Abstract
Recent years have been transformational in regard to the perception of the health risks and benefits of cannabis with increased acceptance of use. This has unintended neurodevelopmental implications given the increased use of cannabis and the potent levels of Δ9-tetrahydrocannabinol today being consumed by pregnant women, young mothers and teens. In this Review, we provide an overview of the neurobiological effects of cannabinoid exposure during prenatal/perinatal and adolescent periods, in which the endogenous cannabinoid system plays a fundamental role in neurodevelopmental processes. We highlight impaired synaptic plasticity as characteristic of developmental exposure and the important contribution of epigenetic reprogramming that maintains the long-term impact into adulthood and across generations. Such epigenetic influence by its very nature being highly responsive to the environment also provides the potential to diminish neural perturbations associated with developmental cannabis exposure.
There has been a large societal shift in recent years in the perception of harm from cannabis use coinciding with its widespread legalization for recreational and/or medicinal use in most states in the USA and many countries worldwide. Despite important societal outcomes of these changes, such as the decriminalization of cannabis use, the potential for harm particularly for vulnerable populations has raised concern as more teens and pregnant women use cannabis today. This is also pertinent given the exponential increase over the years in the concentration of Δ9-tetrahydrocannabinol (THC; the main psychoactive intoxicant) in the cannabis used recreationally and sold in dispensaries (BOX 1). Brain development is a dynamic process that extends well beyond the prenatal period, where full maturity is not achieved until early adulthood. The endocannabinoid (eCB) system (ECS) (BOX 1), which mediates the actions of THC, plays a critical regulatory role throughout all developmental stages, from the determination of cell fate and neuronal migration to the regulation of signalling pathways and synaptic transmission in the mature central nervous system (CNS). Therefore, the supraphysiological impact on the ECS by cannabis exposure during critical periods of development could change the normal trajectory of cellular processing and neurocircuitry, leading to behavioural disturbances later in life. In this Review, we provide a general overview of the ECS (which has been extensively reviewed) and describe the neurodevelopmental effects of cannabis/THC on the basis of animal and human studies relevant to neuropsychiatric risk. While evident discrepancies exist in the literature and not all studies observe adverse cannabis outcomes, we focus on animal findings pertinent to human cannabis studies reporting neurobehavioural disorders in order to gain neurobiological insights germane to risk. We highlight epigenetic mechanisms as a critical link underlying the molecular processes that maintain protracted effects of developmental cannabis exposure, not just during one’s lifetime but across generations.
Box 1 |. The mutable cannabis plant.
The cannabis plant has been used for thousands of years for its psychoactive and medicinal properties152, and in 1964, Δ9-tetrahydrocannabinol (THC) was discovered to be the primary psychoactive component contributing to its euphorigenic effects84,153. Today, more than 120 cannabinoids (for example, THC, cannabidiol (CBD) and cannabinol) have been identified in the cannabis plant, with more than 440 additional compounds, including terpenoids, that make up the complex, synergistic ‘entourage’ effects of the plant154. The two major types of cannabis plants are Cannabis sativa (most abundant subspecies and generic term often used to denote cannabis) and Cannabis indica, which were typically characterized by high THC and low CBD (non-intoxicating cannabinoid) levels and low THC and high CBD levels, respectively. In recent years, thousands of cannabis strains have been bred with many hybrids, resulting in varied cannabinoid constituents. Thus the names C. sativa and C. indica no longer reflect their initial content and related psychotropic effects. Additionally, THC concentrations in C. sativa, the most commonly used recreational plant, which ranges from 1.5% to 4% in the 1980s and 1990s155, have increased substantially in recent years, with some strains having as much as 29% THC156. Moreover, new routes of concentrated cannabis products such as ‘dabs’ which contain upwards of 76% THC157,158 have been introduced to increase the speed and intensity of the ‘high’.
The endocannabinoid system
The ECS plays a broad and critical role in numerous developmental processes. Briefly, it consists of cannabinoid receptors (CBRs; CB1R and CB2R) and endogenous ligands (eCBs), including the most studied 2-arachidonoylglycerol (2-AG) and anandamide (AEA), as well as the proteins responsible for their transport, synthesis (diacylglycerol lipase (DAGL) and N-acylphosphatidylethanolamine-selective phospholipase D (NAPE-PLD)) and degradation (monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH)). eCBs, which are lipidic neuro-modulators, can interact with other receptors, including transient receptor potential vanilloid 1 (TRPV1), peroxisome proliferator-activated receptor (PPAR) and G-protein-coupled receptor 55 (GPR55), to regulate synaptic transmission (for a review, see REF.1).
A substantial literature highlights fundamental roles of eCBs and CBRs in key developmental processes, including neurogenesis, glial formation, neuronal migration, axonal elongation, fasciculation (axonal bundling), synaptogenesis and synaptic pruning2–4. While considerable gaps in knowledge remain regarding granular mechanisms by which the ECS regulates some of these processes, significant insights have been gained into specific expression patterns linked to the functional and structural maturation of the CNS (FIG. 1) relevant to cannabis exposure during these periods.
Fig. 1 |. Neurodevelopmental pattern of the endocannabinoid system in humans and rodents.
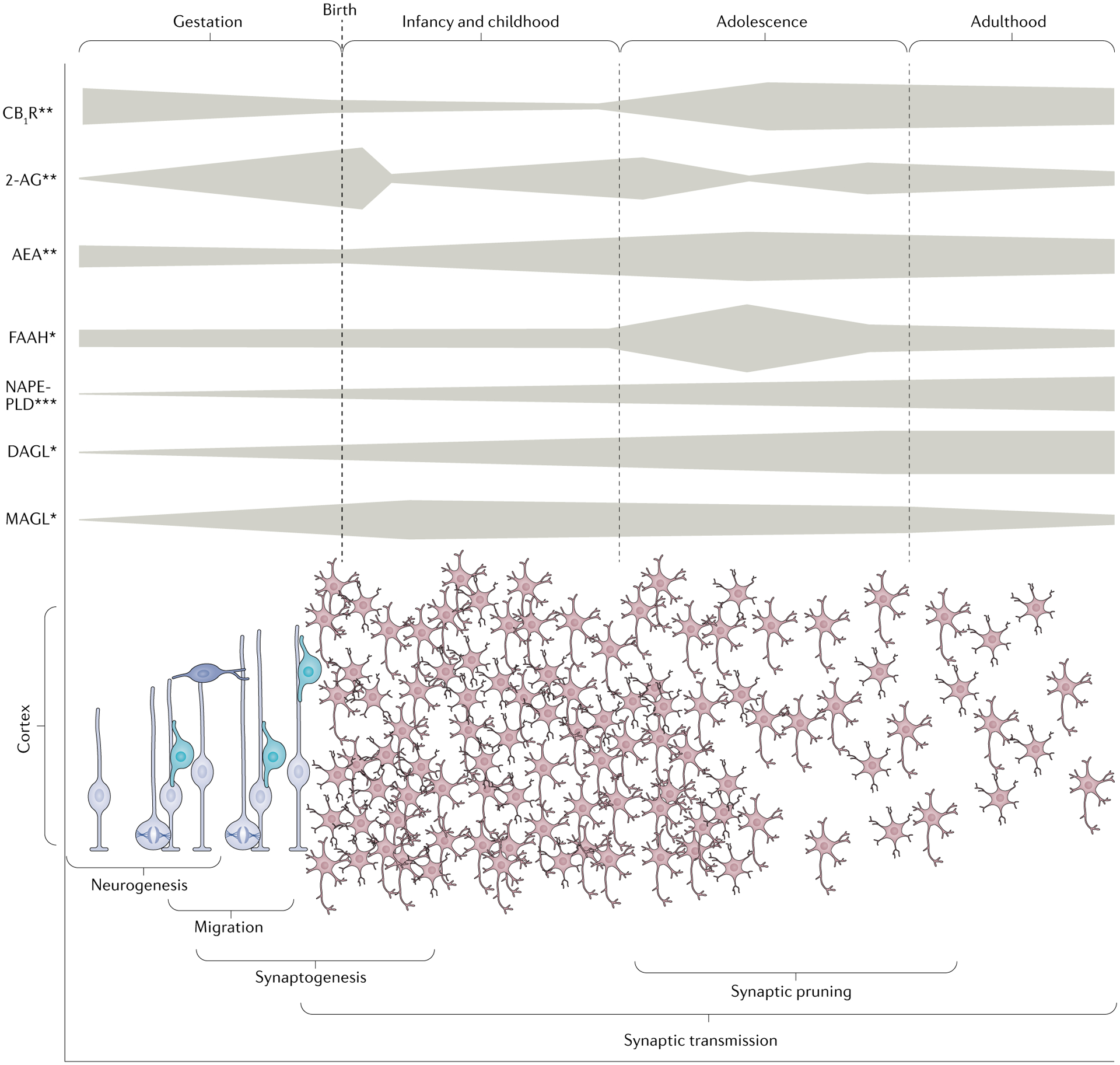
Top panel: Schematic overview of the developmental pattern of the expression of cannabinoid receptor type 1 (CB1R), endocannabinoid (eCB) ligands (2-arachidonyolglycerol (2-AG) and anandamide (AEA)), synthesis enzymes (diacylglycerol lipase (DAGL) and N-acylphosphatidylethanolamine-selective phospholipase D (NAPE-PLD)) and degradative enzymes (monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH)) detected in the brain (single asterisk, human; two asterisks, rodent; three asterisks, human and rodent). In rodents, the Cnr1 gene, encoding CB1R, exhibits its highest expression during early development and then expression decreases until adulthood8. Consistently, CB1R binding progressively increases from early gestation to reach maximal densities during adolescence in various brain regions (striatum, limbic forebrain and ventral mesencephalon) before decreasing to sustained high levels in the adult brain149. In humans, CB1R appears to have a similar developmental pattern from mid-to-late gestation to adulthood in homologous brain areas9 with an extremely high density in adulthood9,16,150. The eCB ligands also have distinct ontogenic patterns. While AEA expression exhibits a progressive increase from late gestation in rodents to reach maximal levels during adolescence, 2-AG expression peaks just after birth and fluctuates in early and late adole scence before reaching its adult levels8,19,20. In rodents, the AEA-synthesizing enzyme NAPE-PLD is first expressed in late gestation2, and human studies show that NAPE-PLD mRNA expression in the prefrontal cortex increases steadily over the lifespan, while expression of FAAH, the enzyme that degrades AEA, peaks during adolescence before maintaining stable levels into adulthood151. The ontogenic presence of 2-AG requires active DAGL and MAGL. While the expression profiles throughout development for MAGL and DAGL have not been well studied in rodents, the human prefrontal cortex exhibits a progressive increase of DAGL mRNA levels until early adulthood, whereas MAGL expression reaches its peak in early childhood before to declining in early adulthood151. Bottom panel: Illustration of several neurodevelopment events which occur from gestation to early adulthood that are regulated by eCB signalling (see the top panel). The gestation period is characterized by neuronal generation and migration followed in a later stage by synaptogenesis and synaptic transmission that continues into infancy, childhood and adulthood. The adolescent period is characterized by synaptic pruning (elimination of synapses) that shapes the mature brain architecture with dynamic fluctuations of components of the eCB system, including CB1R expression, eCB ligands and FAAH activity during early and late adolescence.
CB1R and CB2R are the primary targets of THC, with CB1R having a prominent role in CNS development given its abundant expression in the developing brain in contrast to CB2R, the role of which is aligned mainly with cells of microglial/macrophage lineage5,6. CB1Rs are present and functional from at least the ninth gestational week in humans, a period that overlaps with the initiation of cortical development, and from gestational day 11 in rodents5–7. In both rodents and humans, there is a transient presence of CB1Rs on white matter neuronal fibres during embryonic stages8–10, potentially reflecting CB1R on axons as they grow and migrate to their final site in establishing neuronal pathways, or their presence on non-neuronal cells (astrocytes and oligodendrocytes) that guide neuronal migration and axonal elongation. CB1R, expressed by diverse pluripotent cells, regulates cell differentiation and proliferation3,11, with expression correlated with neural differentiation12. Rodent studies have clearly demonstrated that CB1R expression and eCB signalling in postmitotic neurons play critical roles in neuronal migration and differentiation of glutamate and GABAergic cortical cells, cholinergic basal forebrain neurons, GABAergic cerebellar cells and hypothalamic neurons13. Moreover, the expression of CB1R by progenitor cells can control the neuron-to-glia ratio in the brain, and alterations in CB1R expression during fetal development can modify the connectivity between brain regions such as the cerebral cortex and hippocampus2,3. The developmental expression of CB1R is dynamic postnatally into adolescence before high levels are established in early adulthood, where CB1R is ubiquitously expressed and becomes the most abundant G-protein-coupled receptor14–16. CB1Rs in adulthood are primarily enriched in the cerebral cortex, basal ganglia, hippocampus and cerebellum14 and are predominantly localized to the synapse on presynaptic terminals17 of both glutamatergic and GABAergic cells18.
eCB ligands also have distinct developmental patterns, with divergent ontogenic bioavailability of the two prominent ligands — AEA and 2-AG. AEA has been shown to be critical during early stages of pregnancy for embryo implantation in the uterus, whereas increasing 2-AG levels during embryonic development correlate with cell differentiation and axonal elongation in the CNS3. After reaching a peak at postnatal day 1 (PND1), 2-AG levels remain steady until adolescence when they fluctuate (high in both early and late adolescence) before stabilizing in adulthood8,19. In contrast, AEA concentrations gradually increase from gestational day 21 and reach maximal levels during adolescence in most brain regions that have been studied8,19,20. In the mature brain, the ECS has been mainly described for its role in neurotransmission modulation and synaptic plasticity21. eCBs are retrograde messengers synthesized in the postsynaptic neuron in response to specific events. The biosynthesis of AEA is triggered by increased intracellular calcium or cAMP levels in the postsynaptic neuron, whereas 2-AG is produced after postsynaptic depolarization and the increase of calcium influx following the activation of voltage-gated Ca2+ channels. Once released from the postsynaptic site, eCBs are transported across the synaptic cleft to activate their main target, presynaptic CB1Rs. As a Gi/o-protein-coupled receptor, CB1R activation by eCBs inhibits synaptic transmission by decreasing the probability of neurotransmitter release. Therefore, the activation of CB1Rs inhibits Ca2+ influx through voltage-gated Ca2+ channels and activates presynaptic K+ channels and cAMP/PKA signalling, resulting in depression of synaptic transmission22. The modulation of these ion channels by cannabinoid agonists regulates synaptic strength of excitatory and inhibitory neurons. Through this feedback modulation of synaptic transmission, eCBs display a powerful regulatory role of synaptic function in numerous brain areas controlling synaptic excitability on different timescales so as to regulate a wide range of behaviours, including cognitive processes, motor function, emotions, reward, memory and food intake23.
Cannabis exposure during gestation
Prenatal THC exposure is often associated with reduced Cnr1 mRNA expression during fetal development11,24, which would have significant downstream consequences considering the major role of the ECS in cell proliferation, migration, differentiation and in maturation of neurons relevant to the development of various neural systems. Indeed, prenatal exposure of rodent models (corresponding to the first and second trimesters in humans; gestational day 5 to gestational day 20 in rodents) to CBR agonists has repeatedly been shown to alter glutamatergic, GABAergic, dopaminergic, opioidergic and serotonergic systems25. Importantly, a number of the impairments observed with THC exposure in animal models are also evident in the brains of human fetuses with in utero cannabis exposure, such as decreased mRNA expression of dopamine D2 receptor in the nucleus accumbens (NAc) and amygdala26,27 as well as altered opioid receptor and opioid neuropeptide mRNA expression in the amygdala, mediodorsal thalamus and striatum28–30. These and other findings emphasize that several cannabis-associated effects evident on the human developing brain can be causally ascribed to THC despite the many challenges of human studies.
Synaptic plasticity is critically dependent on the precision of synaptic connections and structural organization to express its full functional working range later in life. In utero, cannabis has been shown to compromise cytoskeletal stability that can lead to erroneous neurite growth and corticofugal development. For instance, prenatal THC exposure reduces the hippocampal and striatal expression of SCG10 (also known as stathmin 2), a microtubule-binding protein in axons, an alteration also detected in cannabis-exposed human fetuses24. Disturbingly, the reduction of SCG10 expression is associated with reorganization of the fetal cortical circuitry, deficits that last into adulthood causing rearrangements in the localization of CB1Rs and reduced long-term depression (LTD) in the hippocampus24.
In addition to biochemical and molecular evidence, morphological experiments have also confirmed that maternal exposure to cannabinoids affects proliferation, neurite branching31–33 and migration and differentiation of GABAergic and glutamatergic neurons34. Nanoscale insights are now also being revealed by the recent use of super-resolution imaging of the synapse, which demonstrates that THC exposure during gestation causes sex-specific molecular crowding at the presynaptic active zone of GABAergic terminals in the ventral tegmental area (VTA) of male rats, leading to decreased neurotransmitter release35. This further aligns with a decrease in the ratio of voltage-gated Ca2+ channels to CB1Rs detected at inhibitory synaptic terminals after maternal THC exposure35.
The functional consequences of prenatal cannabinoid exposure are also reflected in the efficiency, activity and expression of receptors, channels, enzymes and other molecules essential for the expression of synaptic plasticity (FIG. 2). Given the crucial role of glutamatergic neurotransmission in synaptic plasticity, most studies have focused on this transmitter system in relation to fetal cannabinoid exposure. Such investigations provide evidence that baseline and evoked levels of glutamate are reduced in vivo in the hippocampus36 and frontal cortex37 in young and adult rats prenatally exposed to a synthetic high-affinity CBR agonist, WIN-55,212–2. Similar results are evident in cortical cell cultures from PND1 pups exposed to WIN-55,212–2 in utero, accompanied by a reduction in the function of NMDA receptors33. Glutamate receptors are necessary for the expression of long-term potentiation (LTP) and LTD, and synaptic strength is mediated by the addition or removal of synaptic AMPA receptors38. Recently, adult male rats, but not adult female rats, with prenatal THC exposure were shown to have an increased AMPA/NMDA ratio associated with changes in the composition of receptor subunits in the VTA35 and reduced mRNA expression of prefrontal cortex (PFC) perisynaptic mGlu5 receptors, crucial effectors for the expression of the eCB-mediated LTD39. The various synaptic disturbances identified in the hippocampus11,40,41, PFC39 and VTA35 due to prenatal cannabinoid exposure range from short-term (depolarization-induced suppression of inhibition, DSI) to long-term (NMDA-mediated LTP, NMDA-mediated LTD and eCB-mediated LTD) forms of synaptic plasticity.
Fig. 2 |. Impact of developmental THC exposure on synaptic plasticity in adulthood.
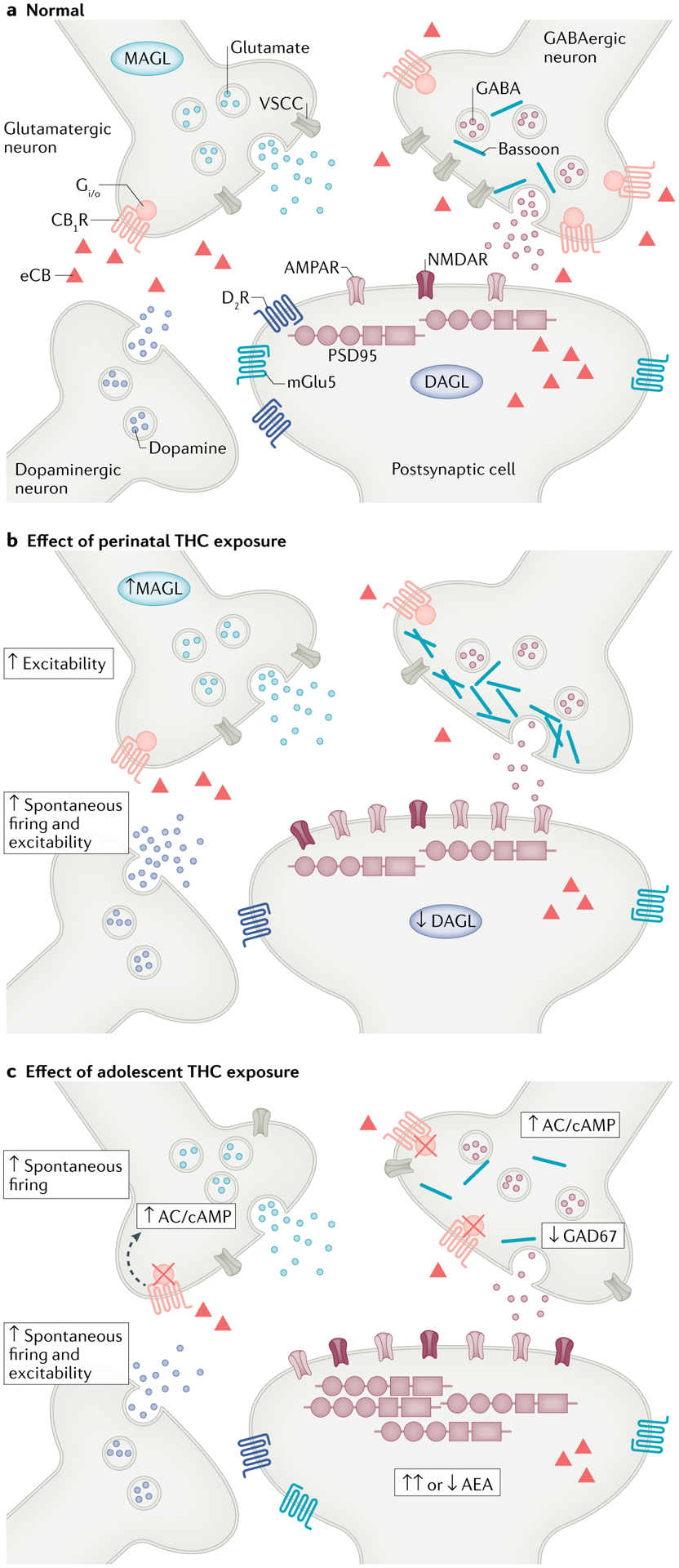
Schematic overview of the synapse under normal conditions or after perinatal or adolescent Δ9-tetrahydrocannabinol (THC) exposure based on rodent models. a | During normal conditions, in the absence of THC exposure, endocannabinoid (eCBs) synthesized postsynaptically are released into the synaptic cleft and influence GABAergic and glutamatergic synapses by binding to cannabinoid receptor type 1 (CB1R) expressed presynaptically. In this way, the eCB system tightly regulates these synapses as well as other neurotransmitter systems such as the dopamine system (for example, indirectly regulated by cannabinoid receptor on inhibitory GABA terminals that synapse onto dopaminergic neurons; not shown in figure). b | After prenatal THC exposure, GABAergic mechanisms are significantly abrogated due to a decrease in voltage-sensitive calcium channel (VSCC) density, synaptic ‘crowding’ by bassoon scaffolding proteins and reduced GABA release. In contrast, glutamatergic and dopaminergic inputs are sensitized (as measured by increased firing and excitability), which is paired with shifts in postsynaptic receptor expression, including reduced dopamine D2 receptor (D2R) and metabotropic glutamate receptor 5 (mGlu5) levels and increased AMPA/NMDA ratios, hallmarks of compensatory downregulation. Moreover, prenatal THC exposure impacts the expression of eCB system enzymes (increased monoacylglycerol lipase (MAGL) and decreased diacylglycerol lipase (DAGL) expression), resulting in a reduction of eCB bioavailability (represented by the red triangles). c | Similarly, adolescent THC exposure results in significant reductions in GABAergic tone via reductions in the expression of the critical GABA synthesis enzyme glutamate decarboxylase 67 (GAD67) as well as greater firing and excitability of presynaptic glutamatergic neurons and dopaminergic inputs. In the postsynaptic cell, expression of AMPA and NMDA receptors is increased in animals with adolescent THC exposure. Although the same biological markers are not always studied in perinatal and adolescent models, adolescent THC exposure also alters the architecture of the synapse, as evident in increased expression of postsynaptic density protein 95 (PSD95) at glutamatergic synapses. In addition, adolescent THC exposure leads to disruptions of key eCB system components, including reduced expression of CB1Rs, impaired CB1R G protein functionality (as depicted by red crosses) associated with increased expression of second messengers such as adenyl cyclase (AC) and cyclic adenosine monophosphate (cAMP) and abnormal levels of anandamide (AEA). AMPAR, AMPA receptor; NMDAR, NMDA receptor.
Disruption in the gain and loss of synaptic efficiency induced in different cell types and neurotransmitter systems is relevant to the behavioural outcomes associated with in utero cannabinoid exposure. Interestingly, Frau and collaborators demonstrated sex-specific hypersms implicated in the effects of cannaexcitability of dopaminergic neurons due to an excitatory–inhibitory imbalance and reduced synaptic inhibition onto VTA dopaminergic neurons, highlighting a switch from LTD to LTP in juvenile males prenatally exposed to THC35. Such changes in dopaminergic neurons that project from the VTA to the NAc could explain the increased vulnerability for enhanced drug intake seen in adult male rats exposed to THC during gestation26,28. Plasticity deficits such as the alterations in hippocampal LTP36 and LTD24 and inhibition of DSI41 as well as sex-dimorphic impaired hippocampal oscillations resulting in aberrant microcircuit function in males due to a decrease in the population of inhibitory (CCK-positive) interneurons40 would be predictive of altered cognitive functions such as memory, learning and social interaction. Indeed, there is evidence that the PFC of adult male rats with prenatal THC exposure is characterized by an abolition of the eCB-mediated LTD and increased excitability in pyramidal neurons correlated with deficits in socialization39.
Dysfunction apparent in synaptic circuits within mesocorticolimbic pathways linked to prenatal cannabinoid exposure in preclinical models could be relevant to behavioural outcomes evident in human studies of cannabis exposure during fetal life. For instance, findings in preclinical studies echo observations of deficits in learning, memory, attention and aggressive behaviour assessed in children and adolescents exposed to maternal cannabis use42. Neuroimaging studies have also documented altered neuronal functioning in cortical regions of young adults with prenatal cannabis exposure in relation to working memory43. Moreover, alterations of the dopaminergic system revealed by animal models could reflect the increase in the expression of depressive symptoms44, anxiety-like behaviours45 and drug use observed during childhood, adolescence46 and young adulthood47. TABLE 1 provides a summary of the molecular and behavioural effects described for gestational cannabinoid exposure in humans and animal models.
Table 1 |.
Effects associated with exposure to cannabis or cannabinoid agonists during gestation and/or lactation in humans and animal models in relation to behavioural and neurobiological outcomes during exposure and later in life
| Gestation (0–40 weeks for humans, GD0–GD22 for rodents) | Infancy and childhood (0–11 years for humans, PND1–PND25 for rodents) | Adolescence (12–18 years for humans, PND28–PND65 for rodents) | Adulthood (>18 years for humans, >PND70 for rodents) |
|---|---|---|---|
| Humans | |||
| ↓ D2R mRNA (NAc and amygdala)26 | ↑ Sleep disturbances146,147 | ↑ Delinquency148 | ↑ Drug use47 |
| ↓ SCG10 (also known as stathmin 2) mRNA (hippocampus and striatum)24 | ↓ Cognition (verbal reasoning, attention, memory)42 | ↑ Drug-seeking behaviours46 | ↓ Executive functions43 |
| ↓ CB1R mRNA (cerebrum)24 | ↑ Aggression and impulsivity149,150 | ↓ Visual memory and integration151 | ↑ Neuronal functioning in cortical regions during a visuospatial task43 |
| ↓ DAGL mRNA (cerebrum)24 | ↑ Depressive and anxious behaviours44,45 | - | - |
| ↑ MAGL mRNA (cerebrum)24 | - | - | - |
| Rodents | |||
| ↓ CB1R mRNA and function in cortical neurons11 | Change in social communication33*,61,66* | ↓ Memory retention36* | ↓ Social interaction39,41,60 |
| ↓ D2R mRNA in NAc26 | ↑ Locomotor activity66* | ↑ Social anxiety152 | ↑ Social discrimination60 |
| - | GABA switch delayed in PFC61 | ↑ Sensitivity to THC challenge35 | ↑ Opioid sensitivity29,57 |
| - | ↓ NMDAR functions33* | ↓ Glutamate levels in PFC and hippocampus31,36*,37*,55 | ↓ Memory33*,36*,40,55 |
| - | ↓ CB1R density in hippocampus40 | ↓ NMDA-mediated LTP in hippocampus36* | Change in motor functions11,36* |
| - | - | ↑ Excitability of dopaminergic neurons in VTA35 | ↓ LTD and LTP in PFC and hippocampus35,36*,39,60 |
| - | - | ↓ GABA release in the VTA35 | ↓ DSI in hippocampus41* |
| - | - | ↑ AMPA/NMDA ratio in VTA35 | Change in pyramidal neuronal excitability of PFC39,60 |
| - | - | ↓ Glutamatergic inputs (number and/or strength) onto dopaminergic neurons in VTA35 | ↓ mGlu5,TRPV1 and DAGL mRNA in PFC39*,60 |
| - | - | Change in long-term plasticity polarity35 | ↓ D2R mRNA in NAc26 |
| - | - | - | ↑ DOPAC/DA ratio in NAc and VTA58 |
| - | - | - | ↓ Glutamate levels (basal and evoked)36*,55 |
| - | - | - | Change in CB1R density in cortex and hippocampus59 |
| - | - | - | ↓ CB1R function in striatum and hippocampus63,153* |
| - | - | - | ↓ GABA levels (basal and evoked) in hippocampus63 |
The Δ9-tetrahydrocannabinol (THC) animal studies cited used low to moderate THC doses. CB1R, cannabinoid receptor type 1; DA, dopamine; DAGL, diacylglycerol lipase; DOPAC, dihydroxyphenylacetic acid; D2R, dopamine D2 receptor; DSI, depolarization- induced suppression of inhibition; GABA, γ-aminobutyric acid; GD, gestational day; LTP, long-term potentiation; LTD, long-term depression; MAGL, monoacylglycerol lipase; mGlu5, metabotropic glutamate receptor 5, NAc, nucleus accumbens; NMDAR, NMDA receptor; PFC, prefrontal cortex; PND, postnatal day; TRPV1, transient receptor potential vanilloid 1; VTA, ventral tegmental area.
The cannabinoid receptor agonist WIN-55,212–2 was used.
Cannabis exposure during lactation
In the USA, it is estimated that 15% of breastfeeding women use cannabis48. Despite this high incidence, the effects of cannabis during lactation on children have not been well studied. As lipidic molecules, cannabinoids such as THC can easily be transferred through the maternal milk during breastfeeding49. A recent study reported that ~2.5% of maternal THC is transferred to the infant50 depending on the chronicity and the concentration of THC in the cannabis used. Such exposure may have significant consequences given the early postnatal burst in synaptogenesis that occurs during this period when women breastfeed their children. Synaptogenesis, which begins during gestation, peaks in midchildhood, followed by activity-dependent pruning of excessive synapses later in childhood that continues through adolescence and into early adulthood51. Brain development during childhood that is marked by the emergence of increased neural connectivity is highly sensitive to environmental stimuli and activity-dependent experiences such as drug exposure.
To our knowledge, only two small studies have investigated the consequences of cannabis use during breastfeeding, with conflicting results on motor and mental performance in 1-year old children52,53. Those studies were conducted more than 30 years ago, when the concentrations of THC in available products were likely much lower than today (BOX 1). Given the evident challenges with human studies, particularly for this developmental epoch, animal models are critical to provide neurobiological insights. Most animal models though have used ‘perinatal’ protocols that include both the prenatal period and the postnatal period, considering that the first 10 postnatal days in rodents correspond to the third trimester of pregnancy in humans54. Such studies have observed results similar to those from strictly prenatal models with alterations of glutamatergic31,55,56, opioidergic57, dopaminergic58 and cannabinoid59 function and expression in mesocorticolimbic brain areas with cannabinoid exposure extended to postnatal days (gestational day 5 to PND24). Moreover, the perinatal exposure also induces higher morphine self-administration57 as well as deficits in memory, social recognition, olfactory and inhibitory avoidance tasks55. The similar long-lasting disturbances caused by these prenatal and perinatal models strongly suggest that the early trimesters of pregnancy are crucial windows of cannabinoid sensitivity for many long-term outcomes documented to date. However, while the deficits in CB1R mRNA expression after a prenatal THC exposure are mainly evident during fetal life11,24, perinatal THC exposure leads to long-lasting alterations in different brain areas in adulthood59. To differentiate the effects of THC exposure restricted to the late trimester and early infancy periods of development, a few studies have begun to strictly examine exposure during lactation in rodent models60,61. One line of these studies has focused on the GABAergic system since GABA signalling is critical for normal spontaneous network activity and for maturation and refinement of neuronal networks62. Moreover, perinatal THC exposure has been shown to reduce basal and stimulated GABA release in the hippocampus of adult rats63, whereas prenatal exposure to a CBR agonist increases the number of migrating GABAergic interneurons during early gestation34. Importantly, although GABA is known as the main inhibitory neurotransmitter in the CNS, it starts its developmental journey being excitatory in the immature brain. The GABA switch from excitatory to inhibitory transmission relies on a developmentally and genetically regulated expression of the sodium–potassium–chloride transporter (NKCC1) and potassium–chloride co-transporter 2 (KCC2). This shift from depolarization to hyperpolarization is due to a reduction of the intracellular levels of chloride during the second week of postnatal development in rodents64,65. Recent studies now provide evidence that exposure to THC during the lactation period (PND1 to PND10) delays the GABA switch in the PFC60. This reprogramming of the GABAergic trajectory correlates with retarded KCC2 gene upregulation necessary for the GABA excitatory to inhibitory switch. Moreover, the delay in GABA maturation is associated with aberrant ultrasonic vocalizations, reflective of impaired early social communication and aversive affective state, in the pups. Abnormality in this first form of cognition and emotional distress in rodents is also evident after prenatal cannabinoid exposure33,66. Lactation-delivered THC also induced electrophysiological disturbances that persist into adulthood, where a hypoexcitability of pyramidal neurons (layers 5 and 6) is evident and is associated with impairment of several forms of synaptic plasticity in the PFC, including a total abolishment of NMDA-mediated LTP and eCB-mediated LTD, with augmentation in the magnitude of mGlu2/3-mediated LTD60,61. Both LTP and eCB-mediated LTD are restored by inhibition of MAGL, the degradative enzyme for 2-AG, suggesting a deficit in 2-AG bioavailability as contributing to the long-term effects of THC exposure during lactation.
While there remains a large gap in knowledge regarding the specific contribution of cannabis exposure during infancy, particularly with breastfeeding, initial preclinical lactation models appear to suggest an impact on neural network assembly, disturbance of the physiological trajectory of major neurotransmitter systems, dysregulation of the balance between excitation–inhibition balance leading to dysfunction in synaptic plasticity and behavioural disruptions as in adults.
Second-hand cannabis exposure
Another aspect of postnatal cannabis exposure that deserves attention is second-hand exposure during the juvenile period. The evidence that second-hand cannabis smoke has effects on development is extremely limited compared with the evidence for second-hand tobacco smoke. However, it has been documented that there are significant concentrations of THC and other THC metabolites in oral fluid, blood and/or urine samples of children exposed to second-hand cannabis smoke67. Cannabis use among parents with children at home in the USA increased from 4.9% to 6.8% between 2002 and 2015, with an increase to 17.4% among cigarette-smoking parents68. Moreover, air particle monitoring in almost 300 homes of families with at least one child younger than 14 years showed that 15.1% had documented indoor cannabis smoking69. These trends are disconcerting given that toddlers exposed to second-hand cannabis smoke display cognitive and emotional problems70. While these and other studies suggest significant effects of second-hand cannabis exposure on children, there continues to be a lag in preclinical studies to address gaps in knowledge regarding potential short-term and long-term neurobiological effects and that can circumvent the multiple challenging variables of human developmental studies.
Cannabis exposure during adolescence
Adolescence is a distinct period of maturation, wherein many of the juvenile neurobiological processes and behaviours recede, but a significant amount of refinement occurs, specifically in areas relevant for cognitive function, reward and emotionality. Indeed, due to processes nearing final maturation within the PFC, striatum and amygdala, adolescence is considered a critical period especially susceptible to reward and affective processes. For clinical studies that document an increased association between adolescent cannabis use and psychiatric risk, such conditions include anxiety, depression, addiction and psychosis disorders71,72. Neurobiologically, even under conditions with a lack of outward differences in volumetric or white matter perturbations, structural morphology has been shown to correlate with cannabis use patterns such as age of onset73. Furthermore, activation differences are evident in several brain regions, including the PFC, striatum and amygdala73. Animal studies in which complex factors (for example, genetics, environment and THC concentration) can be partly controlled, as compared with human investigations, demonstrate that adolescent THC exposure can impact normal neurodevelopmental processes causally linked to protracted behavioural effects relevant to humans.
As depicted in FIG. 1, the ECS undergoes significant changes during adolescence, with a critical involvement in synaptic pruning, a process characteristic of this developmental period needed to establish the ultimate architecture of the mature brain. THC exposure during adolescence is well documented to change the normal ontogenic profile of the ECS. For example, the normal correlations seen between AEA and 2-AG levels throughout the adolescent period in the PFC and NAc are discordant in THC-exposed animals19. Such differences may relate to findings that adolescent THC exposure leads to a premature peaking of AEA levels in the NAc in male rats in midadolescence, achieving levels comparable to those in adulthood19. Similarly, AEA levels that are normally increased in the PFC in late adolescence are reduced by escalating, high-dose THC exposure in female animals74. Unlike AEA, neither moderate nor high THC exposure alters 2-AG levels19,74, suggesting unique sensitivity of AEA signalling in the PFC and NAc to adolescent THC exposure. On a physiological level, adolescent THC exposure inhibits the normal eCB-mediated LTD seen in medial PFC layer 2/3, an effect reversed by increased AEA bioavailability through the chronic administration of an FAAH inhibitor75.
Adolescent THC exposure also significantly impacts the CB1R, with important contributions of dose and sex on CB1R expression and functionality. For example, whereas moderate dosing elevates CB1R levels in the amygdala of THC-exposed rats76, escalating, very high doses decrease CB1R levels in this region as well as in multiple brain regions, including the PFC, striatum, hippocampus, thalamus, substantia nigra, VTA and cerebellum74,77,78 in adulthood regardless of sex. These latter results are concomitant with the functional reductions in CB1R G protein activity (as measured by [35S] GTPɣS) in these regions77. When both sexes are studied together, the strongest changes in CB1R functionality are seen in females77. Female rats are also more sensitive than male rats to adolescent THC exposure, showing pronounced depression-like behaviour, social avoidance and memory deficits78–81, whereas male rats exhibit working memory deficits and greater sensitivity to THC’s rewarding effects78,82. Notably, male rats show reduced CB1R levels in glutamatergic terminals in the VTA, which may contribute to their greater reward responsivity78. In contrast, female rats have elevated CB1R levels in the amygdala after moderate doses but reduced levels after higher THC exposure77, a key structure in emotional regulation. Female rats not only exhibit overall greater changes in the ECS after adolescent exposure, but also in THC metabolism during the adolescent dosing period, with higher levels of 11-OH-THC82,83, a metabolite with significant CB1R activity84. These sex differences are in line with greater susceptibility for divergent psychiatric vulnerability in humans, where females with cannabis use show greater susceptibility for depression, and males appear more prone to addiction, including greater risk of development of cannabis use disorder72.
In addition to modulating the ECS, adolescent THC exposure has profound effects seen in adulthood on the excitatory and inhibitory mechanisms, which have been studied mainly in the PFC. Chronic exposure to escalating THC levels during adolescence significantly downregulates GAD67, the rate-limiting enzyme for GABA synthesis85, suggesting reduced GABA tone later in life. Interestingly, activating GABAA receptors in the PFC reverses THC-induced anxiety, a phenotype linked to reduced GABA levels86,87. In contrast, excitatory tone in the adult PFC is sensitized with adolescent THC exposure, with medial PFC pyramidal neurons demonstrating greater spontaneous firing86. The in vivo induction of glutamate release, by pharmacological inhibition of NMDA receptor, is also potentiated in medial PFC neurons in THC-exposed animals87. Adolescent THC exposure also increases the levels of postsynaptic glutamatergic markers in the PFC, including PSD95 (REF.88), NMDA receptor subunits GluN2A and GluN2B, and the AMPA receptor GluA1 subunit89,90. Importantly, adolescent THC exposure not only increases the overall levels of these glutamatergic markers in adulthood but also alters the normal developmental trajectory of the expression of glutamate receptor subunits that have functional significance for synaptic plasticity. For example, GluN2A, a subunit whose level progressively increases during development, reaches its highest levels in late adolescence in rats exposed to a low dose of THC. In contrast, GluN2B, a marker of developing neurons, whose level usually declines from midadolescence into adulthood, remains at an elevated level as a consequence of prior THC exposure. These findings indicate that adolescent THC exposure not only leads to premature maturation of key plasticity markers, which matches the premature pruning of dendritic spines91, but also simultaneously attenuates the normal reduction in NMDA receptor GluN2B content, delaying another facet of normal development. Altogether, the resulting abrogation of GABAergic markers when paired with elevated levels of glutamatergic effectors suggests significantly increased pyramidal excitatory output. Additionally, high-dose adolescent THC-induced reduction of eCB-mediated LTD from layer 5 pyramidal cells is reversed by increasing AEA bioavailability75. Furthermore, dendritic spines and arborizations in pyramidal layer 2/3 neurons (which project to subcortical structures such as the amygdala) are significantly reduced even after low-dose adolescent exposure74,92, accompanied by a marked reorganization of genes related to neuronal development and synaptic plasticity in young adulthood91. Altogether, these results suggest increased excitatory tone (FIG. 2), but potentially reduced top-down control and diminished intercortical coordination of output signals.
Collectively, adolescent THC-induced alterations in excitatory–inhibitory balance have significant implications for behavioural outcomes, which are particularly relevant to psychosis-related conditions, for which a substantial body of evidence documents improper processing of the cortical signal-to-noise ratio93,94. A battery of rodent studies demonstrate that adolescent THC exposure increases psychosis-like behavioural phenotypes, including greater sensitivity to phencyclidine-induced locomotor activity, prepulse inhibition impairments, social avoidance and working memory deficits. Activation of GABAA receptors in the medial PFC of THC-exposed rats reversed these behavioural effects86. Dopaminergic activity also plays a key role in the ‘noisy cortex’ hypothesis of psychosis, wherein dopaminergic activity leads to greater sensitivity of glutamatergic neurons and increased salience attribution. Consistently, a history of adolescent THC exposure sensitizes VTA dopaminergic cell firing and excitability80. Moreover, oral consumption of THC during adolescence reduces CB1R expression in the VTA, specifically on glutamatergic inputs, which would be expected to render excitatory inputs to the VTA less sensitive to eCB inhibition, resulting in enhanced glutamatergic tone and increased dopamine levels78. Interestingly, the marked reorganization of developmentally regulated THC gene networks in layer 2/3 pyramidal neurons of the adolescent exposure rat model mimics gene networks dysregulated in the PFC of individuals with diagnosed schizophrenia91.
Beyond affective and psychosis-like conditions, a history of adolescent cannabis use is associated with increased susceptibility to drugs of abuse. Preclinical models have recapitulated these results, with adolescent THC exposure associated with increased sensitivity to cocaine95, heroin28,96–98 and cannabinoids99 later in life. In addition to the changes to the dopaminergic system described above, which has long been implicated in mediating the formation and maintenance of proaddictive behaviour100, adolescent THC exposure also increases expression of the gene encoding endogenous opioid proenkephalin (Penk) in the NAc28, and increased Penk expression is causally associated with increased heroin self-administration98. Aspects of genetics and behavioural traits also appear to play a role in adolescent THC-induced opioid sensitivity. For instance, high-dose adolescent THC exposure increased heroin responding in ‘addiction-prone’ rats (Lewis rats)96 compared with Wistar rats97. Overall, collective results in the field suggest that the long-term impact of adolescent THC/cannabis exposure on future drug sensitivity may depend on multiple factors, including behavioural traits, genetics, amount of THC (including frequency of exposure) and sex.
Epigenetics, the link across time
The long-term consequences of developmental cannabinoid exposure naturally raise questions about the underlying molecular mechanisms that maintain such protracted effects. Epigenetic factors are considered the most likely candidates to explain enduring phenotypic changes since the epigenome provides the cellular context for environmental influences, including cannabinoid exposure, to functionally alter genes and related behaviours101–103. The original definition in the 1950s stated “an epigenetic trait is a stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence”104,105. Today, the term ‘epigenetics’ is typically used to describe molecular biological mechanisms that modulate gene expression without altering the genetic code. The main epigenetic mechanisms implicated in the effects of cannabinoids are summarized in BOX 2. Epigenetics is not only crucial for normal development but can also create the most well-characterized biological frameworks known to maintain aberrant neuronal processing and plasticity over long periods. Generally, the interaction between genes and regulatory genomic DNA elements, epigenetic modifiers and transcriptional factors determines the expression state of genes. The network of these processes is tightly coordinated during development and within different organ systems, including the brain106,107. The ECS, similarly to other biological systems, is highly regulated by epigenetics108,109, and these processes are critical in controlling various aspects of both short-term and long-term synaptic communication and plasticity in early life110, the adult brain111 and various neuropsychiatric disorders91.
Box 2 |. Main epigenetic mechanisms implicated in the effects of cannabinoids.
DNA methylation
Methyl groups are added directly to the DNA. The role of DNA methylation is dependent upon genomic location, developmental stage, cell type and disorder. Methylation in promoter regions and transcriptional regulatory sequences has frequently been associated with gene silencing, whereas methylation within the gene body is less understood and may act as either a positive effector or a negative effector159,160.
Histone modifications
Histone proteins are associated with ~150 bp of DNA to form the nucleosome, the basic unit of chromatin. Histones are subject to well-characterized covalent modifications, including acetylation, methylation, phosphorylation, ubiquitination and sumoylation161 and a variety of more recently discovered structures in the brain162. The modifications can influence both the accessibility of genomic regions and the binding of transcription factors to the DNA.
Non-coding rNAs
These functional RNA molecules are transcribed from DNA and regulate gene expression at the transcriptional and post-transcriptional levels163. While the exact genomic targets of specific non-coding RNAs remain to be characterized, the multiple non-coding RNAs are mechanistically intriguing given the variety of tissue-specific cellular and developmental processes that can be influenced by them164. Some non-coding RNAs even persist during the maturation of germ cells and in early embryo development and thus are interesting candidates for the propagation of cannabinoid effects across generations165.
Despite the role of epigenetic factors in neurodevelopmental processes and in maintaining molecular memories throughout life, there remains a surprising dearth of studies regarding epigenetic mechanisms in relation to developmental cannabis/THC exposure (TABLE 2). Of the known epigenetic mechanisms, most information relevant to the neurodevelopmental effects of cannabinoids has been accrued regarding histone modifications. Our group revealed distinct alterations in the histone modification profile of the NAc in adult rats with prenatal THC exposure26,112. The disturbances were characterized by decreased levels of the trimethylation of lysine 4 on histone H3 (H3K4me3; a transcriptionally permissive mark), increased levels of dimethylation of lysine 9 on histone H3 (H3K9me2; a repressive mark) and decreased RNA polymerase II association with the promoter and coding regions of the dopamine D2 receptor gene (Drd2) in the NAc. Importantly, THC-related chromatin modifications, which would be predictive of reduced transcription, were linked to disturbances of the Drd2 mRNA expression in both humans and rodents and persisted into adulthood. These findings are intriguing since reduction of the dopamine D2 receptor function and mRNA levels is characteristic of individuals with substance use disorders113, suggesting that early cannabinoid exposure potentially enhances addiction vulnerability through altering the epigenetic processes at the Drd2 locus. Unfortunately, this remains the only published study to date to investigate direct epigenetic effects of prenatal THC exposure. Thus, the epigenetic relationship to other molecular alterations documented with prenatal THC exposure is currently unknown.
Table 2 |.
Overview of studies on the epigenetic dysregulation of the brain in association with cannabinoid exposure
| Cannabinoid | Exposure period | Brain region and rodent subject studied | Epigenetic alteration | Effecta | Ref. |
|---|---|---|---|---|---|
| THC | Prenatal | Adult male rat NAc | H3K4me2, H3K9me3 Promoter, gene body | ↓ Drd2 mRNA levels | 26 |
| THC | Adolescent | Adult male rat NAc shell | H3K9me2, H3K9me3 Promoter, gene body | ↑ Penk mRNA levels | 98 |
| THC | Adolescent | Adult female rat PFC | H3K9me3 Global levels, promoters | ↓ mRNA expression of genes related to ECS and synaptic plasticity | 75b |
| WIN-55,212–2 cannabinoid agonist | Adolescent | Adult male mouse hippocampus | DNA methylation Intragenic | ↑ DNA methylation and ↓ mRNA expression of Rgs7 | 147 |
| WIN-55,212–2 cannabinoid agonist | Adolescent, adult | Adult male rat PFC | Chromatin accessibility (ATAC-seq) Promoter, gene body | ↑ Accessibility at Npas2 and splicing | 115 |
| HU-210 cannabinoid agonist | Adolescent | Adolescent male rat entorhinal cortex | microRNAs | Altered expression of various microRNAs | 117 |
| THC | Adolescent, adult | Adolescent and adult female rat hippocampus, NAc and amygdala | H3K4me2, H3K9me3, H3K14ac Global levels | Brain region-specific and age-specific alterations of histone modifications at different times after exposure | 90b |
| THC | Adolescent; Preconception (both parents) | Adult male and female rat NAc | CpG DNA methylation at promoters, intergenic regions, especially in gene bodies | Altered methylation at loci implicated in synaptic plasticity, including the Dlg4 gene network | 120 |
| THC | Preconception (father) | Adult rat NAc with THC exposure (father only) | DNA methylation Promoter | ↓ DNA methylation of synaptic Dlgap2 | 139 |
| WIN-55,212–2 cannabinoid agonist | Preconception (father) | Adult male rat PFC | DNA methylation Global levels | ↑ DNA methylation and ↑ DNMT expression | 148 |
ATAC-seq, assay for transposase-accessible chromatin using sequencing; DNMT, DNA methyltransferase; ECS, endocannabinoid system; H3K4me2, dimethylation of lysine 4 on histone H3; H3K9me2, dimethylation of lysine 9 on histone H3; H3K9me3, trimethylation of lysine 9 on histone H3; H3K14ac, acetylation of lysine 14 on histone H3; NAc, nucleus accumbens; PFC, prefrontal cortex; THC, Δ9-tetrahydrocannabinol.
Arrows indicate the direction of change; in several large-scale studies different directions were observed, depending on the conditions, locus or RNA.
The functional significance of histone modifications can be transcriptional repression (H3K4me2 and H3K9me3) and transcriptional activation (H3K14ac). Enhanced chromatin accessibility correlates with increased mRNA expression. The potential roles of DNA methylation differ by locus and can regulate both transcription and splicing. MicroRNAs are involved in post-transcriptional repression.
Very high dose THC (in excess of levels normally used by humans) used in the study.
Knowledge is also limited regarding epigenetic factors in relation to adolescent THC exposure, but several reports have described persistent disturbances associated with exposure during this period. For instance, enduring reduction of repressive epigenetic marks, H3K9me2 and H3K9me3, has been detected at the opioid neuropeptide Penk locus in the NAc of adult rats following adolescent THC exposure, concomitant with increased Penk mRNA levels and enhanced heroin self-administration behaviour in adulthood98. One of the strongest pieces of evidence for a critical role of epigenetic disturbances linked to developmental THC exposure comes from leveraging an unbiased strategy of RNA sequencing of pyramidal layer 2/3 neurons111. The results revealed that low-dose THC exposure during adolescence leads to a profound reorganization of the transcriptome (only 5% overlap with the normal developmental gene expression signature) predominantly related to histone modification, chromatin remodelling and synaptic plasticity gene networks, matching morphological alterations of dendritic spines. Importantly, the history of adolescent THC exposure substantially enhanced the coordination between epigenetic and dendritic/synaptic plasticity-related genes, with the prominent epigenetic modifications associated with these networks being H3K4me3 and KMT2A, a histone methyltransferase with specific activity at H3K4 (REF.111). These findings are intriguing given that H3K4 methyltransferases are essential for neurogenesis114.
A comprehensive set of investigations by Prini et al. has also highlighted epigenetic differences on specific global chromatin modifications within mesocorticolimbic brain regions (PFC, hippocampus, NAc and amygdala) depending on whether THC exposure (escalating moderate to high dose) occurred during adolescent or adulthood75,90. In line with the concept of higher vulnerability of the immature brain to external stimuli, there was a more robust and complex response to adolescent THC exposure as compared with adult exposure on repressive histone methylation (H3K9me2 and H3K9me3) and an acetylation mark of active transcription (H3K14ac). Moreover, alterations of H3K9me3 in the PFC were associated with cognitive impairments75, and pharmacological blockade of H3K9me3 during adolescent exposure to THC prevented the development of cognitive deficits, demonstrating the causal contribution of epigenetic mechanisms to the long-term behavioural effects induced by adolescent THC exposure.
An apparent greater sensitivity of the epigenetic machinery to adolescent versus adult cannabinoid exposure was also demonstrated with WIN-55,212–2 (REF.115). Adolescent, but not adult, rats exposed to this CBR agonist developed increased sensitization to cocaine correlated with chromatin remodelling, histone hyper-acetylation and decreased levels of histone deacetylase (HDAC6) expression. There were also alterations of chromatin accessibility involving genes such as Npas2, which encodes a transcription factor that regulates GABAergic neurotransmission. Once again, these preclinical data demonstrate that adolescent cannabinoid exposure significantly reprogrammes the epigenetic and behavioural responses to cannabinoids.
In addition to chromatin alterations, direct methylation of the DNA also occurs as a consequence of adolescent WIN-55,212–2 exposure, which led to memory deficits in adulthood accompanied by increased hippocampal AEA levels116. DNA hypermethylation was observed at the intragenic region of Rgs7, a gene involved in synaptic plasticity via G-protein-coupled receptor signalling cascades, and the deficits observed in hippocampus-dependent learning and memory processes were attributed to reduced expression of Rgs7. Abnormal expression of short non-coding microRNAs has also been detected in adult animals with adolescent cannabinoid treatment, which was exacerbated by prior maternal stress117, but the functional developmental consequences remain to be elucidated.
Although not extensive to date, current research suggests that cannabis exposure during multiple stages of development can alter epigenetic mechanisms to change the trajectory of individual vulnerability to neurobiological and phenotypic dysregulation later in life. FIGURE 3 illustrates a simplified model based on various lines of evidence for the role of chromatin accessibility and other epigenetic mechanisms as major factors in the regulation of normal brain development118, which can be affected by developmental cannabinoids. The fact that epigenetic modifications are linked to THC-induced neural and behavioural phenotypes has important treatment implications and can account for some inconsistent findings regarding developmental effects of cannabis/THC since epigenetic modifications are reversible and thus sensitive to environmental conditions.
Fig. 3 |. Overview of proposed epigenetic effects induced by exogenous cannabinoids on the developing brain and epigenetic reprogramming of the germ line.
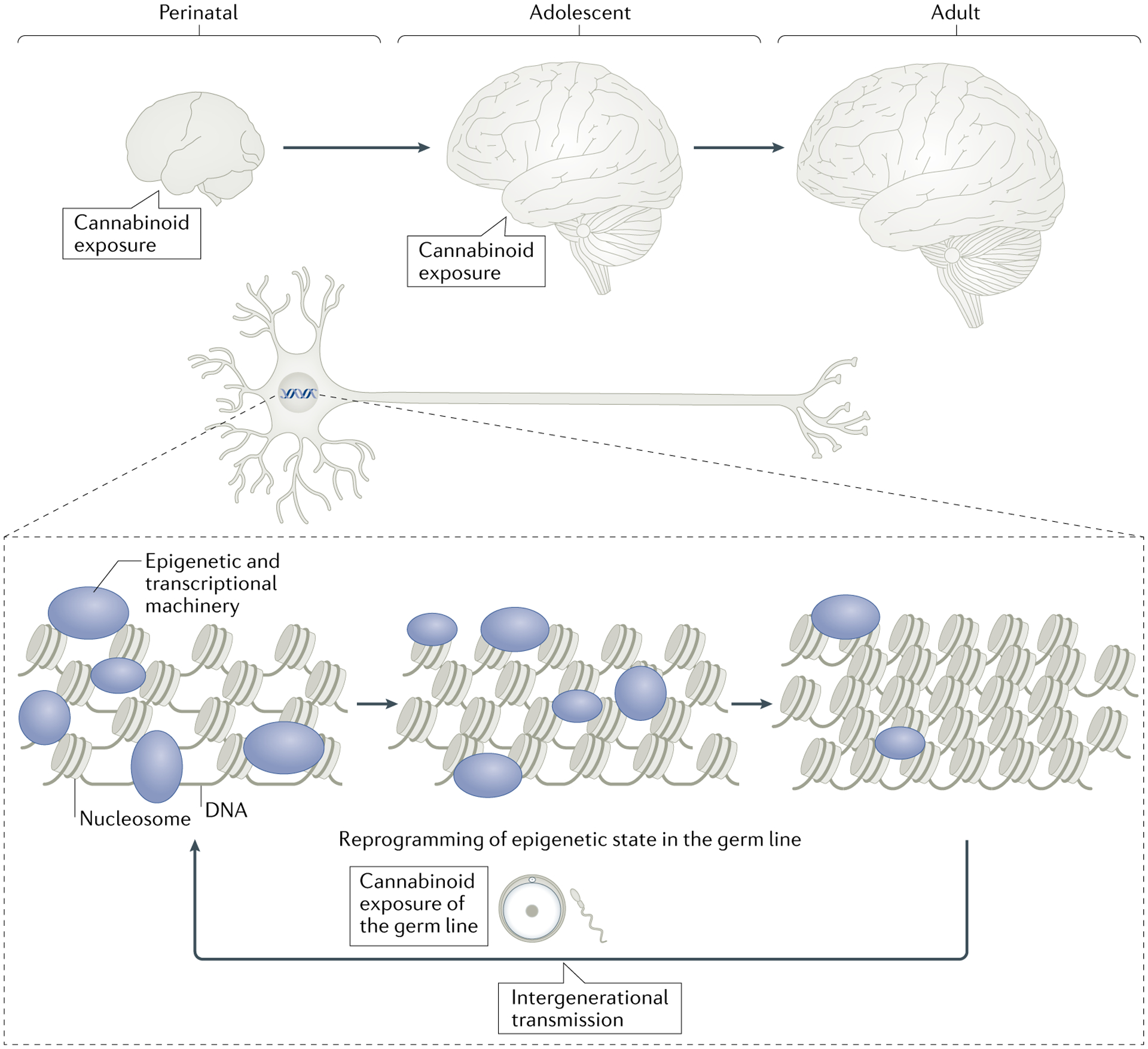
Epigenetic mechanisms, which occur on DNA in the nucleus of cells to regulate gene expression, are sensitive to cannabinoid exposure. During perinatal and adolescent stages of development, the chromatin structure of gene loci functionally related to synaptic plasticity (depicted by the synapse) is in a more open state, regulated by epigenetic and transcriptional machinery (represented by various coloured shapes) that can be altered by external stimuli such as cannabinoid exposure. As development progresses, this higher sensitivity of epigenetic responsiveness declines, rendering the adult brain less vulnerable to external influences. Marked epigenetic remodelling occurs during germ cell production, and reprogrammed germ cells are sensitive to the effects of cannabis. Such epigenetic interference by cannabis may escape the normal reprogramming of the germ line and thereby lead to intergenerational transmission, affecting the offspring from the earliest stages of development. The exact epigenetic mechanisms contributing to intergenerational transmission remain to be elucidated.
Intergenerational impact
In addition to the direct effects of cannabis exposure during development on the brain, there is growing evidence that cannabis may also act through the germ line to shape synaptic development and behaviour across generations. The first attempt to address this possibility was our cross-generational study where male and female rats were intermittently exposed to a low dose of THC during adolescence and mated during adulthood. Surprisingly, their F1 male progeny displayed increased work effort to self-administer heroin and abnormal behaviours during heroin withdrawal119. As male offspring (females were not tested) were cross-fostered in these studies, these intergenerational phenotypes were likely inherited through the germ line. Neurobiologically, the adult F1 generation with parental adolescent THC exposure also had abnormal mRNA expression of CBR, dopamine receptor and glutamatergic receptor genes in the striatum and altered LTD measured electrophysiologically101. Both male and female progeny exhibit significant glutamatergic disturbances in the dorsal striatum in adulthood, and these effects appear stronger in females101. Genome-wide alterations of DNA methylation in the NAc have also been documented in adult offspring of THC-exposed parents, indicating robust intergenerational epigenetic reprogramming120. These effects were more prominent for synaptic plasticity-related genes in a functional network centred on Dlg4 (REF.120). Dlg4 encodes PSD95, a membrane-associated guanylate kinase scaffolding protein located in neural postsynaptic densities and important for regulating dopamine–glutamate interactions121. Epigenetic dysregulation of Dlg4 has been linked to abnormal glutamatergic transmission involved in morphine conditioning122, consistent with the earlier observation of increased heroin self-administration in adult offspring with parental THC exposure119. Many of the differentially methylated genes in the Dlg4 network have been speculated to be susceptibility genes for psychiatric conditions such as schizophrenia, depression, autism and obsessive–compulsive disorder123,124.
Multiple other studies addressing intergenerational effects of cannabinoids have now been reported but with key differences between experimental paradigms and in outcomes. For instance, a study similarly examining adolescent THC exposure in both parents, but in a different rats strain, reported reduced heroin reinforcement behaviour in male offspring125. The effects of THC exposure before conception in either the father or the mother have also been evaluated in independent studies. THC exposure limited to females (when as adolescents) diminished the reward-facilitating effects of THC and amphetamine in their male offspring (females were not tested)126. In contrast, adolescent exposure of female rats to WIN-55,212–2 long before conception increased morphine sensitivity in both male and female offspring127,128. Comparatively, males exposed to low or moderate doses of THC during adulthood sired offspring with reduced attentional performance, adolescent hyperactivity, and impaired learning and memory in both sexes129,130. More research is clearly needed, but these findings suggest there may be unique cross-generational effects of cannabinoid exposure imparted through the male and female germ lines. Additionally, the cross-generational effects of THC documented thus far pertain to intergenerational effects (that is, in the F1 generation) where the offspring are derived from germ cells directly exposed to THC. It will be important to determine whether the effects of THC can be transferred transgenerationally (that is, in the F2 generation for males and the F3 generation for females) independently of this direct germ line exposure.
The observations of cross-generational THC effects have spurred intense interest in the underlying epigenetic mechanisms allowing germ line transmission. Until recently, male gametes were believed to deliver little to no epigenetic memory to the fertilized oocyte. However, with the advancement of next-generation sequencing technology allowing enhanced resolution in methylation, chromatin modification and non-coding RNA analyses, studies are beginning to characterize a unique, environmentally responsive and functional epigenetic landscape in sperm39. Comparatively, environmentally responsive epigenetic mechanisms in the female germ line have been understudied, in part due to technical challenges131 that should diminish as single-cell/low-cell-number technologies become more widely adopted.
The ECS plays critical roles not only in the development of a variety of somatic cells and physiological systems but also in reproduction. An extensive literature has detailed the importance of the ECS to germ line development and function132,133. It is known that both male and female reproductive tissues express CBRs and eCBs. Cannabis-using women are known to produce lower-quality oocytes, which is associated with lower pregnancy rates134. In males, THC can disrupt the normal development of sperm cells135,136, with both cannabis use in humans and THC exposure in rodents associated with reduced sperm counts and altered sperm motility137. Recent evidence also extends these documented effects to epigenetic mechanisms; cannabis use is associated with altered DNA methylation in human sperm138. Furthermore, THC exposure directly affects methylation at several gene loci in the rat model, including some genes differentially methylated in the sperm of cannabis users138. Most notably, Dlgap2, which has neuronal functions, including synapse organization and signalling, was hypomethylated in human cannabis user sperm, a finding validated in THC-exposed rat sperm139. Intriguingly, this locus was similarly hypomethylated in THC-exposed rodent NAc139. Moreover, several of the THC-affected loci in rat sperm were associated with genes differentially methylated in the offspring brain of adolescent THC-exposed rats identified by Watson et al.120, suggesting a relationship between THC-responsive epigenetic patterning in the germ line and THC responsive epigenetic patterning in the adult brain39.
Of other germ line epigenetic mechanisms, sperm microRNAs and other types of non-coding RNA have gained recognition as causal mechanisms driving intergenerational effects of paternal preconception exposures such as chronic stress and high-fat diet in rodents140–145. Additionally, recent studies have revealed that the sperm features a complex chromatin organization at genomic regulatory regions, such as enhancers that associate with chromatin structure in somatic cells during postnatal development146. Thus, future studies exploring the constellation of these implicated mechanisms will elucidate how the THC-responsive germ line epigenome may reshape early neuronal development to promote sustained alterations in synaptic function and behaviour across the lifetime.
Conclusion
While debates mount regarding the developmental effects of cannabis, accumulating evidence from various lines of research demonstrates that there is potential for exposure during prenatal and adolescent periods to change the trajectory of various neurobiological systems. The evidence also clearly identifies perturbation of synaptic plasticity as a common characteristic of the adult brain despite THC exposure limited to early developmental periods. Importantly, the causal disturbances of neural processes in adulthood by early cannabinoid exposure are linked to behavioural phenotypes predictive of psychiatric and addiction risk. Despite the growing literature, there nevertheless remain large gaps in knowledge regarding individual differences related to early cannabinoid exposure, including genetics, sex (including aspects of puberty that have not been addressed), other environmental insults during development (for example, trauma/stress, toxins and other drugs) and the amount/frequency of exposure (including the combination of various cannabinoids with THC in different strains being consumed today). Systematic studies of these factors are also critical to identify individuals at potential risk so as to optimize early interventions that could prevent the full onset of adult psychopathology. Nevertheless, it is important to emphasize that any such psychopathology need not be deterministic. The marked epigenetic reprogramming induced by THC, and that could account for the parental environment to be transmitted across generations, emphasizes that the observed neurobiological disturbances and behaviours can be reversed. The dynamic nature of epigenetic mechanisms in contrast to static genetic inheritance could thus provide opportunities to counter negative consequences of developmental cannabis exposure.
Acknowledgements
The authors were supported by NIDA grants DA030359 and DA050403 (G.R.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Lu HC & Mackie K Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 10.1016/j.bpsc.2020.07.016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berghuis P et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316, 1212–1216 (2007). [DOI] [PubMed] [Google Scholar]; This study demonstrates the crucial role of the eCB signalling in axonal guidance and in the formation of accurate synaptic connections.
- 3.Maccarrone M, Guzmán M, Mackie K, Doherty P & Harkany T Programming of neural cells by (endo) cannabinoids: from physiological rules to emerging therapies. Nat. Rev. Neurosci 15, 786–801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulder J et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl Acad. Sci. USA 105, 8760–8765 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley NE, Hansson S, Harta G & Mezey E Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 82, 1131–1149 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Zurolo E et al. CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience 170, 28–41 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Biegon A & Kerman IA Autoradiographic study of pre-and postnatal distribution of cannabinoid receptors in human brain. Neuroimage 14, 1463–1468 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Berrendero F, Sepe N, Ramos JA, Di Marzo V & Fernández-Ruiz JJ Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 33, 181–191 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Mato S, Del Olmo E & Pazos A Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci 17, 1747–1754 (2003). [DOI] [PubMed] [Google Scholar]; This is an important study showing functional G protein coupling of CBRs in the human fetal brain.
- 10.Romero J et al. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse 26, 317–323 (1997). [DOI] [PubMed] [Google Scholar]
- 11.de Salas-Quiroga A et al. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc. Natl Acad. Sci. USA 112, 13693–13698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harkany T et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci 28, 83–92 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Keimpema E, Calvigioni D & Harkany T Endocannabinoid signals in the developmental programming of delayed-onset neuropsychiatric and metabolic illnesses. Biochem. Soc. Trans 41, 1569–1576 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Mackie K Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol 10.1007/3-540-26573-2_10 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Herkenham M et al. Cannabinoid receptor localization in brain. Proc. Natl Acad. Sci. USA 87, 1932–1936 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the anatomical expression of CB1R and its being the most abundant G protein receptor in the adult brain.
- 16.Wang X, Dow-Edwards D, Keller E & Hurd YL Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694 (2003). [DOI] [PubMed] [Google Scholar]; This is the first study to characterize the distinct anatomical pattern of CNR1 expression in the human fetal brain.
- 17.Freund TF, Katona I & Piomelli D Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev 83, 1017–1066 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Marsicano G & Lutz B Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci 11, 4213–4225 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Ellgren M et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur. Neuropsychopharmacol 18, 826–834 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee TT, Hill MN, Hillard CJ & Gorzalka BB Temporal changes in N-acylethanolamine content and metabolism throughout the peri-adolescent period. Synapse 67, 4–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo PE, Younts TJ, Chávez AE & Hashimotodani Y Endocannabinoid signaling and synaptic function. Neuron 76, 70–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M & Watanabe M Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev 89, 309–380 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Meyer HC, Lee FS & Gee DG The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology 43, 21–33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tortoriello G et al. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 33, 668–685 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides mechanistic evidence of the molecular underpinnings of prenatal THC exposure with regard to neuronal differentiation and axonal guidance evident in the rodent model and in the human fetal brain.
- 25.Higuera-Matas A, Ucha M & Ambrosio E Long-term consequences of perinatal and adolescent cannabinoid exposure on neural and psychological processes. Neurosci. Biobehav. Rev 55, 119–146 (2015). [DOI] [PubMed] [Google Scholar]
- 26.DiNieri JA et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 70, 763–769 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Dow-Edwards D, Anderson V, Minkoff H & Hurd YL In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol. Psychiatry 56, 909–915 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Ellgren M, Spano SM & Hurd YL Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32, 607–615 (2007). [DOI] [PubMed] [Google Scholar]; This is the first study to demonstrate that adolescent THC exposure increases opioid self-administration in adulthood and opioid receptor signalling in the NAc.
- 29.Spano MS, Ellgren M, Wang X & Hurd YL Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiatry 61, 554–563 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Dow-Edwards D, Anderson V, Minkoff H & Hurd YL Discrete opioid gene expression impairment in the human fetal brain associated with maternal marijuana use. Pharmacogenomics J. 6, 255–264 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Castaldo P et al. Altered regulation of glutamate release and decreased functional activity and expression of GLT1 and GLAST glutamate transporters in the hippocampus of adolescent rats perinatally exposed to Delta(9)-THC. Pharmacol. Res 61, 334–341 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Ferraro L et al. Short-and long-term consequences of prenatal exposure to the cannabinoid agonist WIN55,212–2 on rat glutamate transmission and cognitive functions. J. Neural Transm 116, 1017–1027 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Antonelli T et al. Prenatal exposure to the CB1 receptor agonist WIN 55,212–2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 15, 2013–2020 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Saez TM, Aronne MP, Caltana L & Brusco AH Prenatal exposure to the CB1 and CB2 cannabinoid receptor agonist WIN 55,212–2 alters migration of early-born glutamatergic neurons and GABAergic interneurons in the rat cerebral cortex. J. Neurochem 129, 637–648 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Frau R et al. Prenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenolone. Nat. Neurosci 22, 1975–1985 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the sex-specific impact of prenatal THC exposure on the dopaminergic system in the VTA of preadolescent male rats that increased their sensitivity to a single THC injection re-exposure. Moreover, the study shows that postnatal treatment with pregnenolone can reverse the synaptic and behavioural deficits.
- 36.Mereu G et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc. Natl Acad. Sci. USA 100, 4915–4920 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castaldo P et al. Prenatal exposure to the cannabinoid receptor agonist WIN 55,212–2 increases glutamate uptake through overexpression of GLT1 and EAAC1 glutamate transporter subtypes in rat frontal cerebral cortex. Neuropharmacology 53, 369–378 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Bassani S, Folci A, Zapata J & Passafaro M AMPAR trafficking in synapse maturation and plasticity. Cell Mol. Life Sci 70, 4411–4430 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bara A et al. Sex-dependent effects of in utero cannabinoid exposure on cortical function. eLife 10.7554/eLife.36234 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; In addition to demonstrating sex-specific effects of prenatal THC exposure on PFC functions and social behaviour, this study is the first to show that eCB-mediated LTD in the PFC is mediated by distinct receptors in males and females.
- 40.de Salas-Quiroga A et al. Long-term hippocampal interneuronopathy drives sex-dimorphic spatial memory impairment induced by prenatal THC exposure. Neuropsychopharmacology 45, 877–886 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vargish GA et al. Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Mol. Psychiatry 22, 56–67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grant KS, Conover E & Chambers CD Update on the developmental consequences of cannabis use during pregnancy and lactation. Birth Defects Res. 10.1002/bdr2.1766 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith AM et al. Prenatal marijuana exposure impacts executive functioning into young adulthood: An fMRI study. Neurotoxicol. Teratol 58, 53–59 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Gray KA, Day NL, Leech S & Richardson GA Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol. Teratol 27, 439–448 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Leech SL, Larkby CA, Day R & Day NL Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J. Am. Acad. Child Adolesc. Psychiatry 45, 223–230 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Porath AJ & Fried PA Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol. Teratol 27, 267–277 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Sonon KE, Richardson GA, Cornelius JR, Kim KH & Day NL Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol. Teratol 47, 10–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergeria CL & Heil SH Surveying lactation professionals regarding marijuana use and breastfeeding. Breastfeed. Med 10, 377–380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Reyes M & Wall ME Presence of delta9-tetrahydrocannabinol in human milk. N. Engl. J. Med 307, 819–820 (1982). [DOI] [PubMed] [Google Scholar]
- 50.Baker T et al. Transfer of inhaled cannabis into human breast milk. Obstet. Gynecol 131, 783–788 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Kolb B, Mychasiuk R, Muhammad A & Gibb R Brain plasticity in the developing brain. Prog. Brain Res 207, 35–64 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Tennes K et al. Marijuana: prenatal and postnatal exposure in the human. NIDA Res. Monogr 59, 48–60 (1985). [PubMed] [Google Scholar]
- 53.Astley SJ & Little RE Maternal marijuana use during lactation and infant development at one year. Neurotoxicol. Teratol 12, 161–168 (1990). [DOI] [PubMed] [Google Scholar]
- 54.Spear LP & File SE Methodological considerations in neurobehavioral teratology. Pharmacol. Biochem. Behav 55, 455–457 (1996). [DOI] [PubMed] [Google Scholar]
- 55.Campolongo P et al. Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict. Biol 12, 485–495 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Suárez I et al. Down-regulation of the AMPA glutamate receptor subunits GluR1 and GluR2/3 in the rat cerebellum following pre-and perinatal delta9-tetrahydrocannabinol exposure. Cerebellum 3, 66–74 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Vela G et al. Maternal exposure to delta9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 807, 101–109 (1998). [DOI] [PubMed] [Google Scholar]
- 58.González B et al. Effects of perinatal exposure to delta 9-tetrahydrocannabinol on operant morphine-reinforced behavior. Pharmacol. Biochem. Behav 75, 577–584 (2003). [DOI] [PubMed] [Google Scholar]
- 59.García-Gil L, Romero J, Ramos JA & Fernández-Ruiz JJ Cannabinoid receptor binding and mRNA levels in several brain regions of adult male and female rats perinatally exposed to delta9-tetrahydrocannabinol. Drug. Alcohol. Depend 55, 127–136 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Scheyer AF, Borsoi M, Pelissier-Alicot AL & Manzoni OJJ Perinatal THC exposure via lactation induces lasting alterations to social behavior and prefrontal cortex function in rats at adulthood. Neuropsychopharmacology 10.1038/s41386-020-0716-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheyer AF et al. Cannabinoid exposure via lactation in rats disrupts perinatal programming of the gamma-aminobutyric acid trajectory and select early-life behaviors. Biol. Psychiatry 87, 666–677 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to demonstrate that exposure to THC during lactation delays the developmental excitatory-to-inhibitory GABA polarity switch in the rat PFC.
- 62.Hensch TK Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci 6, 877–888 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Beggiato S et al. Long-lasting alterations of hippocampal GABAergic neurotransmission in adult rats following perinatal Δ9-THC exposure. Neurobiol. Learn. Mem 139, 135–143 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Rivera C et al. The K+/Cl−co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Kaila K, Price TJ, Payne JA, Puskarjov M & Voipio J Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci 15, 637–654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manduca A et al. Sex-specific behavioural deficits induced at early life by prenatal exposure to the cannabinoid receptor agonist WIN55, 212–2 depend on mGlu5 receptor signalling. Br. J. Pharmacol 177, 449–463 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holitzki H, Dowsett LE, Spackman E, Noseworthy T & Clement F Health effects of exposure to second-and third-hand marijuana smoke: a systematic review. CMAJ Open 5, E814–E822 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodwin RD et al. Trends in cannabis and cigarette use among parents with children at home: 2002 to 2015. Pediatrics 10.1542/peds.2017-3506 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Posis A et al. Indoor cannabis smoke and children’s health. Prev. Med. Rep 14, 100853 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eiden RD et al. Pre-and postnatal tobacco and cannabis exposure and child behavior problems: bidirectional associations, joint effects, and sex differences. Drug Alcohol Depend. 185, 82–92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Renard J, Krebs MO, Le Pen G & Jay TM Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci 8, 361 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubino T, Zamberletti E & Parolaro D Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J. Psychopharmacol 26, 177–188 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Blest-Hopley G, Colizzi M, Giampietro V & Bhattacharyya S Is the adolescent brain at greater vulnerability to the effects of cannabis? A narrative review of the evidence. Front. Psychiatry 11, 859 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rubino T et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol. Dis 73, 60–69 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Cuccurazzu B et al. Adult cellular neuroadaptations induced by adolescent THC exposure in female rats are rescued by enhancing anandamide signaling. Int. J. Neuropsychopharmacol 21, 1014–1024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silva L, Black R, Michaelides M, Hurd YL & Dow-Edwards D Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: the unique susceptibility of the prepubescent animal. Neurotoxicol. Teratol 58, 88–100 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Rubino T et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33, 2760–2771 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Kruse LC, Cao JK, Viray K, Stella N & Clark JJ Voluntary oral consumption of Delta(9)-tetrahydrocannabinol by adolescent rats impairs reward-predictive cue behaviors in adulthood. Neuropsychopharmacology 44, 1406–1414 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abela AR, Rahbarnia A, Wood S, Le AD & Fletcher PJ Adolescent exposure to Delta9-tetrahydrocannabinol delays acquisition of paired-associates learning in adulthood. Psychopharmacology 236, 1875–1886 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Renard J et al. Adolescent cannabinoid exposure induces a persistent sub-cortical hyper-dopaminergic state and associated molecular adaptations in the prefrontal cortex. Cereb. Cortex 27, 1297–1310 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Cha YM, Jones KH, Kuhn CM, Wilson WA & Swartzwelder HS Sex differences in the effects of delta9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav. Pharmacol 18, 563–569 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Ruiz CM et al. Pharmacokinetic, behavioral, and brain activity effects of Δ9-tetrahydrocannabinol in adolescent male and female rats. Neuropsychopharmacology 10.1038/s41386-020-00839-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiley JL & Burston JJ Sex differences in Δ9-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci. Lett 576, 51–55 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pertwee RG Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol 147, S163–S171 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee SE, Lee Y & Lee GH The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch. Pharm. Res 42, 1031–1039 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Renard J et al. Adolescent THC exposure causes enduring prefrontal cortical disruption of GABAergic inhibition and dysregulation of sub-cortical dopamine function. Sci. Rep 7, 11420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zamberletti E et al. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol. Dis 63, 35–47 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Rubino T et al. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox. Res 15, 291–302 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Zamberletti E et al. Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharmacol. Res 111, 459–470 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Prini P, Penna F, Sciuccati E, Alberio T & Rubino T Chronic Delta(8)-THC exposure differently affects histone modifications in the adolescent and adult rat brain. Int. J. Mol. Sci 10.3390/ijms18102094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprehensive study characterizing the impact of developmental THC exposure on histone modifications in a brain region-specific and age-specific manner, leading to either transcriptional activation or repression.
- 91.Batool S et al. Synapse formation: from cellular and molecular mechanisms to neurodevelopmental and neurodegenerative disorders. J. Neurophysiol 121, 1381–1397 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Miller ML et al. Adolescent exposure to Delta(9)-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol. Psychiatry 24, 588–600 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winterer G et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am. J. Psychiatry 161, 490–500 (2004). [DOI] [PubMed] [Google Scholar]
- 94.Winterer G & Weinberger DR Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 27, 683–690 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Friedman AL, Meurice C & Jutkiewicz EM Effects of adolescent Delta9-tetrahydrocannabinol exposure on the behavioral effects of cocaine in adult Sprague-Dawley rats. Exp. Clin. Psychopharmacol 27, 326–337 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Lecca D et al. Adolescent cannabis exposure increases heroin reinforcement in rats genetically vulnerable to addiction. Neuropharmacology 166, 107974 (2020). [DOI] [PubMed] [Google Scholar]
- 97.Stopponi S et al. Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur. Neuropsychopharmacol 24, 1037–1045 (2014). [DOI] [PubMed] [Google Scholar]
- 98.Tomasiewicz HC et al. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol. Psychiatry 72, 803–810 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scherma M et al. Adolescent Delta(9)-tetrahydrocannabinol exposure alters WIN55,212–2 self-administration in adult rats. Neuropsychopharmacology 41, 1416–1426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Volkow ND, Wise RA & Baler R The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci 18, 741–752 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Szutorisz H, Egervari G, Sperry J, Carter JM & Hurd YL Cross-generational THC exposure alters the developmental sensitivity of ventral and dorsal striatal gene expression in male and female offspring. Neurotoxicol. Teratol 58, 107–114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Szutorisz H & Hurd YL Epigenetic effects of cannabis exposure. Biol. Psychiatry 79, 586–594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Szutorisz H & Hurd YL High times for cannabis: Epigenetic imprint and its legacy on brain and behavior. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2017.05.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baedke J The epigenetic landscape in the course of time: Conrad Hal Waddington’s methodological impact on the life sciences. Stud. Hist. Philos. Biol. Biomed. Sci 44, 756–773 (2013). [DOI] [PubMed] [Google Scholar]
- 105.Van Speybroeck L From epigenesis to epigenetics: the case of C. H. Waddington. Ann. N. Y. Acad. Sci 981, 61–81 (2002). [PubMed] [Google Scholar]
- 106.Dambacher S, de Almeida GP & Schotta G Dynamic changes of the epigenetic landscape during cellular differentiation. Epigenomics 5, 701–713 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Dillon N Factor mediated gene priming in pluripotent stem cells sets the stage for lineage specification. Bioessays 34, 194–204 (2012). [DOI] [PubMed] [Google Scholar]
- 108.D’Addario C, Di Francesco A, Pucci M, Finazzi Agro A & Maccarrone M Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 280, 1905–1917 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Meccariello R et al. The epigenetics of the endocannabinoid system. Int. J. Mol. Sci 21, 10.3390/ijms21031113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weaver IC Integrating early life experience, gene expression, brain development, and emergent phenotypes: unraveling the thread of nature via nurture. Adv. Genet 86, 277–307 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Bayraktar G & Kreutz MR Neuronal DNA methyltransferases: epigenetic mediators between synaptic activity and gene expression? Neuroscientist 24, 171–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris CV, DiNieri JA, Szutorisz H & Hurd YL Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. Eur. J. Neurosci 34, 1574–1583 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Volkow ND & Morales M The brain on drugs: from reward to addiction. Cell 162, 712–725 (2015). [DOI] [PubMed] [Google Scholar]
- 114.Lim DA et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature 458, 529–533 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scherma M et al. Cannabinoid exposure in rat adolescence reprograms the initial behavioral, molecular, and epigenetic response to cocaine. Proc. Natl Acad. Sci. USA 117, 9991–10002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tomas-Roig J et al. Chronic exposure to cannabinoids during adolescence causes long-lasting behavioral deficits in adult mice. Addict. Biol 22, 1778–1789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hollins SL, Zavitsanou K, Walker FR & Cairns MJ Alteration of imprinted Dlk1-Dio3 miRNA cluster expression in the entorhinal cortex induced by maternal immune activation and adolescent cannabinoid exposure. Transl. Psychiatry 4, e452 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weaver IC et al. Stress and the emerging roles of chromatin remodeling in signal integration and stable transmission of reversible phenotypes. Front. Behav. Neurosci 11, 41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szutorisz H et al. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology 39, 1315–1323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the first to demonstrate that preconception exposure to THC affects neurobehavioural and neurophysiological phenotypes in the offspring.
- 120.Watson CT et al. Genome-wide DNA methylation profiling reveals epigenetic changes in the rat nucleus accumbens associated with cross-generational effects of adolescent THC exposure. Neuropsychopharmacology 40, 2993–3005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Bartolomeis A & Tomasetti C Calcium-dependent networks in dopamine-glutamate interaction: the role of postsynaptic scaffolding proteins. Mol. Neurobiol 46, 275–296 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Wang Z, Yan P, Hui T & Zhang J Epigenetic upregulation of PSD-95 contributes to the rewarding behavior by morphine conditioning. Eur. J. Pharmacol 732, 123–129 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Spiers H et al. Methylomic trajectories across human fetal brain development. Genome Res. 25, 338–352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilson ME & Sengoku T Developmental regulation of neuronal genes by DNA methylation: environmental influences. Int. J. Dev. Neurosci 31, 448–451 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hempel BJ et al. Cross-generational THC exposure alters heroin reinforcement in adult male offspring. Drug. Alcohol. Depend 212, 107985 (2020). [DOI] [PubMed] [Google Scholar]
- 126.Pitsilis G, Spyridakos D, Nomikos GG & Panagis G Adolescent female cannabinoid exposure diminishes the reward-facilitating effects of Delta9-tetrahydrocannabinol and d-amphetamine in the adult male offspring. Front. Pharmacol 8, 225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vassoler FM, Johnson NL & Byrnes EM Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. J. Psychopharmacol 27, 1015–1022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Byrnes JJ, Johnson NL, Schenk ME & Byrnes EM Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. J. Psychopharmacol 26, 1348–1354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holloway ZR et al. Paternal factors in neurodevelopmental toxicology: THC exposure of male rats causes long-lasting neurobehavioral effects in their offspring. Neurotoxicology 78, 57–63 (2020). [DOI] [PubMed] [Google Scholar]
- 130.Levin ED et al. Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicol. Teratol 74, 106806 (2019). [DOI] [PubMed] [Google Scholar]
- 131.Yang Q et al. Highly sensitive sequencing reveals dynamic modifications and activities of small RNAs in mouse oocytes and early embryos. Sci. Adv 2, e1501482 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morris A The endocannabinoid system in human testes. Nat. Rev. Endocrinol 15, 684–685 (2019). [DOI] [PubMed] [Google Scholar]
- 133.Walker OS, Holloway AC & Raha S The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res 12, 3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klonoff-Cohen HS, Natarajan L & Chen RV A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. Am. J. Obstet. Gynecol 194, 369–376 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Banerjee A, Singh A, Srivastava P, Turner H & Krishna A Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defects Res. B Dev. Reprod. Toxicol 92, 195–205 (2011). [DOI] [PubMed] [Google Scholar]
- 136.Maccarrone M, Rapino C, Francavilla F & Barbonetti A Cannabinoid signalling and effects of cannabis on the male reproductive system. Nat. Rev. Urol 18, 19–32 (2021). [DOI] [PubMed] [Google Scholar]
- 137.Payne KS, Mazur DJ, Hotaling JM & Pastuszak AW Cannabis and male fertility: a systematic review. J. Urol 202, 674–681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murphy SK et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 10.1080/15592294.2018.1554521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to demonstrate the effects of THC/cannabis on DNA methylation in human sperm.
- 139.Schrott R et al. Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics 15, 161–173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gapp K et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci 17, 667–669 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodgers AB & Bale TL Germ cell origins of posttraumatic stress disorder risk: the transgenerational impact of parental stress experience. Biol. Psychiatry 78, 307–314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gapp K et al. Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Mol. Psychiatry 10.1038/s41380-018-0271-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grandjean V et al. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep 5, 18193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Q et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Benito E et al. RNA-dependent intergenerational inheritance of enhanced synaptic plasticity after environmental enrichment. Cell Rep. 23, 546–554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jung YH et al. Chromatin states in mouse sperm correlate with embryonic and adult regulatory landscapes. Cell Rep. 18, 1366–1382 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tomas-Roig J et al. Chronic exposure to cannabinoids during adolescence causes long-lasting behavioral deficits in adult mice. Addict. Biol 10.1111/adb.12446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ibn Lahmar Andaloussi Z, Taghzouti K & Abboussi O Behavioural and epigenetic effects of paternal exposure to cannabinoids during adolescence on offspring vulnerability to stress. Int. J. Dev. Neurosci 72, 48–54 (2019). [DOI] [PubMed] [Google Scholar]
- 149.Rodríguez de Fonseca F, Ramos JA, Bonnin A & Fernández-Ruiz JJ Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4, 135–138 (1993). [DOI] [PubMed] [Google Scholar]
- 150.Glass M, Dragunow M & Faull RL Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299–318 (1997). [DOI] [PubMed] [Google Scholar]; This is a seminal early study showing the expression of CB1R in the human brain in different developmental periods and in adulthood.
- 151.Long LE, Lind J, Webster M & Weickert CS Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 13, 87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zias J et al. Early medical use of cannabis. Nature 363, 215 (1993). [DOI] [PubMed] [Google Scholar]
- 153.Mechoulam R & Hanus L A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids 108, 1–13 (2000). [DOI] [PubMed] [Google Scholar]
- 154.ElSohly MA, Radwan MM, Gul W, Chandra S & Galal A Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod 103, 1–36 (2017). [DOI] [PubMed] [Google Scholar]
- 155.ElSohly MA et al. Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997. J. Forensic Sci 45, 24–30 (2000). [PubMed] [Google Scholar]
- 156.Chandra S et al. New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur. Arch. Psychiatry Clin. Neurosci 10.1007/s00406-019-00983-5 (2019). [DOI] [PubMed] [Google Scholar]
- 157.Raber JC, Elzinga S & Kaplan C Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J. Toxicol. Sci 40, 797–803 (2015). [DOI] [PubMed] [Google Scholar]
- 158.Stogner JM & Miller BL Assessing the dangers of “dabbing”: mere marijuana or harmful new trend? Pediatrics 136, 1–3 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Armstrong MJ, Jin Y, Allen EG & Jin P Diverse and dynamic DNA modifications in brain and diseases. Hum. Mol. Genet 28, R241–R253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Greenberg MVC & Bourc’his D The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol 20, 590–607 (2019). [DOI] [PubMed] [Google Scholar]
- 161.Bhaumik SR, Smith E & Shilatifard A Covalent modifications of histones during development and disease pathogenesis. Nat. Struct. Mol. Biol 14, 1008–1016 (2007). [DOI] [PubMed] [Google Scholar]
- 162.Chan JC & Maze I Nothing is yet set in (hi)stone: novel post-translational modifications regulating chromatin function. Trends Biochem. Sci 45, 829–844 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iyengar BR et al. Non-coding RNA interact to regulate neuronal development and function. Front. Cell. Neurosci 8, 47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yoshino Y & Dwivedi Y Non-coding RNAs in psychiatric disorders and suicidal behavior. Front. Psychiatry 11, 543893 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Legoff L, D’Cruz SC, Tevosian S, Primig M & Smagulova F Transgenerational inheritance of environmentally induced epigenetic alterations during mammalian development. Cells 10.3390/cells8121559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


