or with NSE slightly improved the sensitivity of lung cancer detection from 75% to 83% (Table 4).
Table 3.
Association between the identification of CTCs and lung cancer.
| Lung cancer | Nonlung cancer | Marginal row totals | |
|---|---|---|---|
| Positive CTCs | 9 | 0 | 9 |
| Negative CTCs | 3 | 6 | 9 |
| Marginal column totals | 12 | 6 | 18 |
CTCs: circulating tumor cells. The Fisher’s exact test statistic value was 0.009, reaching a statistically significant level (P < .05).
Table 4.
Comparison of the Diagnostic Performance for Lung Cancer Between the CTCs and Examined Tumor-Related Markers.
| Method | Number of cases | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| CTC | 9 | 75 | 100 |
| CA125 | 1 | 8.3 | 100 |
| CEA | 0 | 0 | 100 |
| NSE | 4 | 25 | 83 |
| CA125 + CEA + NSE | 5 | 33 | 83 |
| CTC + CA125 | 10 | 83 | 100 |
| CTC + CEA | 9 | 75 | 100 |
| CTC + NSE | 10 | 83 | 83 |
CA125: cancer antigen 125; CEA: carcinoembryonic antigen; CTC: circulating tumor cell; NSE: neuron-specific enolase.
Discussion
For the management of pulmonary nodules, CT follow-up is recommended by both Fleischner and British Thoracic Society guidelines; the frequency of CT follow-up is depending on the size, number, and density of the nodules.13 However, the neoplasms are needed to be distinguished from other lesions by further examinations and are growing during the follow-up period. CTCs were considered as a potential biomarker to provide preliminary pathological information (eg, tumor or nontumor) for early stage of cancers. Recently, the method for detection of CTCs in lung cancers has been improved by several aspects, including isolation devices for CTC capture,14 three-dimensional imaging for translocation of specific DNA fragments,15,16 and others. Further analysis of DNA translocation could be achieved using CTCs from transient cell culture.17
Previous studies have shown that the number of CEP8+ CTCs was significantly higher in patients with lung cancer than with benign lung lesions and was similar across the disease stage of lung cancer.11,18 In this study, this CEP8+ CTCs detection was applied to patients with a solitary pulmonary nodule first identified by CT imaging. We found that detection of CEP8+ CTCs was significantly associated with the diagnosis of lung cancer (P = .009) in 18 patients with solitary pulmonary nodule. Furthermore, the diagnosis performance of CEP8+ CTCs for lung cancer was 75% of sensitivity and 100% of specificity, which was similar with that of previous studies (sensitivity: 68.39-82.2%, specificity: 86.9%-100%).11,18 The present results suggested that the clinical utility of CEP8+ CTCs in early detection of lung cancer may be further extended to the patient population enrolled in this study.
On the other hand, we noted that the diagnosis performance of examined serum tumor-related markers is not as great as previously reported. CA125 is a sensitive but not specific marker for a number of benign and malignant conditions;19 the sensitivity and specificity for lung cancer in published reports were 28.9% to 52.6% and > 90%, respectively.20-22 The sensitivity of CEA and NSE in the literature was 40.7% to 62.1% and 19.1% to 40.8%.20,21,23 In this study, the sensitivity of each serum tumor-related marker was 0% to 25%. Moreover, the diagnosis performance of CA125/CEA/NSE combination (sensitivity: 61.9%-79.5%; specificity: 72.7%-78.4%) previously reported was better than that in this study.20,21 One possible reason may be the differences in the enrolled population (eg, disease stage). In this study, patients with solitary pulmonary nodule first found by CT were enrolled. This time point was earlier than most of published studies, which retrospectively enrolled patients with definitive diagnosis of lung cancer for analysis of serum tumor-related markers. Therefore, CEP8+ CTCs may potentially be a useful biomarker for lung cancer detection at an early stage of the disease, compared to the examined serum tumor-related markers.
There are some limitations in this study. First, this single-center study had a small sample size. Second, the reference value of CEP8+ CTCs for lung cancer diagnosis is not well established. Further studies are needed to confirm the diagnosis performance of CEP8+ CTCs for lung cancer.
In conclusion, this study showed that CEP8+ CTCs may be served as an adjunctive technique for early detection of lung cancer in patients with solitary pulmonary nodule and may be helpful for the disease management.
Abbreviations
- CTCs
circulating tumor cells
- CA125
cancer antigen 125
- CEA
carcinoembryonic antigen
- NSE
neuron-specific enolase
Footnotes
Authors’ Note: This study was approved by the institutional review board of Zhuhai People’s Hospital (IRB number: LW-[2020] no. 17) and registered (clinical trial registration number: 20200731105109530). Written informed consents were obtained from all patients.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ouqi Liu https://orcid.org/0000-0003-0225-6897
References
- 1.WHO. https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111(6):1710-1717. doi: 10.1378/chest.111.6.1710 [DOI] [PubMed] [Google Scholar]
- 3.Pinsky PF. Lung cancer screening with low-dose CT: a world-wide view. Transl Lung Cancer Res. 2018;7(3):234-242. doi: 10.21037/tlcr.2018.05.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho HIJ-Y. Chapter three - lung cancer biomarkers. Adv Clin Chem. 2015;72:107-170. [DOI] [PubMed] [Google Scholar]
- 5.Cainap C, Pop LA, Balacescu O, Cainap SS. Early diagnosis and screening in lung cancer. Am J Cancer Res. 2020;10(7):1993-2009. [PMC free article] [PubMed] [Google Scholar]
- 6.Krebs MG, Hou JM, Ward TH, Blackhall FH, Dive C. Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther Adv Med Oncol. 2010;2(6):351-365. doi: 10.1177/1758834010378414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Ge F, Cui W, et al. Lung cancer circulating tumor cells isolated by the EpCAM-independent enrichment strategy correlate with cytokeratin 19-derived CYFRA21-1 and pathological staging. Clin Chim Acta. 2013;419:57-61. doi: 10.1016/j.cca.2013.01.015 [DOI] [PubMed] [Google Scholar]
- 8.Ning N, Zhan T, Zhang Y, et al. Improvement of specific detection of circulating tumor cells using combined CD45 staining and fluorescence in situ hybridization. Clin Chim Acta. 2014;433:69-75. doi: 10.1016/j.cca.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 9.Wang PP, Liu SH, Chen CT, et al. Circulating tumor cells as a new predictive and prognostic factor in patients with small cell lung cancer. J Cancer. 2020;11(8):2113-2122. doi: 10.7150/jca.35308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D, Yang X, Li Y, et al. Clinical significance of circulating tumor cells and metabolic signatures in lung cancer after surgical removal. J Transl Med. 2020;18(1):243. doi: 10.1186/s12967-020-02401-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong CH, Tong D, Zhou ZQ, et al. Performance evaluation of detecting circulating tumor cells and tumor cells in bronchoalveolar lavage fluid in diagnosis of peripheral lung cancer. J Thorac Dis. Apr 2018;10(Suppl 7):S830-S837. doi: 10.21037/jtd.2017.12.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-2542. doi: 10.1056/NEJMcp012290 [DOI] [PubMed] [Google Scholar]
- 13.Sanchez M, Benegas M, Vollmer I. Management of incidental lung nodules <8 mm in diameter. J Thorac Dis. 2018;10(Suppl 22):S2611-S2627. doi: 10.21037/jtd.2018.05.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Kulasinghe A, Bogseth A, O'Byrne K, Punyadeera C, Papautsky I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst Nanoeng. 2019;5:8. doi: 10.1038/s41378-019-0045-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulasinghe A, Lim Y, Kapeleris J, Warkiani M, O'Byrne K, Punyadeera C. The Use of three-dimensional DNA fluorescent In situ hybridization (3D DNA FISH) for the detection of anaplastic lymphoma kinase (ALK) in Non-small cell lung cancer (NSCLC) circulating tumor cells. Cells. 2020;9(6). doi: 10.3390/cells9061465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulasinghe A, Kapeleris J, Kimberley R, et al. The prognostic significance of circulating tumor cells in head and neck and non-small-cell lung cancer. Cancer Med. 2018;7(12):5910-5919. doi: 10.1002/cam4.1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapeleris J, Kulasinghe A, Warkiani ME, et al. Ex vivo culture of circulating tumour cells derived from non-small cell lung cancer. Transl Lung Cancer Res. 2020;9(5):1795-1809. doi: 10.21037/tlcr-20-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Tian X, Gao L, et al. Clinical significance of circulating tumor cells and tumor markers in the diagnosis of lung cancer. Cancer Med. 2019;8(8):3782-3792. doi: 10.1002/cam4.2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miralles C, Orea M, Espana P, et al. Cancer antigen 125 associated with multiple benign and malignant pathologies. Ann Surg Oncol. 2003;10(2):150-154. doi: 10.1245/aso.2003.05.015 [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Zhang P, Wu R, Lu K, Zhou H. Identifying the best marker combination in CEA, CA125, CY211, NSE, and SCC for lung cancer screening by combining ROC curve and logistic regression analyses: is It feasible? Dis Markers. 2018;2018:2082840. doi: 10.1155/2018/2082840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Lu J, Ren H, et al. Combining multiple serum biomarkers in tumor diagnosis: a clinical assessment. Mol Clin Oncol. 2013;1(1):153-160. doi: 10.3892/mco.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ZQ, Huang LS, Zhu B. Assessment of seven clinical tumor markers in diagnosis of Non-small-cell lung cancer. Dis Markers. 2018;2018:9845123. doi: 10.1155/2018/9845123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina R, Marrades RM, Auge JM, et al. Assessment of a combined panel of Six Serum tumor markers for lung cancer. Am J Respir Crit Care Med. 2016;193(4):427-437. doi: 10.1164/rccm.201404-0603OC [DOI] [PubMed] [Google Scholar]