Abstract
Objective:
To examine the role of RET in renal malignancy, in particular papillary renal cell carcinoma.
Materials and Methods:
A cohort of 111 archival renal samples was used consisting of 94 renal cancers (66 papillary renal cell carcinoma, 18 conventional clear cell carcinoma, 10 chromophobe renal cell carcinoma), 4 benign oncocytomas and 13 normal kidney tissues. RET protein expression was examined by immunohistochemistry and expression levels were correlated with clinicopathological and patient survival data.
Results:
Positive RET staining was seen in 34/66 (52%) papillary renal cell carcinomas, 4/10 (40%) chromophobe carcinomas, 4/4 (100%) oncocytomas and 11/13 (85%) normal kidney samples. All 18 cases of conventional clear cell carcinoma had negative RET staining. RET expression was associated with low Fuhrman nuclear grade.
Conclusions:
RET protein may be contributing in part to an adaptation of a papillary growth pattern in certain renal malignancies. Given the possible therapeutic benefit of small molecule inhibitors of RET activation, further work needs to be done to highlight the functional relevance of RET protein expression in papillary renal cell carcinoma.
Keywords: RET, papillary renal cell carcinoma, GDNF
Background
Renal cancer is a heterogeneous disease with diverse morphological features. The WHO International Histological Classification of Kidney Tumours divides renal cell carcinoma (RCC) into clear cell (conventional), papillary, chromophobe, collecting duct and unclassified subtypes [1, 2]. Papillary RCC is the second most common subtype accounting for 10-15% of renal cancers, [3] with an estimated annual incidence of 5000 cases in the US alone [4]. Papillary RCC is characterised morphologically by the presence of fibrovascular cores lined by papillae of malignant epithelial cells. It can be sub-classified into two types [5] based on cell type: Type 1 tumours have small cuboidal cells with basophilic cytoplasm and small uniform nuclei; Type 2 tumours have large cells with eosinophilic cytoplasm and demonstrate pseudostratification. In general, type 2 tumours have a poorer prognosis than type 1 tumours [6].
RET (rearranged during transfection) proto-oncogene is a tyrosine kinase receptor which is coded for on chromosome 10q11.2 and consists of an extracellular cadherin motif, a cysteine rich domain and an intracellular tyrosine kinase (TK) domain.[7-10] Glial cell line-derived neurotrophic factor (GDNF) family ligands (a subclass of the transforming-growth factor-β (TGF-β) superfamily) signal through a multicomponent complex consisting of glycosylphosphatidylinositol (GFRά1-4) and RET tyrosine kinase [11]. The GDNF/RET signalling pathway has an important role in regulating germ cell differentiation and in the development of the peripheral nervous system where it promotes neuronal survival, differentiation and migration [11, 12]. Moreover, RET is expressed in the embryonic nephric ducts of the kidney where GDNF acts as a morphogen to trigger ureteric bud outgrowth. RET-deficient mice die shortly after birth from severe defects in enteric neuron and glial cell development and renal agenesis [12, 13]. Germline mutations in c-RET have been implicated in multiple endocrine neoplasia (MEN) types 2A and B, familial medullary thyroid carcinoma [14, 15] and Hirschsprung's disease [16]. Sporadic mutations in RET are associated with the development of papillary thyroid carcinoma [17, 18]. In addition wild-type RET expression has been identified in a variable proportion of papillary tumours, and may have a role in thyroid tumourigenesis [19-21].
Little is known about RET expression in papillary tumours from renal (i.e. non-neuroendocrine) origin. As RET is associated with renal development and papillary thyroid cancer, the primary objective of this study was to investigate the possibility that RET protein may be expressed in papillary renal tumours.
Methods
Case Selection
Ethical approval for the study was obtained from the corresponding Institutional Review Boards. A total of 111 formalin-fixed paraffin-embedded samples (66 papillary RCC (25 type I, 10 type II, 31 type not otherwise specified), 18 clear cell RCC, 10 chromophobe RCC, 4 oncocytomas and 13 normal kidney tissues) were retrieved from the Renal Cancer TAPCD Core, The Dana Farber Cancer Institute (Boston, MA) and The Department of Histopathology, AMNCH (Tallaght, Dublin, Ireland). H&E slides of all tumours were reviewed by two histopathologists (SS & RF), original diagnoses confirmed and classified according to UICC (International Union against Cancer) criteria. 85/111 samples were arrayed in triplicate on a tissue microarray; a further 26 samples of papillary RCC were available as individual slides. Clinical follow-up data to date was available for 38/66 papillary RCC samples. Table 1 lists the clinicopathological characteristics of the cases selected.
Table 1:
Relationship between RET expression and clinicopathological characteristics of papillary RCC
| Characteristics | Cases | Ret Protein | P* | |
|---|---|---|---|---|
| Age | High | Low | ||
| >50 | 28 | 6 | 22 | 1 |
| ≤50 | 10 | 2 | 8 | |
| Stage | ||||
| I-II | 21 | 14 | 7 | 0.46 |
| III-IV | 13 | 11 | 2 | |
| Unknown | 4 | |||
| Furhman Nuclear Grade | ||||
| II | 15 | 14 | 1 | 0.05 |
| III-IV | 16 | 9 | 7 | |
| Unknown | 7 | |||
| Type | ||||
| I | 25 | 13 | 12 | 0.96 |
| II | 10 | 6 | 4 | |
| Unknown | 3 | |||
| Lymph Node Metastases | ||||
| Y | 9 | 7 | 2 | 1 |
| N | 23 | 19 | 4 | |
| Unknown | 6 | |||
| Lymphovascular Invasion | ||||
| Y | 7 | 4 | 3 | 0.39 |
| N | 27 | 22 | 5 | |
| Unknown | 4 | |||
| Margin Status | ||||
| Positive | 5 | 3 | 2 | 0.84 |
| Negative | 28 | 21 | 7 | |
| Unknown | 5 | |||
| Preoperative Chemotherapy | ||||
| Yes | 15 | 1 | 14 | 1 |
| No | 4 | 0 | 4 | |
| Unknown | 19 | |||
| Recurrence | ||||
| Yes | 20 | 7 | 13 | 0.63 |
| No | 3 | 0 | 3 | |
| Unknown | 15 | |||
| Family history of renal cancer | ||||
| Yes | 2 | 1 | 1 | 0.85 |
| No | 11 | 9 | 2 | |
| Unknown | 25 | |||
| Smoking | ||||
| Yes | 9 | 6 | 3 | 1 |
| No | 5 | 4 | 1 | |
| Unknown | 24 | |||
Two-tailed Fischer’s Exact Test
Immunohistochemistry
Mouse monoclonal IgG antibody directed against the C-terminal (containing the TK domain) of the human RET oncoprotein (Labvision/Neomarkers Corp., CA, USA) was used to detect wild type RET as previously described [22, 23]. Negative controls replaced primary antiserum with nonimmune bovine serum. Antibody was standardized to a 1:15 dilution. 4μm tissue sections were cut, dewaxed and incubated in absolute methanol solution with 0.3ml of hydrogen peroxide for 30 minutes. Antigen retrieval was carried out by boiling the slides in 0.01mM citrate buffer, pH 6.0, for 90 sec. Slides were then treated with blocking serum for 10 minutes after which they were incubated with primary antibody at 25°C for 60 minutes, followed by biotinylated antirabbit IgG (Vector Laboratories, Inc, Burlingame, CA, USA) and premixed ABC reagent (Vector Laboratories, Inc, Burlingame, CA, USA).
Chromogen detection was performed with diaminobenzdine (DAKO Corp., Carpiteria, CA, USA) solution (0.5ml of stock DAB in 4.5ml of Tris buffer with 20μl of hydrogen peroxide). Slides were counterstained with hematoxylin. Two pathologists (RF and SF, blinded to the original diagnosis) scored the sections independently. A modified visual semiquantification method was used as previously described, [24] using a two-score system for immunointensity (II) and immunopositivity (IP). II and IP scores were summated. The semiquantification for II was scored on a scale of: 0, negative; 1, weak; 2, moderate; 3, strong. The semiquantification for percentage of IP cells was scored on a scale of 1 (1–10%), 2 (11–40%), 3 (41–70%) and 4 (>70%). This produced an immunoreactivity score ranging from 0 to +7. Scores from all cores from one case were averaged. A cut-off value of greater than 2 (to exclude focal weak staining) was used to determine immunohistochemical positivity.
Statistical Analysis
All statistical analysis of immunohistochemical studies was performed with both Analyse-it® (Analyse-it Software, Ltd, Leeds, UK) and MedCalc™ Software (MedCalc Software, Mariakerke, Belgium). Multiple and two sample comparisons were performed with the Kruskal-Wallis and Mann-Whitney rank sum tests. The association between RET expression and patient clinicopathological characteristics was examined by means of a Fischer’s Exact test. All tests were two-tailed, and the significance level was set at p ≤ 0.05.
Results
Immunohistochemistry
Normal renal tubules showed predominantly diffuse cytoplasmic staining for RET (staining was present in both proximal and distal convoluted tubules). Eighty-five percent of normal renal tissue samples (11 of 13) showed immunoreactivity of >2, and the mean RET immunoreactivity in all renal tissue samples (n = 13) was 5.5. Clear cell RCC showed absent staining for RET. One-hundred percent of samples showed immunoreactivity of <2, and the mean RET immunoreactivity in all samples (n = 18) was 0.1.
Fifty-two percent of papillary RCC (34 of 66) showed immunoreactivity of >2, and the mean RET immunoreactivity in all papillary RCC (n = 66) was 2.5. The mean immunoreactivity in all positive papillary RCC cases (n=34) was 4.7, a greater than forty-fold increase over clear cell RCC. Forty percent of chromophobe RCC (4 of 10) showed immunoreactivity of >2, and the mean RET immunoreactivity in all chromophobe RCC (n=10) was 2.4. The mean immunoreactivity in all positive chromophobe RCC cases (n=4) was 4.7, a greater than forty-fold increase over clear cell RCC. All cases of oncocytoma (n=4) showed immunoreactivity of >2, and the mean RET immunoreactivity in all oncocytomas was 6.9, a greater than sixty-fold increase over clear cell RCC. Figure 1 demonstrates RET protein expression in the different histotypes. The difference in RET immunoreactivity between histological subtypes was statistically significant (p<0.0001, Kruskall-Wallis test). The results of the immunohistochemical analysis of RET expression are summarized in Table 2 and 3.
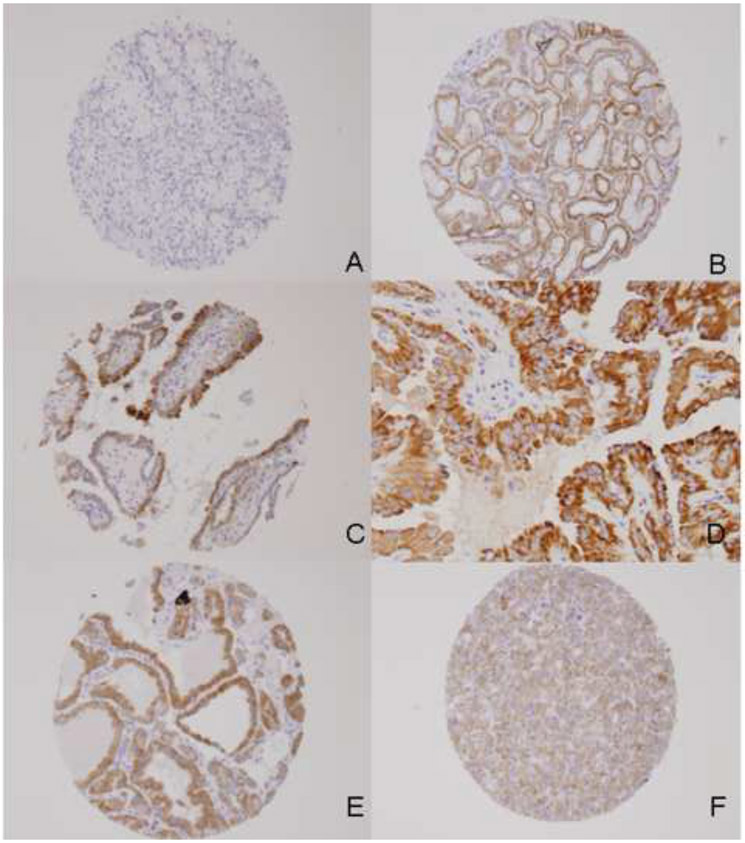
Figure 1:
Expression of RET in normal kidney and RCC: No staining (‘0’) for clear cell RCC X10 (A). Strong diffuse cytoplasmic staining for RET in normal renal tubules X10 (B), papillary RCC Type 1 X10 (C), papillary RCC Type 2 X20 (D), benign oncocytoma X10 (E), moderate diffuse cytoplasmic staining for RET in chromophobe RCC X10 (F).
Table 2:
Expression of RET in renal cell carcinoma
| Histology | No. of cases |
Mean ± SEM | Immunoreactivity score | ||
|---|---|---|---|---|---|
| Negative ret | Positive ret | ||||
| 0 | 0.1-2.0 | 2.1-7.0 | |||
| Normal renal tubules | 13 | 5.5 ± 0.5 | 0(0%) | 2(15%) | 11(85%) |
| Papillary RCC | 66 | 2.5 ± 0.3 | 26(39%) | 6(9%) | 34(52%) |
| Clear cell RCC | 18 | 0.1 ± 0.1 | 17(94%) | 1(6%) | 0(0%) |
| Chromophobe RCC | 10 | 2.4 ± 0.8 | 3(30%) | 3(30%) | 4(40%) |
| Oncocytoma | 4 | 6.9 ± 0.1 | 0(0%) | 0(0%) | 4(100%) |
The difference in immunoreactivity between all groups is statistically significant (p<0.0001, Kruskall-Wallis test).
Table 3:
Pairwise comparisons of RET expression in papillary RCC to normal kidney and the various tumour subtype cohorts.
| Test | p-value* |
|---|---|
| Papillary RCC ≠ normal kidney | 0.0002 |
| Papillary RCC ≠ Clear cell carcinoma | <0.0001 |
| Papillary RCC ≠ Chromophobe carcinoma | 0.88 |
| Papillary RCC ≠ Oncocytoma | 0.004 |
Mann-Whitney U test.
RET Expression in PRCC samples correlates with Clinicopathological Features
Our next investigation was to consider whether protein expression levels of RET had any relationship with clinicopathological characteristics of papillary RCC. We found that there was a statistically significant relationship between RET expression and Fuhrman nuclear grade (Table 1). Cases with positive RET expression showed significantly greater association with lower Fuhrman grade than cases with negative RET expression (p=0.05, Fischer’s Exact test).
Discussion
Specific mutations in the RET oncogene have been associated with defined morphological variants of thyroid cancer such as medullary or papillary thyroid carcinoma. RET expression in non-neuroendocrine tumours has not been widely addressed. In the present study we report RET expression in primary renal tumours, specifically papillary RCC. We demonstrate a significant association between positive RET expression and low Fuhrman nuclear grade in papillary RCC. With regards to other clinicopathological features of papillary RCC, RET positivity tended to be associated with other favorable prognostic indicators such as low tumour stage, histologic Type 1, absence of nodal metastases and lymphovascular invasion and negative surgical margins, although none of these associations reached the level of statistical significance.
RET protein is involved in early renal morphogenesis; therefore it is not wholly surprising to find RET expression in primary renal tumours. Recently, RET expression has been reported in breast, pancreatic and lung tumours [25-28]. RET was found to be functional in a panel of breast cancer cell lines characterised by their dependence on endocrine signalling. GDNF significantly enhanced the proliferation of cells maintained in suspension in a RET-dependent manner and significantly increased anchorage-independent growth in soft agar, results which indicate that RET modulates the oncogenicity of breast tumour cells and may contribute to their invasive potential [25]. Similarly, GDNF and another RET ligand artemin have been implicated in pancreatic tumour cell invasion in vitro [26]. Moreover, the G691S RET polymorphism was detected in human pancreatic tumours and increased GDNF-induced pancreatic cell invasion [27]. A recent high-throughput oncogene mutation profiling study of 17 cancer types detected a RET mutation in a primary non-small cell lung tumour, which was classified as a low frequency event; [28] however this study did not describe RET mutation in their cohort of renal cancer tumours.
We also demonstrate RET expression in a small number of oxyphilic renal tumours (i.e. chromophobe RCC and benign oncocytoma). Oncocytic tumours are characterized by the presence of oxyphil cells which are large polygonal cells with hyperchromatic nuclei and eosinophilic granular cytoplasm. The appearance of the cytoplasm is related to the large number of mitochondria present or rarely due to the abundance of granular endoplasmic reticulum. Of interest, RET activating mutations have previously been found in oncocytic thyroid tumours [29-31]. One group speculate that such an occurrence may have occurred subsequent to genetic events determining oncocytic metaplasia [30].
It is interesting to speculate that RET is maybe contributing in part to an adaptation of a papillary growth pattern in certain renal malignancies. Many organs in the body are made up of a network of branched tubules. Cells are responsive to positional signals from their local cellular microenvironment that govern where and when branches are formed. The RET receptor tyrosine kinase is activated by GDNF and controls outgrowth and invasion of the ureteric bud in the developing kidney. In renal epithelia activation of RET results in chemotaxis as RET expressing cells invade the surrounding GDNF expressing tissue. The PI3K/PTEN axis has a critical role in shaping the epithelial branches in the developing kidney in response to RET activation [32]. PTEN helps regulate cellular chemotaxis by antagonising the PI3K signalling pathway. Of note, PTEN mutations have been described in human primary renal cell carcinomas,[33] with reduction in PTEN protein occurring in papillary RCC [34]. It is interesting to hypothesize that this in turn may lead to loss of PTEN mediated suppression of RET induced cell migration and hence the adoption of a papillary morphology. Moreover, recent agents that target the mTOR/PI3K/PTEN axis such as temsirolimus have proven of clinical benefit in patients with metastatic papillary renal cell carcinoma, again suggesting a close relationship between RET and PTEN[35]. Recently, crosstalk between activated RET and the MET proto-oncogene has been demonstrated in human thyrocytes leading to neoplastic induction and development of a proinvasive phenotype [36]. It is interesting to speculate that a putative association may also exist in the kidney where activating MET mutants has been previously identified in both hereditary and sporadic papillary RCC [37-39]. Further work needs to be done to highlight the functional relevance of RET expression in papillary RCC, especially as there are now a number of small molecule inhibitors of RET activation available [40].
Conclusions
We demonstrate for the first time RET expression in primary renal tumours, specifically papillary RCC. RET protein may be contributing in part to an adaptation of a papillary growth pattern. Given the possible therapeutic benefit of inhibitors of RET activation, further work needs to be done to highlight the functional relevance of RET protein expression in papillary RCC.
Acknowledgements
Dr. Richard Flavin was sponsored by a HRB Clinical Research Fellow Ireland grant number CRT/2006/010.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Eble JN, Sauter G, Epstein JI, Sesterhenn LA. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon:IARC Press.; 2004. [Google Scholar]
- 2.Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997;80(5):987–989. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol 1997;183(2):131–133. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55(1):10–30. [DOI] [PubMed] [Google Scholar]
- 5.Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 1997;10(6):537–544. [PubMed] [Google Scholar]
- 6.Mejean A, Hopirtean V, Bazin JP, et al. Prognostic factors for the survival of patients with papillary renal cell carcinoma: meaning of histological typing and multifocality. J Urol 2003;170(3):764–767. [DOI] [PubMed] [Google Scholar]
- 7.Ishizaka Y, Itoh F, Tahira T, et al. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989;4(12):1519–1521. [PubMed] [Google Scholar]
- 8.Iwamoto T, Taniguchi M, Asai N, Ohkusu K, Nakashima I, Takahashi M. cDNA cloning of mouse ret proto-oncogene and its sequence similarity to the cadherin superfamily. Oncogene 1993;8(4):1087–1091. [PubMed] [Google Scholar]
- 9.Takahashi M, Buma Y, Hiai H. Isolation of ret proto-oncogene cDNA with an amino-terminal signal sequence. Oncogene 1989;4(6):805–806. [PubMed] [Google Scholar]
- 10.Takahashi M, Buma Y, Iwamoto T, Inaguma Y, Ikeda H, Hiai H. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene 1988;3(5):571–578. [PubMed] [Google Scholar]
- 11.Takahashi M The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 2001;12(4):361–373. [DOI] [PubMed] [Google Scholar]
- 12.Manie S, Santoro M, Fusco A, Billaud M. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet 2001;17(10):580–589. [DOI] [PubMed] [Google Scholar]
- 13.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 2002;3(5):383–394. [DOI] [PubMed] [Google Scholar]
- 14.Asa SL. How familial cancer genes and environmentally induced oncogenes have changed the endocrine landscape. Mod Pathol 2001;14(3):246–253. [DOI] [PubMed] [Google Scholar]
- 15.Jhiang SM. The RET proto-oncogene in human cancers. Oncogene 2000;19(49):5590–5597. [DOI] [PubMed] [Google Scholar]
- 16.Romeo G, Ronchetto P, Luo Y, et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature 1994;367(6461):377–378. [DOI] [PubMed] [Google Scholar]
- 17.Grieco M, Santoro M, Berlingieri MT, et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 1990;60(4):557–563. [DOI] [PubMed] [Google Scholar]
- 18.Santoro M, Carlomagno F, Hay ID, et al. : Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J Clin Invest 1992;89(5):1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fluge O, Haugen DR, Akslen LA, et al. Expression and alternative splicing of c-ret RNA in papillary thyroid carcinomas. Oncogene 2001;20(7):885–892. [DOI] [PubMed] [Google Scholar]
- 20.Kjellman P, Learoyd DL, Messina M, et al. Expression of the RET proto-oncogene in papillary thyroid carcinoma and its correlation with clinical outcome. Br J Surg 2001;88(4):557–563. [DOI] [PubMed] [Google Scholar]
- 21.Mai KT, Landry DC, Thomas J, Yazdi HM, Perkins DG, Odell PF. Ret oncogene protein expression in papillary thyroid carcinoma and related lesions. Tumori 2001;87(3):166–172. [DOI] [PubMed] [Google Scholar]
- 22.Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol 2001;14(4):338–342. [DOI] [PubMed] [Google Scholar]
- 23.Rebelo S, Domingues R, Catarino AL, et al. Immunostaining and RT-PCR: different approaches to search for RET rearrangements in patients with papillary thyroid carcinoma. Int J Oncol 2003;23(4):1025–1032. [PubMed] [Google Scholar]
- 24.Nisolle M, Gillerot S, Casanas-Roux F, Squifflet J, Berliere M, Donnez J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum Reprod 1999;14(11):2844–2850. [DOI] [PubMed] [Google Scholar]
- 25.Boulay A, Breuleux M, Stephan C, et al. The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res 2008;68(10):3743–3751. [DOI] [PubMed] [Google Scholar]
- 26.Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg 2006;244(2):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawai H, Okada Y, Kazanjian K, et al. The G691S RET polymorphism increases glial cell line-derived neurotrophic factor-induced pancreatic cancer cell invasion by amplifying mitogen-activated protein kinase signaling. Cancer Res 2005;65(24):11536–11544. [DOI] [PubMed] [Google Scholar]
- 28.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet 2007;39(3):347–351. [DOI] [PubMed] [Google Scholar]
- 29.Cheung CC, Ezzat S, Ramyar L, Freeman JL, Asa SL. Molecular basis off hurthle cell papillary thyroid carcinoma. J Clin Endocrinol Metab 2000;85(2):878–882. [DOI] [PubMed] [Google Scholar]
- 30.Chiappetta G, Toti P, Cetta F, et al. The RET/PTC oncogene is frequently activated in oncocytic thyroid tumors (Hurthle cell adenomas and carcinomas), but not in oncocytic hyperplastic lesions. J Clin Endocrinol Metab 2002;87(1):364–369. [DOI] [PubMed] [Google Scholar]
- 31.Musholt PB, Imkamp F, von Wasielewski R, Schmid KW, Musholt TJ. RET rearrangements in archival oxyphilic thyroid tumors: new insights in tumorigenesis and classification of Hurthle cell carcinomas? Surgery 2003;134(6):881–889; discussion 889. [DOI] [PubMed] [Google Scholar]
- 32.Kim D, Dressler GR. PTEN modulates GDNF/RET mediated chemotaxis and branching morphogenesis in the developing kidney. Dev Biol 2007;307(2):290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo K, Yao M, Kobayashi K, et al. PTEN/MMAC1/TEP1 mutations in human primary renal-cell carcinomas and renal carcinoma cell lines. Int J Cancer 2001;91(2):219–224. [DOI] [PubMed] [Google Scholar]
- 34.He L, Fan C, Gillis A, et al. Co-existence of high levels of the PTEN protein with enhanced Akt activation in renal cell carcinoma. Biochim Biophys Acta 2007;1772(10):1134–1142. [DOI] [PubMed] [Google Scholar]
- 35.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–2281. [DOI] [PubMed] [Google Scholar]
- 36.Cassinelli G, Favini E, Degl'Innocenti D, et al. RET/PTC1-driven neoplastic transformation and proinvasive phenotype of human thyrocytes involve Met induction and beta-catenin nuclear translocation. Neoplasia 2009; 11(1):10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffers M, Schmidt L, Nakaigawa N, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci U S A 1997;94(21):11445–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvi A, Marchina E, Benetti A, Grigolato P, De Petro G, Barlati S. Germline and somatic c-met mutations in multifocal/bilateral and sporadic papillary renal carcinomas of selected patients. Int J Oncol 2008;33(2):271–276. [PubMed] [Google Scholar]
- 39.Schmidt L, Junker K, Nakaigawa N, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 1999;18(14):2343–2350. [DOI] [PubMed] [Google Scholar]
- 40.Plaza-Menacho I, Mologni L, Sala E, et al. Sorafenib functions to potently suppress RET tyrosine kinase activity by direct enzymatic inhibition and promoting RET lysosomal degradation independent of proteasomal targeting. J Biol Chem 2007;282(40):29230–29240. [DOI] [PubMed] [Google Scholar]