Abstract
Purpose of Review:
To review diagnosis, clinical characteristics and treatment of Multisystem Inflammatory Syndrome in Children (MIS-C) associated with SARS-CoV-2.
Recent findings:
MIS-C emerged in spring 2020 as a hyperinflammatory syndrome following SARS-CoV-2 exposure in children. Despite growing awareness of MIS-C, diagnosis remains challenging due to the range of phenotypes and severity. Fever accompanied by shock, cardiac dysfunction, gastrointestinal symptoms, or mucocutaneous signs suggestive of Kawasaki disease (KD), especially in the presence of known or suspected COVID-19 exposure, should trigger consideration of MIS-C. However, clinical presentations are highly varied, and may overlap with other infectious diseases. Clinicians must maintain a high index of suspicion for MIS-C and be aware that patients may develop coronary artery aneurysms and myocarditis even with few or no KD symptoms. More precise diagnostic criteria and specific biomarkers are needed to aid diagnosis. IVIG is first-line therapy, and steroids should be considered as initial adjunctive treatment for patients with severe manifestations or other risk factors. Prompt treatment is essential, as patients may worsen acutely, though overall prognosis is reassuring.
Summary:
MIS-C associated with SARS-CoV-2 has varied clinical manifestations. Clinicians must be aware of the common presentation and potential for decompensation and cardiac sequalae to guide appropriate evaluation and treatment.
Keywords: Multisystem Inflammatory Syndrome in Children (MIS-C), COVID-19, SARS-COV2, Kawasaki, pediatric
Introduction
Multisystem Inflammatory Syndrome in Children (MIS-C) is one of the most concerning manifestations of SARS-CoV-2 infection in children. Despite increasing awareness of MIS-C, diagnosis remains challenging due to the shared symptomatology with acute COVID-19 and other febrile illnesses of childhood coupled with the lack of specific biomarkers for MIS-C. Given the risk of cardiovascular sequalae and progression to multisystem organ involvement and death, prompt recognition and treatment of MIS-C is essential. In this review, we discuss the emergence, clinical manifestations, and treatment of MIS-C.
Emergence of MIS-C
During the first months of the COVID-19 pandemic, reports of SARS-CoV-2 infection in children were reassuring, with low infection rates and few severe cases [1]. However, in late April 2020, alarming reports came from the United Kingdom of children presenting with hyperinflammatory shock and features of Kawasaki disease (KD) suspected to be related to SARS-CoV-2 exposure. Eventually, this entity would become known as MIS-C [2–3*]. In the first series describing this syndrome, 5 of 8 children required mechanical ventilation, one developed a giant coronary aneurysm, and one died, highlighting the critical nature of MIS-C [3*]. All patients had positive SARS-CoV-2 serologies. An early case series from northern Italy, a European epicenter of COVID-19, reported a 30-fold increase in KD cases during 2020 compared to the 5 years preceding the COVID-19 pandemic [4*]. These children also presented with severe disease, with unusually high rates of macrophage activation syndrome and KD Shock Syndrome compared to KD in the pre-pandemic era. Eighty percent of children in the Italian study were seropositive for SARS-CoV-2, strengthening the presumed association with prior COVID-19 infection.
Additional studies from Europe and New York followed, with similarly high rates of shock and SARS-CoV-2 antibody positivity [5,6*,7]. As MIS-C gained recognition, subsequent reports characterized a spectrum of disease severity, ranging from fever and systemic inflammation to critical illness [8,9*,10].
Case Definitions
Despite growing recognition, MIS-C is difficult to define. The Royal College of Paediatrics, Centers for Disease Control (CDC) and World Health Organization (WHO) criteria are presented in Table 1 [11–13]. These case definitions were developed to expedite reporting of MIS-C to local health authorities, and thus are intentionally broad. Further, all were developed based on early reports which overrepresent children with the most severe phenotypes, and therefore may miss milder MIS-C. They are not validated for clinical diagnostic purposes, and may capture other febrile diseases. Given the differences in case definitions, studies that utilize different inclusion criteria are not necessarily comparable.
Table 1.
MIS-C Case Definitions
| Royal College of Paediatrics and Child Health11 | Centers for Disease Control12 | World Health Organization13 | |
|---|---|---|---|
| Fever | Persistent fever >38.5°C | Fever >38.0°C for ≥24 hours, or report of subjective fever lasting ≥24 hours | Fever > 3 days |
| Evidence of SARS-CoV2 Infection or Exposure | SARS-CoV-2 PCR testing may be positive or negative | Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks prior to the onset of symptoms | Evidence of COVID-19 (RT-PCR, antigen test or serology positive), or likely contact with a person with COVID-19 |
| Clinical Features | Inflammation (neutrophilia, elevated CRP and lymphopenia) AND evidence of single or multi-organ dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurological disorder) with additional features |
Laboratory evidence of inflammation AND Multisystem (>2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurological) |
Elevated markers of inflammation AND Two of the following: Rash/ mucocutaneous signs; Hypotension or shock; Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities; Coagulopathy; Acute gastrointestinal problems |
| Alternative Diagnoses | Exclusion of any other microbial cause | No alternative plausible diagnoses | No other obvious microbial cause of inflammation |
| Level of Care | Not specified | Hospitalization required | Not specified |
Epidemiology
Cases of MIS-C peak 2–6 weeks after highest community incidence of SARS-CoV-2 infections. In series with data on MIS-C patients’ antecedent SARS-CoV-2 infections, MIS-C occurred a median of 21–25 days following initial respiratory symptoms [6*,9*]. Male predominance is reported in a majority of studies. Most children are previously healthy, though asthma and obesity are common [6*,9*]. Though initially described as a pediatric syndrome, nearly identical clinical presentations are reported in adults in increasing numbers [14*].
Despite high rates of SARS-CoV-2 infections in China early in the pandemic, very few cases of MIS-C have been reported in east Asia. A review of KD cases from Tokyo showed no increase in the prevalence of KD after the start of the COVID-19 pandemic, nor in rates of myocardial dysfunction [15]. However, in recent months, MIS-C cases have been observed in multiple other regions around the world, including Iran, India, South Korea, South Africa, and Latin America [16–20]. The rarity of MIS-C in east Asia is not fully understood, with some theories suggesting that differences in the immunogenicity of SARS-CoV-2 variants or host-related factors may explain this observation.
Racial/Ethnic Disparities
As early as the first U.K. series, racial and ethnicity disparities in the incidence of MIS-C have been apparent [3]. Subsequent reports consistently demonstrate disproportionate rates of MIS-C in Black and Hispanic children. In the USA, 63% of MIS-C cases reported by the CDC were in Black or Hispanic children [21]. In New York, Black and Hispanic children were hospitalized with both acute COVID-19 and MIS-C at higher rates than White and Asian children (Figure 1) [22]. It is difficult to determine if there is an additional preponderance of MIS-C cases beyond the disproportionately elevated risk of acute COVID-19 in Black and Hispanic communities. A large series evaluating MIS-C and pediatric acute COVID-19 found that children with MIS-C were more likely to be Black compared to non-Hispanic White children [23*]. The reason for this increased MIS-C risk in Black children is not fully understood. Access to care for acute COVID-19 among Black children may play a role, as the reported rate of COVID-19 hospitalizations, though elevated for Black adults, underestimates the even larger disparity in the number of COVID-19 cases treated at home [24–25]. This situation may be exacerbated by lack of testing in predominantly Black communities, which leads to inaccurate data on COVID-19 community prevalence [26]. Socioeconomic status has been shown to be associated with pediatric acute COVID-19 infection, suggesting that risk factors such as parental occupation, public transportation use and crowding may be mediators of these disparities [27].
Figure 1. Racial and Ethnic Distribution of Cases.
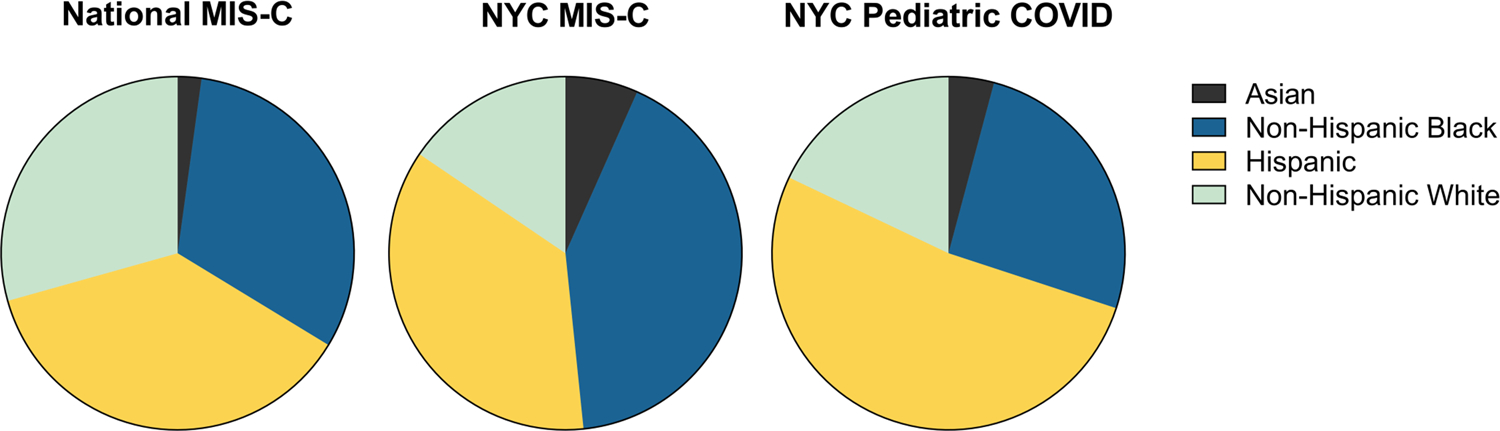
* Data from Centers for Disease Control and Prevention. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States https://www.cdc.gov/mis-c/cases/index.html and Lee, E. H., Kepler, K. L., Geevarughese, A., Paneth-Pollak, R., Dorsinville, M. S., Ngai, S., & Reilly, K. H. (2020). Race/Ethnicity Among Children With COVID-19-Associated Multisystem Inflammatory Syndrome. JAMA Netw Open
Clinical Characteristics of MIS-C
While fever is universal, other clinical features of MIS-C vary, with some children presenting in fulminant shock while others may fulfill incomplete or complete KD criteria. Per CDC criteria, hospitalization is required, and most children in early series were admitted to the intensive care unit for vasopressor or respiratory support. However, other studies have reported children with only fever and systemic inflammation without end organ involvement, highlighting the broad spectrum of disease [8,10].
Cardiac
Myocarditis is one of the most alarming manifestations of MIS-C. While similar to KD shock syndrome, ventricular dysfunction occurs at higher rates in MIS-C, ranging from 34% to 62% in large series [6*, 8,9*,10, 28]. Patients frequently present with elevated markers of cardiac injury including troponin T, B-type natriuretic peptide (BNP), and N-terminal-proBNP [3*,6*,8,9*,10]. Many require vasopressor support, including some children with normal ventricular function, suggesting vasodilatory shock. Reassuringly, most patients recover ventricular function during hospitalization [29,30], though some progress to profound myocardial dysfunction requiring extra corporal membrane oxygenation (ECMO) [7], and several children have died.
Coronary artery aneurysms (CAA) or dilation are reported in variable proportions of patients within the acute period; large cohorts report CAAs (z score >2.5) in around 13% [4*,5,6*,7–8,9*, 23*]. Unlike myocarditis, CAAs do not appear correlated with degree of systemic inflammation [6*]. Most aneurysms in series reporting dimensions are small, though large or giant aneurysms are also reported [3*,6*,8,28]. Some patients developed CAAs following the acute phase of illness, indicating a need for continued echocardiogram monitoring during convalescence [29,31]. While long term data on CAAs in MIS-C are limited, one study reported resolution in over 90% of patients by 30 days [23*].
EKG abnormalities and cardiac arrhythmias, particularly first-degree heart block, are reported in over half of MIS-C patients [30]. Rarely, progression to higher grade heart block is observed; one child had refractory arrythmias necessitating ECMO [3*,32]. Illness severity appears to be associated with atrioventricular (AV) block; in one series, all patients with AV block were admitted to the ICU and had decreased ventricular function [30]. Monitoring EKGs in hospitalized patients every 48 hours for development of arrhythmias is recommended [31]. Long-term cardiac sequalae of myocarditis remains an area of active concern and investigation, and patients require ongoing cardiology follow-up [29].
Gastrointestinal
Gastrointestinal symptoms are reported in the majority of patients with MIS-C and may be the most common manifestation [9*]. Most children have abdominal pain; diarrhea and vomiting are also common [6*,8,28]. Abdominal pain is often severe and may be mistaken for acute appendicitis or testicular torsion [33–36]. In one report, a child with fever and right lower quadrant abdominal pain who underwent appendectomy subsequently developed shock with positive SARS-CoV-2 serologies. Pathology was atypical for acute bacterial appendicitis, with necrotizing lymphadenitis and vasculitis, suggesting MIS-C as the etiology [36]. Abdominal imaging may reveal ascites, adenopathy, and inflammation of the gallbladder or bowel; rarely, bowel wall thickening is profound enough to cause obstruction [6*, 33–34].
Mucocutaneous
KD-like mucocutaneous symptoms are described in multiple large cohorts, most commonly rash (52–63%), conjunctivitis (39–56%), oral mucosa changes (22–42%), and less often hand or foot swelling (9–37%), though some children have no KD features [6*, 8–9*, 23]. Large cervical lymph nodes appear less common (6–10%), though few series confirm node size [6*, 9*]. KD-like presentations appear more common in younger children, and myocarditis is more prevalent in older children and teenagers [6*]. Importantly, children with MIS-C who lack the mucocutaneous stigmata of KD have developed CAAs [8].
Hematologic
Markedly elevated D-dimer levels are common in MIS-C and tend to be higher than in pediatric COVID-19 [37]. Due to the risk of thrombosis in adults with acute COVID-19, there is concern that coagulation abnormalities may confer increased clot risk in MIS-C. While one large series reported deep vein thrombosis or pulmonary embolism in 7% of adolescent patients with MIS-C [9*], other studies report lower rates, including one meta-analysis demonstrating deep vein thrombosis or pulmonary embolus in only 3.5% of patients [38]. It remains unclear if there is an increased risk of thrombosis in MIS-C compared to other pediatric conditions that require intensive care, immobilization, and central venous access. One study showed schistocytes on peripheral blood smear in all MIS-C patients and elevated soluble sC5b-9, suggesting complement activation leading to microangiopathy [39]. This study also showed evidence of endothelial dysfunction in mild acute COVID-19, indicating that this may be a feature of SARS-CoV-2 infection, and not specific to MIS-C.
Neurologic
Headache is common, especially in adolescents. Altered mental status, encephalopathy, weakness, and areflexia are less frequently reported. In a series of 1695 children combining MIS-C and acute COVID, 12% were found to have life-threatening neurologic manifestations including encephalopathy, stroke, and acute cerebral edema; 0.6% died from these complications [40]. Meningismus is also reported, though few children underwent lumbar puncture; in these patients, aseptic meningitis was confirmed with sterile CSF pleocytosis, absent oligoclonal bands and negative SARS-CoV-2 PCR [41–42]. Four children with new neurologic symptoms had MRI signal abnormalities in the corpus collosum; all recovered [42]. Most strokes in MIS-C occurred in patients with other risk factors such as ECMO, bacterial co-infections (Lemierre, mastoiditis), or predisposing conditions (sickle cell). However, strokes are also reported in a small number of previously healthy children [40, 43–44].
Other Organ Systems
The largest MIS-C series to date reports lower respiratory tract involvement in over 40% of patients [23*]. Fifty-two percent of patients with MIS-C were PCR positive, higher than many studies, which may suggest that patients with acute COVID-19 were included in the MIS-C group. Series with lower PCR positivity have reported a relative lack of pulmonary involvement [3*,4–5,6*,8,9*,23*]. The cause of respiratory symptoms in MIS-C may be multifactorial and due to lingering effects of acute COVID-19 pneumonia, direct lung involvement from MIS-C, or from therapies such as fluid resuscitation. Less common symptoms of MIS-C include acute kidney injury, pancreatitis, and arthritis and arthralgia [9*, 45–46].
Differential diagnosis
Clinical features of MIS-C are observed in many other childhood febrile illnesses, making the diagnosis of MIS-C challenging, and it is essential to rule out other infectious and oncologic causes. Empiric treatment for MIS-C while other studies are pending is often necessary in critically ill children. However, clinicians must be vigilant in considering other causes of fever, even in the setting of positive SARS-CoV-2 serologies or PCR, as children initially thought to have features consistent with MIS-C were later found to bacterial or viral infections, notably toxic shock syndrome and bacterial enteritis [47–48].
Lab Findings
Systemic inflammation is required by all case definitions of MIS-C, and found in essentially all reported cases in the literature. Laboratory features include elevated CRP, procalcitonin, ferritin, D-dimer, lactate dehydrogenase, fibrinogen and liver function tests [6*,8,9*]. Neutrophilia with an accompanying lymphopenia and thrombocytopenia is common [3*,4*5,6*,7–8,9*,10,23*]. The majority (80–100%) of patients are SARS-CoV-2 antibody positive [4*,5,6*,8,9*]. Fewer children (20–39%) have positive nasopharyngeal PCR testing, but cycle thresholds are higher in MIS-C than in acute COVID-19, indicating the detected virus may not be replicating [4*,6*, 8,9*, 49].
Treatment
Children with MIS-C require multidisciplinary care from providers with expertise in rheumatology, cardiology, hematology and infectious disease. Given the multi-organ involvement of MIS-C, other subspecialists may be needed, and severely ill children will require admission to intensive care units. In this section, the focus will be on immunomodulatory treatment and anticoagulant use in MIS-C.
As MIS-C emerged, initial treatments were based on the similarity of MIS-C to KD and toxic shock syndrome. Intravenous immunoglobulin (IVIG) is used to prevent CAAs in KD and for immunomodulation in toxic shock [50]. Similarly, glucocorticoids are used in KD shock and refractory KD [51]. Glucocorticoids and IVIG are used in myocarditis, though evidence for these is mixed. By summer 2020, the RECOVERY trial demonstrated benefit of dexamethasone in patients hospitalized acute COVID-19, increasing clinician comfort with this approach [52].
IVIG and glucocorticoids remain the most common treatments for MIS-C, with a recent multicenter report indicating that 77% of patients with MIS-C received IVIG and 69% were treated with systemic steroids [23*]. Similarly, a survey of physicians treating MIS-C reported that IVIG was the most common immunomodulator, though glucocorticoids were preferred for those with severe presentations or IVIG non-responders, and anakinra (58%), infliximab (28%) and tocilizumab (8%) were also used [53].
Consensus treatment guidelines from the American College of Rheumatology (ACR) and the PIMS-TS National Consensus Management Study Group (UK) are presented in Table 2 [30,54*,55*]. Both the ACR and UK guidelines recommend IVIG at a dose of 2 gm/kg as first-line treatment; however, ACR advises against the use of a 2nd dose of IVIG for refractory disease. Adjunctive first-line glucocorticoids are recommended by ACR in patients with organ-threating disease, while their use is limited to children under 12 months of age or those with CAAs by UK guidelines.
Table 2.
Treatment Guidelines
| American College of Rheumatology (USA)55 | PIMS-TS National Consensus Management Study Group (UK)54 | ||
|---|---|---|---|
| Date Published | June 2020, Revised November 2020 | September 2020 | |
|
|
|||
| Population Applied To | Children with MIS-C | Children with PIMS-TS and Kawasaki disease-like phenotype |
Children with PIMS-TS and non-specific phenotype* |
|
|
|||
| IVIG | 2 g/kg based on ideal body weight -First-line therapy in hospitalized MIS-C patients -Second dose of IVIG not recommended |
2 g/kg dosed on ideal body weight - First-line therapy in all PIMS-TS with KD-like phenotype and in all treated non-specific PIMS-TS patients - Second dose considered for children who have not responded to the first dose |
|
| Glucocorticoids | IV methylprednisolone (1–2 mg/kg/day) - Fist-line with IVIG if shock or organ threatening disease - Second-line in refractory disease in other children IV methylprednisolone (10–30 mg/kg/day) - For treatment intensification in refractory disease 2–3 week steroid taper to prevent rebound |
IV methylprednisolone (10–30 mg/kg/day) - First-line with IVIG if < 12 months or coronary artery abnormalities - As second-line in other children |
IV methylprednisolone (10–30 mg/kg/day) -Second-line therapy |
| Additional Immunomodulation | High-dose anakinra if refractory to IVIG and/or steroids | Infliximab if non-responsive to IVIG and steroids |
Third line if non-responsive to IVIG and steroids Consensus not reached; equipoise for tocilizumab, anakinra, and infliximab |
| Anticoagulation | -Low dose ASA in all MIS-C patients without significant bleeding risk until normalization of plt count and confirmed normal coronary arteries. -Anticoagulation if CAA with z-score≥10, documented thrombosis, or Or EF<35% |
- If >12 years, should wear compression stockings - Low-dose ASA for minimum 6 weeks in all patients - Local protocol for management of a thrombotic event - Consult with hematologist re: long-term antiplatelet and anticoagulation therapy if CAA |
|
| Local protocol for KD ASA dosing | |||
| Antimicrobial | Not addressed | If SARS-CoV-2 positive (RT-PCR or antigen), consider remdesivir IV antibiotics in all patients; should be focused or stopped on the basis of the clinical picture and culture results If criteria for toxic shock syndrome met, clindamycin in addition to broad-spectrum antibiotics |
|
Treatment for this group is recommended in patients who have a coronary abnormality, TSS, progressive disease, or fever > 5 days.
At the time of this review, no randomized trials exist to guide clinicians on the most effective MIS-C therapies. One study used propensity scoring to retrospectively compare initial treatment with IVIG alone versus IVIG plus methylprednisolone (1.6–2 mg/kg/day in most) and concluded that in addition to lower rates of treatment failure (defined as persistent or recurrent fever), those treated with glucocorticoids had reduced duration of ICU admission, need for hemodynamic support, and less ventricular dysfunction [56*]. Steroids as adjunctive therapy also showed benefit over IVIG alone in one observational study measuring time to cardiac recovery [57]. At one center, instituting a clinical pathway which led to faster IVIG and glucocorticoid administration was shown to reduce overall and ICU length of stay [58]. In total, these studies suggest a benefit for rapid initiation of treatment and adjunctive steroids as part of first-line treatment in MIS-C.
One of the most pressing clinical questions is the need for anticoagulation in children with MIS-C. As discussed above, although D-dimers are markedly elevated in MIS-C, the true risk for thrombosis in these patients remains uncertain. Based on experience with KD, there is broad agreement that low dose aspirin should be provided to MIS-C patients without significant bleeding risk [50]. Similarly, there is general consensus that anticoagulants should be used in patients with severely decreased ejection fraction, large or giant CAAs, or evidence of clot, as aligned with prior clinical practice [30,54*,55*]. Clinicians should consider prophylactic anticoagulation as would be indicated for degree of critical illness, immobility, and glucocorticoid use. Due to the lack of evidence for universal anticoagulation beyond these indications, treatment based on laboratory evidence of hypercoagulability should be individualized according to patient risk factors. This topic remains controversial, and some guidelines suggest more aggressive anticoagulation based on D-dimer levels, including prophylaxis for all children with D-dimer >5 times the upper limit of normal [59].
Controversies and Questions
Many controversies and questions remain in the diagnosis and treatment of MIS-C. Since its emergence, MIS-C and KD have been compared; however, the relationship between these two syndromes remains unclear. Possibilities include that MIS-C is a particularly severe variant of KD, a subset of MIS-C patients have KD, or the two entities should be considered as different etiologies entirely. Age appears to impact the presentation of MIS-C with higher rates of young children meeting KD clinical criteria, while adolescents more frequently present with myocarditis [6*,60]. Thus, future studies may need to stratify patients by age. While the relationship between these diseases has yet to be fully elucidated, clinicians must approach management decisions with a clear understanding of the differences between MIS-C and pre-pandemic KD. MIS-C patients are at much greater risk of rapid decompensation and developing shock. Further, CAAs have occurred in MIS-C patients who have not met KD clinical criteria. Relatedly, multisystem inflammatory syndrome in adults (MIS-A) is increasingly reported, yet likely remains underrecognized, as older patients have fewer characteristic mucocutaneous features [14*]. Clinicians must maintain a high degree of suspicion for this entity in teenagers and young adults who present with unexplained fever, particularly in the presence of confirmed or suspected COVID-19 exposure in the prior 1–2 months.
Conclusion
Despite advances in our understanding of MIS-C, this disease remains a diagnostic challenge due to the broad range of phenotypes and severity. Fever accompanied by shock, cardiac dysfunction, abdominal pain, or mucocutaneous signs in the presence of known or suspected COVID-19 exposure should trigger prompt evaluation. However, clinicians must be aware that patients may develop severe cardiac and other sequalae even with few or no KD symptoms. More precise diagnostic criteria and specific biomarkers are needed to aid diagnosis, especially as SARS-COV-2 antibody prevalence increases. Prompt treatment is essential, as patients may worsen acutely, though overall prognosis is reassuring. IVIG is first-line therapy, and steroids should be considered as initial adjunctive treatment, especially for patients with severe manifestations or other risk factors. Optimal anticoagulation remains controversial. Multidisciplinary involvement is essential to quality clinical care, and to optimize diagnostic and therapeutic approaches as new data emerge.
Key Points:
Reliably identifying MIS-C remains difficult given the wide spectrum of phenotypes found in affected patients and similarity between MIS-C and other childhood febrile conditions.
Validated diagnostic criteria that can be used in the clinical setting are lacking and need to be developed.
The relationship between pre-pandemic KD and MIS-C remains unclear and while there are similarities in clinical features, patient with MIS-C may develop CAAs and cardiac dysfunction with few or no mucocutaneous features of KD.
While there are no prospective studies comparing treatment approaches in MIS-C, there is evidence to suggest that rapid initiation of IVIG and glucocorticoids is beneficial.
Multisystem inflammatory syndrome associated with SARS-CoV-2 also occurs in adults (MIS-A) and is likely underrecognized.
Acknowledgements:
Dr. Roberts was supported by NIH grant 5T32AI007512–34 and Dr. Henderson was supported by NIAMS K08 AR073339, NIAMS P30 AR070253, an Investigator Award and a Career Development Bridge Funding Award from the Rheumatology Research Foundation, an All Arthritis Grant from the Arthritis National Research Foundation, and a Career Development Award from the Office of Faculty Development at Boston Children’s Hospital.
Footnotes
There was no additional assistance, and the authors have no conflicts of interest to declare.
References and Recommended Reading
- 1.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 Infection in Children. N Engl J Med 2020;382(17):1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PICS Statement. Increased number of reported cases of novel presenta- tion of multi-system inflammatory disease. Paediatric Intensive Care Society: Paediatric Intensive Care Society 2020. https://picsociety.uk/wp-content/uploads/2020/04/PICS-statement-re-novel-KD-C19-presentation-v2-27042020.pdf.Accessed February 16, 2021.
- 3. *Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395(10237):1607–8. First series from UK describing toxic-shock like presentation and coronary involvement following SARS-CoV-2 exposure.
- 4. *Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395(10239):1771–8. Early series from Italy describing Kawasaki-disease like presentations of shock associated with COVID-19.
- 5.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020;369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. *Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med 2020;383(4):347–58. Early series describing MIS-C in New York; describes presenting features by age group.
- 7.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020;142(5):429–36. [DOI] [PubMed] [Google Scholar]
- 8.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 2020;324(3):259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. *Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med 2020;383(4):334–46. First U.S. national, multicenter series describing epidemiology, clinical and laboratory features and treatment of MIS-C.
- 10.Lee PY, Day-Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest 2020;130(11):5942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19 Accessed February 16, 2021. https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims. [DOI] [PubMed]
- 12.Centers for Disease Control and Prevention. Emergency preparedness and response: health alert network. “Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19).” Published May 14, 2020. Accessed February 17, 2021.https://emergency.cdc.gov/han/2020/han00432.asp
- 13.World Health Organization. “Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19” Published May 15, 2020. Accessed February 16, 2021.https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 14. *Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, et al. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep 2020;69(40):1450–6. Reports on series of patients with Multisystem Inflammatory Syndrome in Adults (MIS-A).
- 15.Iio K, Uda K, Hataya H, Yasui F, Honda T, Sanada T, et al. Kawasaki disease or Kawasaki-like disease: Influence of SARS-CoV-2 infections in Japan. Acta Paediatr 2021;110(2):600–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mamishi S, Movahedi Z, Mohammadi M, Ziaee V, Khodabandeh M, Abdolsalehi MR, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect 2020;148:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain S, Sen S, Lakshmivenkateshiah S, Bobhate P, Venkatesh S, Udani S, et al. Multisystem Inflammatory Syndrome in Children With COVID-19 in Mumbai, India. Indian Pediatr 2020;57(11):1015–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe YJ, Choi EH, Choi JW, Eun BW, Eun LY, Kim YJ, et al. Surveillance of COVID-19-Associated Multisystem Inflammatory Syndrome in Children, South Korea. Emerg Infect Dis 2021;27(4):1196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb K, Abraham DR, Faleye A, McCulloch M, Rabie H, Scott C, et al. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health 2020;4(10):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antunez-Montes OY, Escamilla MI, Figueroa-Uribe AF, Arteaga-Menchaca E, Lavariega-Sarachaga M, Salcedo-Lozada P, et al. COVID-19 and Multisystem Inflammatory Syndrome in Latin American Children: A Multinational Study. Pediatr Infect Dis J 2021;40(1):e1–e6. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States Published February 8, 2021. Accessed April 3, 2021.https://www.cdc.gov/mis-c/cases/index.html
- 22.Lee EH, Kepler KL, Geevarughese A, Paneth-Pollak R, Dorsinville MS, Ngai S, et al. Race/Ethnicity Among Children With COVID-19-Associated Multisystem Inflammatory Syndrome. JAMA Netw Open 2020;3(11):e2030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. * Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021;325(11):1074–87. Largest series to date of children with MIS-C and acute COVID-19.
- 24.Azar KMJ, Shen Z, Romanelli RJ, Lockhart SH, Smits K, Robinson S, et al. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In California. Health Aff (Millwood) 2020;39(7):1253–62. [DOI] [PubMed] [Google Scholar]
- 25.Gaffney AW, Himmelstein D, Bor D, McCormick D, Woolhandler S. Home Sick with Coronavirus Symptoms: a National Study, April-May 2020. J Gen Intern Med 2020;35(11):3409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman-Cribbin W, Tuminello S, Flores RM, Taioli E. Disparities in COVID-19 Testing and Positivity in New York City. Am J Prev Med 2020;59(3):326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goyal MK, Simpson JN, Boyle MD, Badolato GM, Delaney M, McCarter R, et al. Racial and/or Ethnic and Socioeconomic Disparities of SARS-CoV-2 Infection Among Children. Pediatrics 2020;146(4). [DOI] [PubMed]
- 28.Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. Acute Cardiovascular Manifestations in 286 Children With Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation 2021;143(1):21–32. [DOI] [PubMed] [Google Scholar]
- 29.Alsaied T, Tremoulet AH, Burns JC, Saidi A, Dionne A, Lang SM, et al. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation 2021;143(1):78–88. [DOI] [PubMed] [Google Scholar]
- 30.Dionne A, Mah DY, Son MBF, Lee PY, Henderson L, Baker AL, et al. Atrioventricular Block in Children With Multisystem Inflammatory Syndrome. Pediatrics 2020;146(5). [DOI] [PubMed] [Google Scholar]
- 31.Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 1. Arthritis Rheumatol 2020;72(11):1791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Assaad I, Hood-Pishchany MI, Kheir J, Mistry K, Dixit A, Halyabar O, et al. Complete Heart Block, Severe Ventricular Dysfunction, and Myocardial Inflammation in a Child With COVID-19 Infection. JACC Case Rep 2020;2(9):1351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahn B, Eze OP, Edelman MC, Chougar CE, Thomas RM, Schleien CL, et al. Features of Intestinal Disease Associated With COVID-Related Multisystem Inflammatory Syndrome in Children. J Pediatr Gastroenterol Nutr 2021;72(3):384–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal Symptoms as a Major Presentation Component of a Novel Multisystem Inflammatory Syndrome in Children That Is Related to Coronavirus Disease 2019: A Single Center Experience of 44 Cases. Gastroenterology 2020;159(4):1571–4.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer JS, Robinson G, Moonah S, Levin D, McGahren E, Herring K, et al. Acute appendicitis in four children with SARS-CoV-2 infection. J Pediatr Surg Case Rep 2021;64:101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson RJ, Chavarria HD, Hacking SM. A Case of Multisystem Inflammatory Syndrome in Children Mimicking Acute Appendicitis in a COVID-19 Pandemic Area. Cureus 2020;12(9):e10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Borrello G, Giraudo I, Bondone C, Denina M, Garazzino S, Linari C, et al. SARS-COV-2-associated coagulopathy and thromboembolism prophylaxis in children: A single-center observational study. J Thromb Haemost 2021;19(2):522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aronoff SC, Hall A, Del Vecchio MT. The Natural History of Severe Acute Respiratory Syndrome Coronavirus 2-Related Multisystem Inflammatory Syndrome in Children: A Systematic Review. J Pediatric Infect Dis Soc 2020;9(6):746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diorio C, McNerney KO, Lambert M, Paessler M, Anderson EM, Henrickson SE, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv 2020;4(23):6051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome. JAMA Neurol 2021. [DOI] [PMC free article] [PubMed]
- 41.Chen TH. Neurological involvement associated with COVID-19 infection in children. J Neurol Sci 2020;418:117096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, et al. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol 2020;77(11):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minen F, Hands C, Mustafa MR, Pienaar A, Lillie J. Thrombophilia in Pediatric Patients with Multisystem Inflammatory Syndrome in Children Secondary to Coronavirus Disease 2019 Supported on Extracorporeal Membrane Oxygenation. Asaio j 2021;67(1):7–11. [DOI] [PubMed] [Google Scholar]
- 44.Beslow LA, Linds AB, Fox CK, Kossorotoff M, Zuñiga Zambrano YC, Hernández-Chávez M, et al. Pediatric Ischemic Stroke: An Infrequent Complication of SARS-CoV-2. Ann Neurol 2020. [DOI] [PubMed]
- 45.Deep A, Upadhyay G, du Pré P, Lillie J, Pan D, Mudalige N, et al. Acute Kidney Injury in Pediatric Inflammatory Multisystem Syndrome Temporally Associated With Severe Acute Respiratory Syndrome Coronavirus-2 Pandemic: Experience From PICUs Across United Kingdom. Crit Care Med 2020;48(12):1809–18. [DOI] [PubMed] [Google Scholar]
- 46.Abbas M, Törnhage CJ. Family Transmission of COVID-19 Including a Child with MIS-C and Acute Pancreatitis. Int Med Case Rep J 2021;14:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell JI, Roberts JE, Dubois M, Naureckas Li C, Sandora TJ, Lamb GS. Non-SARS-CoV-2 Infections Among Patients Evaluated for MIS-C Associated With COVID-19. The Pediatric Infectious Disease Journal 2021;40(2):e90–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dworsky ZD, Roberts JE, Son MBF, Tremoulet AH, Newburger JW, Burns JC. MISTAKEN MIS-C: A CASE SERIES OF BACTERIAL ENTERITIS MIMICKING MIS-C. The Pediatric Infectious Disease Journal 9000;Online First. [DOI] [PMC free article] [PubMed]
- 49.Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest 2020;130(11):5967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017;135(17):e927–e99. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet 2012;379(9826):1613–20. [DOI] [PubMed] [Google Scholar]
- 52.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elias MD, McCrindle BW, Larios G, Choueiter NF, Dahdah N, Harahsheh AS, et al. Management of Multisystem Inflammatory Syndrome in Children Associated With COVID-19: A Survey From the International Kawasaki Disease Registry. CJC Open 2020;2(6):632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. *Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health 2021;5(2):133–41. United Kingdom (PIMS-TS National Consensus Management Study Group) guidelines for the diagnosis and treatment of MIS-C.
- 55. *Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol 2020. Updated American College of Rheumatology Guidelines for the diagnosis and treatment of MIS-C.
- 56. *Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, et al. Association of Intravenous Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone With Course of Fever in Multisystem Inflammatory Syndrome in Children. Jama 2021;325(9):855–64. Retrospective study using propensity matching to compare IVIG plus glucocorticoids versus IVIG alone; showed less treatment failure, improved cardiovascular outcomes and shorter ICU length of stay with glucocorticoids plus IVIG.
- 57.Belhadjer Z, Auriau J, Méot M, Oualha M, Renolleau S, Houyel L, et al. Addition of Corticosteroids to Immunoglobulins Is Associated With Recovery of Cardiac Function in Multi-Inflammatory Syndrome in Children. Circulation 2020;142(23):2282–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jonat B, Gorelik M, Boneparth A, Geneslaw AS, Zachariah P, Shah A, et al. Multisystem Inflammatory Syndrome in Children Associated With Coronavirus Disease 2019 in a Children’s Hospital in New York City: Patient Characteristics and an Institutional Protocol for Evaluation, Management, and Follow-Up. Pediatr Crit Care Med 2021;22(3):e178–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldenberg NA, Sochet A, Albisetti M, Biss T, Bonduel M, Jaffray J, et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19-related illness. J Thromb Haemost 2020;18(11):3099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowley AH. Diagnosing SARS-CoV-2 Related Multisystem Inflammatory Syndrome in Children (MIS-C): Focus on the Gastrointestinal Tract and the Myocardium. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed]


