Abstract
Sleep difficulties are pervasive in autism spectrum disorder (ASD), yet how sleep problems relate to underlying biological mechanisms such as genetic etiology is unclear, despite recent reports of profound sleep problems in children with ASD-associated de novo likely gene disrupting (dnLGD) mutations, CHD8, DYRK1A, and ADNP. We aimed to inform etiological contributions to ASD and sleep by characterizing sleep problems in individuals with dnLGD mutations. Participants (N = 2886) were families who completed dichotomous questions about sleep problems within a medical history interview for their child with ASD (age 3–28 years). Confirmatory factor analyses compared between those with ASD and a dnLGD mutation and those with idiopathic ASD (i.e., no known genetic event, NON) highlighted four domains (sleep onset, breathing issues, nighttime awakenings, and daytime tiredness) with sleep onset as a strong factor for both groups. Overall, participant predictors indicated that internalizing behavioral problems and lower cognitive scores were related to increased sleep problems. Internalizing problems were also related to increase nighttime awakenings in the dnLGD group. As an exploratory aim, patterns of sleep issues are described for genetic subgroups with unique patterns including more overall sleep issues in ADNP (n = 19), problems falling asleep in CHD8 (n = 22), and increased daytime naps in DYRK1A (n = 23). Implications for considering genetically defined subgroups when approaching sleep problems in children with ASD are discussed.
Keywords: autism spectrum disorders (ASD), sleep, factor analysis, genetic subgroups, likely gene disrupting mutations
Autism spectrum disorder (ASD) is characterized by deficits in social communication and the presence of repetitive, restricted patterns of behavior and interests (American Psychiatric Association, 2013). Yet, ASD is a genetically and phenotypically heterogeneous disorder, which often presents with additional co-occurring medical and behavioral problems. Chronic difficulties with sleep are prevalent amongst autistic children and adolescents and are seen at greater frequency and severity than in typically developing children and other developmental disabilities (Couturier et al., 2005; Gregory & Sadeh, 2012; Park et al., 2012). A majority of parents of autistic individuals report sleep issues, including 53% of parents of young autistic children age 2–5 years (Krakowiak, Goodlin-Jones, Hertz-Picciotto, Croen, & Hansen, 2008) and 66.1% of parents with autistic children age 4–10 years (Souders et al., 2009) report at least one sleep problem.
There is a growing consensus of life-long sleep problems in ASD related to difficulties with bedtime and sleep onset, such as difficulty falling asleep, are frequently reported sleep complaints by parents of autistic children (Liu, Hubbard, Fabes, & Adam, 2006), and confirmed by actigraphy and polysomnography (Malow et al., 2006; Souders et al., 2009; Wiggs & Stores, 2004). Additionally, parents report sleep onset issues, including bedtime resistance and the need for parents to lay down with their child in order for them to fall asleep (Liu et al., 2006), as well as recurrent nighttime awakenings (Krakowiak et al., 2008; Liu et al., 2006), including sleepwalking and night terrors (Couturier et al., 2005; Goldman, Richdale, Clemons, & Malow, 2012). These problems may contribute to increased difficulty waking in the morning, excessive daytime tiredness, and more frequent napping (Liu et al., 2006). It is unclear whether autistic daytime tiredness relates to disrupted biological mechanisms responsible for wakefulness or whether it remains a by-product of difficulties with sleep onset, night awakenings, and less night sleep overall (Goodlin-Jones, Tang, Liu, & Anders, 2009; Humphreys et al., 2014). Sleep-disordered breathing may be elevated in ASD (Cortese, Wang, Angriman, Masi, & Bruni, 2020), but it remains unclear whether in autistic individuals are at increased risk for sleep apnea and breathing difficulties at night (Limoges, Mottron, Bolduc, Berthiaume, & Godbout, 2005; Malow et al., 2006).
Despite a recent increase in research exploring the etiology of sleep disruptions in autistic children and their impact on daytime functioning (Adams, Matson, Cervantes, & Goldin, 2014; Taylor, Schreck, & Mulick, 2012), much remains unknown about whether sleep problems are distinct in potential ASD subgroups with a known genetic etiology. With increasing awareness of the genetic contributions to ASD and identification of genetically defined subgroups (Stessman, Bernier, & Eichler, 2014), deeper understanding of genetic mechanisms involved in ASD-related sleep disruptions is also needed. Ongoing advances in genetic sequencing have identified severe de novo likely gene-disrupting (dnLGD) mutations that are associated with ASD symptoms (O’Roak et al., 2012; Iossifov et al., 2014). These genes are believed to have important, seemingly varied impacts on neurological processes indicated in ASD (Bernier et al., 2014; Helsmoortel et al., 2014; Traylor et al., 2012; van Bon et al., 2015), explaining a portion of the phenotypic variability seen amongst affected individuals. This genetic variability may also help explain the differences we see in sleep presentation amongst autistic individuals.
Large scale genomic studies have identified genes tied directly to circadian rhythm pathways (review provided by Veatch, Keenan, Gehrman, Malow, & Pack, 2017) and enrichment of disruptions to specific melatonin pathway genes have been identified amongst autistic individuals with sleep onset difficulties (Veatch, Maxwell-Horn, & Malow, 2015). Despite these associations, recent work did not find a connection between dnLGDs known to impact circadian rhythms (e.g., ASMT, CLOCK, MTNR1B, TIMELESS) and parent-reported sleep problems (Johansson, Dorman, Chasens, Feeley, & Devlin, 2019). Considering the additional biological mechanisms implicated by other non-circadian rhythm genes (see Iossifov et al., 2014), exploring the differences in sleep problem presentation by dnLGD mutation subgroups of ASD may shed light on the phenotypic variability we see in co-occurring sleep conditions and illuminate the specific neurobiological mechanisms that are disrupting sleep in autistic individuals.
Recently, de novo LGD mutations CHD8, ADNP, and DYRK1A have been identified as candidate genes for ASD and appear to have unique phenotypic profiles (Bernier et al., 2014; Iossifov et al., 2014; O’Roak et al., 2012; van Bon et al., 2015; Vandeweyer et al., 2014). Studies of each gene event have described recurrent reports of sleep issues in individuals with these mutations, yet the correspondence between each genetic subgroup and ASD, broadly, is unclear. Chromodomain helicase binding protein (CHD8) is a gene known to be involved in chromatin remodeling and understood to be important for neuronal cell proliferation and regulation (Bernier et al., 2014; O’Roak et al., 2012). Bernier and colleagues (2014) found that individuals with ASD and mutations to CHD8 were reported to have significant difficulty falling asleep and/or staying up for days at a time. Recent cross-species evidence indicates that the sleep maintenance problems in CHD8 are recapitulated in Drosophila mutants affecting kismet (the sole ortholog to human genes CHD8 and CHD7) during early and adult stages (Coll-Tané et al., in press). DYRK1A, or Dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase 1 A, has been linked to cellular signaling and brain development (Iossifov et al., 2014; O’Roak et al., 2012), and frequent nighttime awakenings have been reported for those with DYRK1A mutations (van Bon et al., 2015). Activity-dependent neuroprotective protein (ADNP) gene is involved in cellular differentiation and maturation (Helsmoortel et al., 2014; Vandeweyer et al., 2014). General sleep issues have been recurrently endorsed for children with ADNP mutations, although specific types of sleep problems have yet to be reported (Vandeweyer et al., 2014).
In order to examine the potential genetic role on sleep problems in ASD, it is critical to also consider additional known factors. For instance, there exists variability in the severity and kind of sleep problem/s exhibited by different autistic children, as evidenced by a recent meta-analysis of polysomnography studies (Chen et al., 2020). Given the multifactorial (Posar & Visconti, 2020) and developmental nature (McMakin & Alfano, 2015; Williamson, Mindell, Hiscock, & Quach, 2020) of sleep problems, factors of development, cognition, and behavior may differentially impact sleep presentation in ASD (Malow et al., 2006; Veatch, Maxwell-Horn, et al., 2015). Goldman et al. (2012) found that while the presence of sleep issues did not change as autistic children grew older, the types of sleep problems did. Younger autistic children showed high instances of bedtime resistance, night awakenings, and nightmares, whereas older children and adolescents had greater difficulty with sleep onset, sleep duration, and daytime tiredness. Sleep issues have been repeatedly correlated with reports of increased internalizing and externalizing behaviors in autistic children, such as inattention, hyperactivity, and anxiety (Goldman et al., 2009; Mazurek & Petroski, 2015; Sikora, Johnson, Clemons, & Katz, 2012; Wiggs & Stores, 2004).
The relationship between cognitive ability, or IQ, and sleep issues in those with ASD remains inconclusive. Individuals with intellectual disability are reported to have greater frequency of sleep issues than typically developing individuals (Elrod & Hood, 2015; Piazza, Fisher, & Kahng, 2008; Taylor et al., 2012); however, sleep problems are observed across a range of cognitive abilities regardless of IQ score (Richdale, 1999), potentially indicating the need for a deeper investigation of specific sleep issues to elucidate which specific problems are implicated across the autism spectrum. Given high rates of co-occurring diagnoses alongside ASD, including intellectual disability (Howlin, Goode, Hutton, & Rutter, 2004; Bernier & Gerdts, 2010) and behavioral disorders (e.g., anxiety, inattention/hyperactivity associated with externalizing and internalizing problems; Bauminger, Solomon, & Rogers, 2010), it is critical to consider how these factors interact with shared biological mechanisms (e.g., genetics, neurological circuitry) and contribute to sleep problems.
Sleep processes are facilitated by a series of neurobiological mechanisms, many of which have been found to be impaired in autistic individuals who have poor sleep (Richdale & Schreck, 2009). Evidence of abnormal melatonin synthesis is evident in ASD (see Wu et al., 2020 for review), thought to disrupt melatonin-dependent circadian rhythms and in turn modulate brain metabolism required to process information. Studies have indicated that autistic individuals spend less time in rapid eye movement sleep compared to controls (Buckley et al., 2010), but findings remain inconsistent (Limoges et al., 2005). At a chemical level, fundamental neurotransmitters, such as dopamine, serotonin, and GABA, are disrupted in ASD and have been found to be important in sleep regulation (Buhr et al., 2002; Crocker & Sehgal, 2010; Holst et al., 2014; Veenstra-VanderWeele et al., 2012). However, the extent to which these neurological mechanisms are impaired and sleep is disrupted varies between autistic individuals.
Thus, within this study, we seek to clarify sleep difficulties in ASD associated with the presence of a known genetic etiology, specifically dnLGDs. We present novel, preliminary work utilizing parent-report to understand better understand the genetic contribution to sleep problems. First, we evaluated sleep problems in a large sample of autistic children with and without genetic etiologies using confirmatory factor analysis (CFA). Considering implications of sleep difficulties as part of genetic-first phenotypes, we compared parent reports of sleep related disturbances for autistic youth with a de novo LGD mutation (dnLGD) and autistic youth without a dnLGD (“idiopathic” or no known genetic event; NON). We predicted that certain sleep issues (e.g., bedtime problems) might be more prevalent and symptomatic in ASD, regardless of genetic etiology. We also anticipated that individual participant differences, such as developmental stage, cognition, and behavioral symptoms (specifically parent-reported internalizing and externalizing), might also predict increased sleep issues and vary with the presence of an dnLGD mutation. Second, in efforts to better understand the variable impact of specific dnLGD mutations, we characterized the sleep profiles for three genetically defined subtypes: CHD8, ADNP, and DYRK1A. Due to the rarity of this population, this aim serves as an exploratory investigation to understand what sleep problems are elevated in these three genetic ASD subgroups.
Methods and materials
Participants
Participants (Table 1) were include from two sources. First, Simons Simplex Collection (SSC, Fischbach & Lord, 2010), a genetic consortium study of families with one child diagnosed with ASD (i.e., ASD ascertainment). dnLGD mutation data from participants in the SSC cohort were obtained via published exome sequencing studies (Iossifov et al., 2014), which defined dnLGD events as de novo nonsense, frameshift, or splice-site gene mutations that disrupt intended protein functions. Phenotypic data was downloaded from sfari.org (version 15 of the SSC). Of this SSC cohort, 2771 families completed the portion of the medical history interview related to sleep symptoms without missing data and are included in this sample. Second, participants from the University of Washington’s TIGER study were enrolled based upon the existence of a known dnLGD associated with neurodevelopmental disorders (i.e., genetic ascertainment). Thus, participants were divided into two groups (Table 1): Autistic participants without a known genetic etiology (NON group; n = 2509; all ascertained for ASD via SSC) and participants with a known ASD-associated dnLGD mutation (dnLGD group; n = 378; 264 ascertained for ASD via SSC; 114 genetic ascertainment). Characterization of a subset of dnLGD group with ADNP, CHD8, and DYRK1A mutations is available in Table 2. ASD diagnoses were based upon the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994), Autism Diagnostic Observation Schedule, (ADOS; Lord et al., 2000), and clinician evaluation. Ethical review board approval was obtained and written informed consent was given by each participant’s legal guardian/s.
Table 1.
Full sample characteristics to address primary aim.
| NON (N = 2509) | dnLGD (N = 378) | Group differences | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Male | 2191 | 87.3% | 263 | 69.6% | χ2(1) = 79.8, p<.001 |
| Female | 318 | 12.7% | 115 | 30.4% | |
| ASD Diagnosisa | 2509 | 100.0% | 335 | 88.6% | χ2(1) = 289.7, p<.001 |
| ID Diagnosisa | 803 | 32.0% | 190 | 50.3% | χ2(1) = 47.7, p<.001 |
| Mean | SD | Mean | SD | Group differences | |
| Age (years) | 9 | 3.57 | 9.39 | 4.23 | F(1,2670) = 3.57, p = .063 |
| Age range (years) | 3–17 | 4–28 | |||
| VIQ | 78.55 | 31.1 | 62.3 | 35.9 | F(1, 2664) = 81.77, p<.001 |
| NVIQ | 85.37 | 25.92 | 65.3 | 33.9 | F(1, 2664) = 171.1, p<.001 |
| Internalization | 60.3 | 9.7 | 60.1 | 8.8 | F(1, 2634) = .26, p = .61 |
| Externalization | 56.5 | 10.6 | 56.5 | 10.9 | F(1,2634) = 0, p = .99 |
Diagnoses unclear for 1 dnLGD participant due to remote only participation.
Table 2.
Genetic subgroup characteristics to address exploratory aim.
| ADNP (N = 19) | CHD8 (N = 23) | DYRK1A (N = 23) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Group differences | Description | |
| Male | 11 | 57.89% | 14 | 63.64% | 15 | 65.22% | χ2(1) = .26, p = .88 | |
| Female | 8 | 42.11% | 8 | 36.36% | 8 | 34.78% | ||
| ASD Diagnosisa | 10 | 52.63% | 20 | 90.91% | 20 | 86.96% | χ2(1) = 11.0, p = .026 | ADNP<(DYRK1A, CHD8) |
| ID Diagnosisa | 19 | 100.00% | 11 | 50.00% | 19 | 82.61% | χ2(1) = 14.9, p = .006 | CHD8<(ADNP, DYRK1A) |
| Mean | SD | Mean | SD | Mean | SD | Group differences | Description | |
| Age (years) | 8.10 | 3.81 | 10.28 | 5.21 | 10.99 | 6.35 | F(1,61) = 1.63 p = .20 | |
| Age range (years) | 3–17 | 4–20 | 4–24 | |||||
| VIQ | 28.44 | 21.44 | 51.40 | 35.91 | 43.22 | 32.46 | F(1, 56) = 2.44, p = .51 | |
| NVIQ | 27.13 | 19.10 | 57.55 | 32.89 | 43.78 | 27.04 | F(1, 56) = 5.48, p = .007 | ADNP<CHD8 |
| Internalization | 57.07 | 9.51 | 62.21 | 8.81 | 57.00 | 11.08 | F(1, 44) = 1.61, p = .21 | |
| Externalization | 60.60 | 8.72 | 50.79 | 12.22 | 52.08 | 9.80 | F(1,44) = 4.01, p = .025 | ADNP<CHD8 |
Diagnoses unclear for 1 ADNP participant due to remote only participation.
Measures
Sleep problems were measured by parent report on a clinician-administered 10-item medical history questionnaire yielding yes (“1”) or no (“0”) responses (Simons Simplex Collection Medical History Questionnaire; See Appendix for full questions). All Sleep variables were treated as binary variables. Responses of “not sure” were coded as missing data. A preliminary hierarchical item cluster analysis (Revelle, 1978; 2011) specified the most similar pair of items fit into four clusters which we characterized as the following domains (see Table 3): Sleep onset (difficulty going to bed, difficulty falling asleep, parents laying down), Breathing issues (difficulty breathing at night, sleep apnea diagnosis), Nighttime awakenings (frequent night awakenings, sleepwalking), and Daytime tiredness (difficulty waking tin the morning, daytime tiredness, long or frequent napping). We acknowledge that these domains are similar yet slightly different from the SSC Sleep Interview developed from principle components analysis (Johansson, Rohay, & Chasens, 2018).
Table 3.
Sleep domains and sleep interview item frequencies.
| Sleep domain | Sleep Interview Item | All participants | NON | dnLGD | Group differences χ2 p-value | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Sleep onset | Total (Endorsing at least one) | 1716 | 40.6% | 1488 | 60.1% | 228 | 60.6% | 0.879 |
| Difficulty going to bed (SBP) | 919 | 32.3% | 796 | 32.0% | 123 | 32.6% | 0.868 | |
| Difficulty falling asleep (SBS) | 1206 | 42.7% | 1043 | 42.1% | 163 | 43.4% | 0.681 | |
| Parent lays down (SBD) | 1343 | 46.8% | 1168 | 47.2% | 175 | 46.4% | 0.833 | |
| Breathing issues | Total (Endorsing at least one) | 190 | 10.6% | 156 | 6.4% | 34 | 9.1% | 0.059 |
| Difficulty breathing at night (BPM) | 166 | 6.9% | 135 | 5.5% | 31 | 8.3% | 0.003 | |
| Sleep apnea dx (BAP) | 73 | 3.3% | 57 | 2.3% | 16 | 4.3% | 0.039 | |
| Nighttime awakenings | Total (Endorsing at least one) | 1173 | 21.7% | 996 | 40.3% | 177 | 46.9% | 0.017 |
| Frequent night awakenings (NAF) | 995 | 37.6% | 839 | 33.9% | 156 | 41.4% | 0.005 | |
| Sleepwalking (NAS) | 390 | 12.5% | 349 | 14.2% | 41 | 10.9% | <.001 | |
| Daytime tiredness | Total (Endorsing at least one) | 837 | 14.8% | 705 | 28.5% | 132 | 35.0% | 0.011 |
| Difficulty waking in morning (DDW) | 269 | 12.3% | 208 | 8.4% | 61 | 16.2% | 0.385 | |
| Daytime tiredness (DDS) | 269 | 12.3% | 208 | 8.4% | 61 | 16.2% | <.001 | |
| Long/Frequent napping (DDN) | 500 | 19.9% | 413 | 16.7% | 87 | 23.1% | <.001 | |
IQ scores were derived from the Differential Ability Scales, 2nd Edition (DAS-II; Elliott, Murray, & Pearson, 1990), the Wechsler Scale of Intelligence, 4th Edition (WISC-IV; Wechsler, 2003), or the Mullen Scales of Early Learning (Mullen, 1995). Consistent with prior studies using this dataset (e.g., Grzadzinski, Lord, Sanders, Werling, & Bal, 2018), we opted to extract verbal and nonverbal IQ (VIQ, NVIQ, respectively) separately, as opposed to full-scale IQ, to more fully represent possible disparities in verbal and nonverbal abilities. The DAS-II Verbal Composite score was used for VIQ and the Special Nonverbal Composite was used for NVIQ. For participants who were administered the WISC-IV, the Verbal Comprehension Index was used as a measure of VIQ and the Perceptual Reasoning Index was used as a measure of NVIQ. In the Mullen Scales of Early Learning, VIQ and NVIQ were derived using a process standardized as part of the Simons Simplex Collection. VIQ was calculated by summing verbal subdomains (Receptive Language and Expressive Language), multiplying by 2 and converting to a standard score using Early Learning Composite norms. NVIQ was calculated the same using nonverbal subdomains (Visual Reception and Fine Motor). Internalizing and externalizing behavior T-scores were calculated from parent report on the Child Behavior Checklist (CBCL; Achenbach, 1991).
Confirmatory factor analysis (CFA)
Our primary objective was to examine latent variables associated with sleep issue for children with idiopathic ASD (NON) and children with ASD and an dnLGD mutation (dnLGD). A series of CFAs were conducted with the open-source lavaan() package for latent variable modeling (Rosseel, 2012; Wirth & Edwards, 2007) in R (version 2.15.1) using maximum likelihood. Factor loadings represent standardized regression coefficients between each sleep interview item and latent variables. According to proposed guidelines (Browne & Cudeck, 1993; Kline, 2011; Hu & Bentler, 1999), goodness-of-fit was assessed by examining: root mean squared error of approximation (RMSEA; good fit = below 0.05; acceptable fit = 0.05–0.08; unacceptable fit > 0.1), comparative fit index (CFI; good fit > .9), and the standardized root mean squared residual (SRMR, good fit = below 0.10). Standardized loadings (SL) are reported.
dnLGD mutation profiles of sleep issues
An exploratory objective was to characterize the sleep profiles (in reference to idiopathic ASD) for children with a dnLGD mutation previously associated with ASD and sleep issues. To accomplish this, we calculated the proportion of positive endorsements of individual sleep issues for three dnLGD mutation subgroups: ADNP (n = 19), CHD8 (n = 22), and DYRK1A (n = 22). Subgroup proportions were compared to that of the NON group. Individual sleep issues were chosen for analysis instead of clustered sleep domains in an effort to better detect minor variations in sleep profile accompanying different dnLGD mutations. Bonferroni-correction was applied where noted (alpha threshold of 0.05), and additional uncorrected trends are reported given the preliminary nature of this analysis.
Results
Latent factor structure associated with sleep issues
We conducted a CFA to investigate model fit of three proposed competitive factor structures for both NON and dnLGD groups. In Model 1, all items loaded onto a single latent Sleep Issues variable. Model 2 consisted of a four-factor structure, including Sleep Onset, Breathing Issues, Nighttime Awakenings, and Daytime Tiredness. The sleep interview items that loaded onto these latent variables are reported in Table 3, with notable group differences related to breathing issues, nighttime awakenings, and daytime tiredness. Model 3 examined a hierarchical factor structure in which there was a second-order factor related to general sleep issues (similar to Model 1) and specific first-order factors related to the four latent variables (similar to Model 2). We predicted that all models would robustly characterize the sleep interview items, but anticipated that Model 2 would best fit the data considering that CFA models perform better in multifactorial structures when items are highly overlapping (i.e., items within one domain; Brown & Moore, 2012).
See Table 4 for model fit indices. For the NON group (N = 2509), Models 2 and 3 were good fitting models, with Model 3 being the best fitting model compared to Model 1, χ2(2) = 684.2, p<.0001, and Model 2, χ2(6) = 22.64, p<.0001. For the dnLGD group (N = 378), Model 3 did not converge and Model 2 was the best fitting model compared to Model 1, χ2(2) = 228.7, p<.0001. To have consistent comparisons across groups, we chose to use the latent factor structure for Model 2 for continued analysis (see Figure 1). For both groups, this four-factor solution indicated that items related to sleep onset had the high factor loadings (all standardized loadings > .63), suggesting that these items may be the most impactful sleep-related problem.
Table 4.
Model fit indices. Notes: Models that did not converge are indicated by “n/a”. Italicization and shading indicates value-specified poor model fit.
| NON | dnLGD | |||||||
|---|---|---|---|---|---|---|---|---|
| Model | Latent Variables | Participant Predictors (Variables) | RMSEA | CFI | SRMR | RMSEA | CFI | SRMR |
| Model 1 | Sleep (general) | None | 0.097 | 0.744 | 0.069 | 0.137 | 0.597 | 0.1 |
| Model 2 | Onset, Breathing, Awake, Tiredness | None | 0.041 | 0.962 | 0.029 | 0.047 | 0.961 | 0.045 |
| Model 3 | Sleep (general), Onset, Breathing, Awake, Tiredness | None | 0.043 | 0.955 | 0.032 | n/a | n/a | n/a |
| Model 2 | Onset, Breathing, Awake, Tiredness | Development (AGE) | 0.042 | 0.953 | 0.029 | 0.049 | 0.948 | 0.046 |
| Model 2 | Onset, Breathing, Awake, Tiredness | Behavioral problems (EXT, INT) | 0.039 | 0.955 | 0.027 | 0.051 | 0.939 | 0.049 |
| Model 2 | Onset, Breathing, Awake, Tiredness | Cognition (VIQ, NVIQ) | 0.053 | 0.916 | 0.042 | 0.042 | 0.96 | 0.045 |
| Model 2 | Onset, Breathing, Awake, Tiredness | All (AGE, EXT, INT, VIQ, NVIQ) | 0.048 | 0.911 | 0.037 | 0.048 | 0.93 | 0.045 |
Abbreviations: EXT, Externalizing problems; INT, Internalizing problems; VIQ, Verbal IQ; NVIQ, Nonverbal IQ.
Notes: Models that did not converge are indicated by “n/a”. Italicization and shading indicates value-specified poor model fit. Abbreviations: EXT, Externalizing problems; INT, Internalizing problems; VIQ, Verbal IQ; NVIQ, Nonverbal IQ.
Figure 1.

Confirmatory factor analysis for children with idiopathic (NON) and genetic (dnLGD) etiologies of ASD. Standardized loadings (SL) are reported for both groups for the three-factor model. Factors include difficulties with Sleep Onset, Nighttime Awakenings, and Daytime Tiredness. Sleep Onset domain includes difficulty going to bed (SBP), difficulty falling asleep (SBS), and parent needing to lay down with child to fall asleep (SBD). Nighttime awakening domain includes frequent or prolonged nighttime awakenings (NAF) and sleepwalking (NAS). Daytime Tiredness domain includes difficulty waking in the morning (DDW), longer, more frequent napping than same-age peers (DDN), and daytime tiredness (DDS). Strength of the standardized loadings are depicted as the thickness and brightness of each line for positive (blue) and negative (red) standardized loadings.
Individual participant predictors
After establishing that Model 2 had the best fit, we examined a series of models to determine how individual participant predictors are related to sleep issues. We systematically tested development (Age), behavioral problems (Internalizing, Externalizing), and cognition (Verbal IQ, Nonverbal IQ). Fit indices indicated all three participant predictor models had good fit for both groups (see Table 4). With the exception of the dnLGD cognition model (χ2(2) = 12.44, p = .41), all predictor models performed better than the non-predictor Model 2, p’s <.046. To better clarify the relationship between the predictors on sleep issue for each group, we will describe the three separate predictor models (see Figure 2).
Figure 2.

Confirmatory factor analysis with participant predictors for children with idiopathic (NON) and genetic (dnLGD) etiologies of ASD. Standardized loadings (SL) are reported for both groups for the three-factor model. Strength of the standardized loadings are depicted as the thickness and brightness of each line for positive (blue) and negative (red) standardized loadings.
Development
Despite improving model fit, the Age predictor does not load significantly onto the latent factors (SL < +/− .105).
Behavioral problems
Externalizing and internalizing behavioral problems weakly (p’s<.032, SL greater than +/− .25) impact latent variables for both groups with a few exceptions (NON group: externalization on Breathing problems, p = .28; dnLGD group: externalization on Onset problems, p = .42, internalization on Breathing problems, p = .57). Increased sleep issues were observed for children with internalizing behavioral problems (both groups) and externalizing behavioral problems (NON group). In contrast, dnLGD youth with externalizing behavioral problems were found to have fewer Breathing (SL = −.22), Awakening (SL = −23), and Tiredness (SL = −.15) problems.
Cognition
Neither VIQ or NVIQ were significantly related to sleep issues in the NON group (p’s>.064, SL less than +/− .097) with one exception: children with higher VIQ scores exhibited fewer Awakening issues (SL = −.11, p = .035). Similarly, dnLGD youth with higher NVIQ scores exhibited fewer Awakening issues (SL = −.54, p = .04).
Genetic ASD subgroups
As an exploratory aim, we sought to characterize specific sleep problems for clusters of dnLGD youth with ADNP (n = 19), CHD8 (n = 22), and DYRK1A (n = 22). Figure 3 shows individual item endorsements for these three specific dnLGD subgroups compared to endorsements for the NON group, as well as other dnLGD participants. Closer inspection of sleep issues in individuals with dnLGD mutations to ADNP, CHD8, and DYRK1A preliminarily suggest the emergence of unique sleep issue profiles. The ADNP profiles exhibited significantly elevated sleep issues relative to all other groups, including breathing problems (BPM: n = 8/19, 42.1%, p’s < .012; BAP: n = 5/19, 26.3%, p’s <.0001, Bonferroni-corrected) and increased daytime naps (n = 12/19, 63.2%, p’s <.0001, Bonferroni-corrected). Frequent nighttime awakenings were highly endorsed relative to NON group for ADNP (n = 15/19, 78.9%, p = .0004 Bonferroni-corrected) and CHD8 (n = 14/22, 63.6%, p = .034, Bonferroni-corrected. Trends were noted for other issues. The CHD8 exhibited more problems falling asleep (n = 15/22, 68.2%, p = .042, uncorrected) and requiring the parent to lay down with the child (n = 14/22, 63.6%, p = .049, uncorrected) than the NON group. The DYRK1A group did not vary as much from the NON group with the exception of having increased difficulty breathing at night (n = 4/22, 18.8%, p = 0.11, uncorrected), increased daytime naps (n = 5/22, 22.7%, p = .021, uncorrected), and no issues with sleepwalking (n = 0/22, 0%, p = .057, uncorrected).
Figure 3.
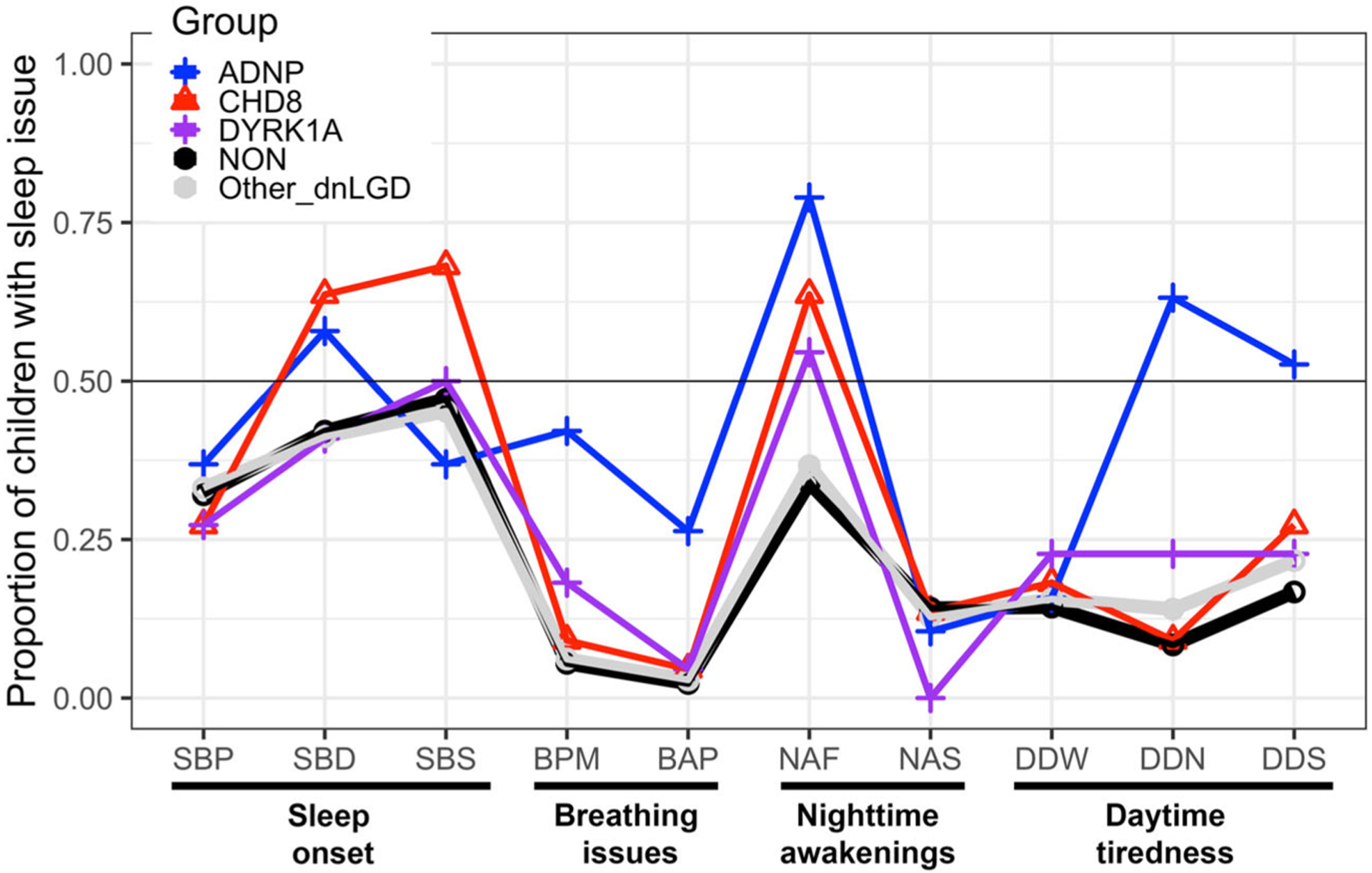
Average endorsement for target dnLGD and comparison groups. Average endorsement for children with dnLGD targets ADNP (n = 19, red circle), CHD8 (n = 22, green triangle), and DYRK1A (n = 22, blue square) compared to children with Other MUT (n = 315, gray line) and NON (n = 2509, black line).
Discussion
We aimed to clarify the kinds of sleep difficulties faced by individuals with genetic etiologies of ASD to better understand the genetic impact on sleep in this population. First, looking at a large sample of autistic individuals with and without a dnLGD mutation, findings from CFA indicate that clustering sleep problems into four symptom categories is a statistically appropriate way to characterize sleep difficulties. Inclusion of a general sleep problem variable as a hierarchical factor model did improve overall model fit for the idiopathic ASD group, but the model failed to converge for the dnLGD group. Because the second-order model failure to converge may be due to sample size issues, it is difficult to interpret differences between groups; thus, subsequent analyses focused on the first-order four factor model. The exceptional model fit of the four-component model for both groups (NON and dnLGD) indicates that the following four factors represent distinct areas of sleep difficulty: sleep onset, breathing issues, nighttime awakenings, and daytime tiredness. This model supports previous research findings that identify primary factors involved in ASD sleep problems and extends our understanding of these effects in genetic subgroups. Within this four-component model, difficulties with sleep onset contributed most strongly to the model (i.e., reliably the largest standardized loading). This finding is consistent with prior work (Liu et al., 2006; Malow et al., 2006; Souders et al., 2009; Wiggs & Stores, 2004), suggesting that trouble with sleep onset is a prominent sleep issue facing autistic individuals. This four-factor model also suggests that challenges with sleep onset and recurrent nighttime awakenings are distinct issues and warrant separate assessment. In previous research, these symptoms have been combined under the term “insomnia” when assessing for sleep problems in ASD, which may miss distinct features of sleep in this population.
Sleep-disordered breathing accounted for very little of variance of sleep problems, supporting previous findings of negligible relationship between breathing difficulties and ASD (Limoges et al., 2005; Malow et al., 2006). However, untreated sleep apnea and the associated increased breathing issues may be untreated or unknown to parents, which may be a contributing factor to daytime tiredness. Considering that individuals with untreated sleep apnea and other medical conditions (e.g., seizures, gastrointestinal problems) are often excluded from sleep research in order to more-specifically understanding mechanism/s (e.g., Goldman et al., 2017), future work will benefit from objectively measuring sleep-disordered breathing in a broader ASD cohort.
The addition of developmental, behavioral, and cognitive predictors to the four-factor model did improve model fit by group. In general, this analysis indicated that internalizing behavioral problems and lower cognitive scores were related to increased sleep issues. For those with idiopathic ASD, increased internalizing problems were associated with increased daytime tiredness. The same relationship was observed in the dnLGD group; additionally, increased internalizing problems relate to increased nighttime awakenings in this group. These results support previous findings that internalizing symptoms, such as anxious or depressive behaviors are associated with difficulty sleeping and reduced energy in youth with anxiety disorders (Alfano, Ginsburg, & Kingery, 2007; Weiner, Meredith Elkins, Pincus, & Comer, 2015).
Considering prior work correlating sleep problems with externalizing behaviors in ASD (Goldman et al., 2009; Mazurek & Petroski, 2015; Sikora et al., 2012; Wiggs & Stores, 2004), it was surprising that children with a dnLGD and externalizing behavioral problems exhibited fewer breathing issues, nighttime awakenings, and daytime issues. Perhaps unique cooccurring disorders and related medication use for individuals with dnLGD mutations impacted incidence of daytime fatigue in this group (Helsmoortel et al., 2014; van Bon et al., 2015). Another possibility is that children with externalizing problem behaviors are more active during the day and thus, have fewer issues with sleep, though the biological consequences of dnLGD mutations may be related to atypical or opposing psychophysiological responses. Autistic youth who demonstrate lower parasympathetic reactivity (i.e., ability to reduce arousal and heart rate) exhibit increased externalizing behaviors (Fenning et al., 2019), which may be related to biological mechanisms of sleep. Future work should target in-depth monitoring of the psychophysiological features to better understand how increased externalizing behaviors (ostensibly by day) are related to sleep.
When cognition is added as a participant predictor to the four-factor model, a relationship between cognition and nighttime awakenings emerges that appears unique to the dnLGD group. Higher nonverbal cognitive ability was related to decreased nighttime awakenings for the dnLGD group, which was a strongest effect of the individual difference predictors (SL = −.54). Variations in cognitive functioning are often accompanied by differences in language ability, emotion regulation skills, and motor mobility, which may be impacting parents’ awareness and understanding of their child’s awakenings at night (Bölte & Poustka, 2002; Wing, 1981). Our observed opposing effects highlight the ambiguity of this relationship between cognition and sleep.
Due to the diversity of genes disrupted in the heterogeneous dnLGD group, closer examination of individuals with recurrent mutations is warranted to clarify observed interactions between participant characteristics and sleep. In our exploratory examination of three previously reported genetically defined subgroups of ASD, unique sleep problem profiles emerged. In particular, reports of difficulty falling asleep are increased for children with CHD8 mutations, while reports of nighttime awakenings are elevated for all three genetic groups. While preliminary, the observed variations in sleep phenotypes for these three subgroups suggest different biological mechanisms. The regulation of chromatin remodeling, a known function of the CHD8 gene, is suggested to be important in the maintenance of circadian rhythms, which are essential to creating typical cycles of sleep (Aguilar-Arnal & Sassone-Corsi, 2013). Mutations to CHD8 may be involved in circadian disruption and thereby help explain why parents report that individuals with CHD8 mutation have such difficulty falling asleep. In one extreme example, one parent reported that their child with a CHD8 mutation stays awake for several days at a time, posing the tremendous challenge of keeping the child safe while parents sleep. New evidence indicates a role for CHD8 in maintaining sleep architecture across developmental stages in kismet, the CHD8/CHD7 Drosophila ortholog (Coll-Tané et al., in press). Critically, this animal model implicates subperineurial glia constituting the blood-brain barrier that could potentially be targeted for therapeutic intervention.
Children with DYRK1A participants had sleep profiles that were largely similar to idiopathic ASD, and the DYRK1A gene is implicated in transcriptional/translational regulation of circadian rhythm proteins. Kurabayashi, Hirota, Sakai, Sanada, and Fukada (2010) found that the knockout of DYRK1A in mice models disrupted the phosphorylation of circadian rhythm-associated gene, CRY2, which resulted in shortened circadian cycles and arrhythmic sleep behaviors. The ADNP sleep profile was significantly elevated relative to all over groups. ADNP has also been linked to gene mechanisms that are implicated in sleep maintenance. Categorized as a Fragile X mental retardation protein (FMRP) associated gene (Iossifov et al., 2014), these genetic disruptions are implicated in disturbed light-dark circadian rhythms and sleep (Xu, Poidevin, Han, Bi, & Jin, 2012; Zhang et al., 2008). Recurrent reports of nighttime awakenings were identified in ADNP participants, a finding that is also common in cases of Alzheimer’s, which has a shared association with ADNP (Malishkevich et al., 2015; Sethi et al., 2015). While more exploration of this relationship is needed, perhaps the sleep-wake cycle mechanisms responsible for sleep maintenance are disrupted by variations in ADNP.
Our findings suggest that genetically defined subgroups may prove informative in understanding sleep in ASD and relevant to consider when individualizing sleep interventions for affected individuals and their families. However, a more extensive exploration of these sleep problems must better characterize extreme problems – both qualitatively, to better understand the disruptive behaviors and the potential origins, but also quantitatively (e.g., frequency of occurrence, level of severity).
Limitations
This study has implications for families of autistic youth with both idiopathic and identified genetic origins. For instance, our findings suggest that sleep onset should be a focus area for generating recommendations and resources for parents of a child with ASD, aligned with current efforts from private foundations and family groups (e.g., Autism Speaks Sleep Toolkit; Coury et al., 2020) and opportunities for clinical intervention (Malow et al., 2012). However, there are several critical limitations to consider. Further investigation of child characteristics (i.e., age, behavior, and cognition), continuous markers of sleep (e.g., actigraphy, polysomnography, and/or parent-reported sleep duration), and other predictors of sleep problems (e.g., metabolism, nutrition, medication) will improve our ability to provide individualized recommendations for families. By building more elaborate clinical profiles for recurrent dnLGD mutations, similar to the preliminary profiles for CHD8, DYRK1A and ADNP, we aim to connect physical manifestation of sleep problems to possible biological mechanisms. For instance, further clinical clarity related to externalizing behaviors is warranted given links between sleep, underlying biological mechanisms, and externalizing disorders such as attention deficit hyperactivity disorder (e.g., Gruber et al., 2012).
Although we did not specifically examine medication interactions due to missing data, continued work should also evaluate the role of medications in both sleep disruption (e.g., selective serotonin-reuptake inhibitors, SSRIs; ritalin) as well as sleep enhancement (e.g., melatonin) to fully understand potential biological interactions between genetic etiology, medication, and sleep. Our study is limited by the heterogeneity within the dnLGD group, which was collapsed across a range of mutations for our primary aim, yet would benefit from better classification based upon specific genetic variants and/or genetic function (e.g., Iossifov et al., 2014). This is an area of further study that shows great promise in explaining the phenotypic variability we see in sleep issues co-occurring with ASD symptoms.
Conclusions
Our results indicate that different domains of sleep difficulties should be considered for autistic youth, specifically that sleep onset and nighttime awakenings should be considered separate domains. This work extends existing knowledge of predictors and factors associated with sleep problems by characterizing these problems in ASD in the context of underlying genetic etiology. The knowledge gained by this study will inform ongoing efforts to establish genotype-phenotypes and build biological indicators for ASD, critical pieces required to pursue precision medicine in support of positive health outcomes for ASD.
Acknowledgments
We are grateful to all of the families at the participating Simons Simplex Collection (SSC) sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, R. Goin-Kochel, E. Hanson, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, K. Pelphrey, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, Z. Warren, E. Wijsman). We appreciate obtaining access to phenotypic data on SFARI Base.
Funding
Funding for this study was provided by the National Institutes for Mental Health (#R01MH100047 to R.B. and #R01MH101221 to E.E.E) and the Simons Foundation Autism Research Initiative Grant No. 294112 to E.E.E.
Appendix. Sleep items on medical history questionnaire
| Sleep problems – SSC Medical History questionnaire | Response |
|---|---|
| Bed Time Problems | Yes / No / Not sure |
| Difficulty going to bed? (i.e., longer than 1 hour; tantrums, etc.) | |
| Difficulty falling asleep? | |
| Do you have to lay down with your child to get him/her to fall asleep? | |
| Excessive Daytime Sleepiness | Yes / No / Not sure |
| Unusually tired/sleepy during the day? | |
| Difficulty waking up in the morning? | |
| Very long or frequent naps more often that other children his/her age? | |
| Sleep-disordered Breathing | Yes / No / Not sure |
| Snore more than half the time? | |
| Difficulty breathing at night? | |
| Nighttime Awakenings | Yes / No / Not sure |
| Frequent or prolonged awakenings at night? | |
| Sleepwalking or frequent nightmares? |
Footnotes
Disclosure statement
Caitlin M. Hudac is on the Scientific Advisory Board of the FamiliesSCN2A Foundation. Evan E. Eichler is on the Scientific Advisory Board of DNAnexus, Inc. The remaining authors have no potential conflict of interest to report.
Data availability statement
Data that support the findings of this study are in part available from SFARI Base. Approved researchers can obtain the SSC population dataset described in this study by applying at https://base.sfari.org. The remaining data collected via the TIGER study are available from the corresponding author, C.M.H., upon reasonable request.
References
- Achenbach TM (1991). Child behavior checklist/4–18 (pp. 5). Burlington: University of Vermont. [Google Scholar]
- Adams HL, Matson JL, Cervantes PE, & Goldin RL (2014). The relationship between autism symptom severity and sleep problems: Should bidirectionality be considered? Research in Autism Spectrum Disorders, 8(3), 193–199. doi: 10.1016/j.rasd.2013.11.008 [DOI] [Google Scholar]
- Aguilar-Arnal L, & Sassone-Corsi P (2013). The circadian epigenome: How metabolism talks to chromatin remodeling. Current Opinion in Cell Biology, 25(2), 170–176. doi: 10.1016/j.ceb.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CA, Ginsburg GS, & Kingery JN (2007). Sleep-related problems among children and adolescents with anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 46(2), 224–232. doi: 10.1097/01.chi.0000242233.06011.8e [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Bauminger N, Solomon M, & Rogers SJ (2010). Externalizing and internalizing behaviors in ASD. Autism Research, 3(3), 101–112. doi: 10.1002/aur.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier RA, & Gerdts J (2010). Autism spectrum disorders: A reference handbook. Santa Barbara, CA: ABC-CLIO, LLC. [Google Scholar]
- Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, … Eichler EE (2014). Disruptive CHD8 mutations define a subtype of autism early in development. Cell, 158(2), 263–276. doi: 10.1016/j.cell.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölte S, & Poustka F (2002). The relation between general cognitive level and adaptive behavior domains in individuals with autism with and without co-morbid mental retardation. Child Psychiatry and Human Development, 33(2), 165–172. doi: 10.1023/A:1020734325815 [DOI] [PubMed] [Google Scholar]
- Brown TA, & Moore MT (2012). Confirmatory factor analysis. Handbook of Structural Equation Modeling, 361–379. [Google Scholar]
- Browne MW, & Cudeck R (1993). Alternative ways of assessing model fit. In Bollen KA & Scott Lang L (Eds.), Testing structural models (2nd ed., pp. 136–162). Newbury Park, CA: Sage Publications. [Google Scholar]
- Buckley AW, Rodriguez AJ, Jennison K, Buckley J, Thurm A, Sato S, & Swedo S (2010). Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Archives of Pediatrics & Adolescent Medicine, 164(11), 1032–1037. doi: 10.1001/archpediatrics.2010.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr A, Bianchi MT, Baur R, Courtet P, Pignay V, Boulenger JP, … Sigel E (2002). Functional characterization of the new human GABA(A) receptor mutation beta3(R192H). Human Genetics, 111(2), 154–160. doi: 10.1007/s00439-002-0766-7 [DOI] [PubMed] [Google Scholar]
- Chen X, Liu H, Wu Y, Xuan K, Zhao T, & Sun Y (2020). Characteristics of sleep architecture in autism spectrum disorders: A meta-analysis based on polysomnographic research. Psychiatry Research, 296, 113677. [DOI] [PubMed] [Google Scholar]
- Coll-Tané M, Gong NN, Belfer SJ, van Renssen LV, Kurtz-Nelson EC, Szuperak M, … Schenck A (in press). The CHD8/CHD7/Kismet family links blood-brain barrier glia and serotonin to ASD-associated sleep defects. Scientifice Advances. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Wang F, Angriman M, Masi G, & Bruni O (2020). Sleep disorders in children and adolescents with autism spectrum disorder: Diagnosis, epidemiology, and management. CNS Drugs, 34(4), 415–423. doi: 10.1007/s40263-020-00710-y [DOI] [PubMed] [Google Scholar]
- Coury DL, Murray DS, Fedele A, Hess T, Kelly A, & Kuhlthau KA (2020). The autism treatment network: Bringing best practices to all children with autism. Pediatrics, 145(Supplement 1), S13–S19. doi: 10.1542/2019-1895D [DOI] [PubMed] [Google Scholar]
- Couturier JL, Speechley KN, Steele M, Norman R, Stringer B, & Nicolson R (2005). Parental perception of sleep problems in children of normal intelligence with pervasive developmental disorders: Prevalence, severity, and pattern. Journal of the American Academy of Child & Adolescent Psychiatry, 44(8), 815–822. doi: 10.1097/01.chi.0000166377.22651.87 [DOI] [PubMed] [Google Scholar]
- Crocker A, & Sehgal A (2010). Genetic analysis of sleep. Genes & Development, 24(12), 1220–1235. doi: 10.1101/gad.1913110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD, Murray GJ, & Pearson LS (1990). Differential ability scales. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Elrod MG, & Hood BS (2015). Sleep differences among children with autism spectrum disorders and typically developing peers: A meta-analysis. Journal of Developmental & Behavioral Pediatrics, 36(3), 166–177. doi: 10.1097/DBP.0000000000000140 [DOI] [PubMed] [Google Scholar]
- Fenning RM, Erath SA, Baker JK, Messinger DS, Moffitt J, Baucom BR, & Kaeppler AK (2019). Sympathetic-parasympathetic interaction and externalizing problems in children with Autism spectrum disorder. Autism Research, 12(12), 1805–1816. doi: 10.1002/aur.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, & Lord C (2010). The Simons Simplex Collection: A resource for identification of autism genetic risk factors. Neuron, 68(2), 192–195. doi: 10.1016/j.neuron.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Goldman SE, Alder ML, Burgess HJ, Corbett BA, Hundley R, Wofford D, … Malow BA (2017). Characterizing sleep in adolescents and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 47(6), 1682–1695. doi: 10.1007/s10803-017-3089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SE, Richdale AL, Clemons T, & Malow BA (2012). Parental sleep concerns in autism spectrum disorders: Variations from childhood to adolescence. Journal of Autism and Developmental Disorders, 42(4), 531–538. doi: 10.1007/s10803-011-1270-5 [DOI] [PubMed] [Google Scholar]
- Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, & Malow BA (2009). Defining the sleep phenotype in children with autism. Developmental Neuropsychology, 34(5), 560–573. doi: 10.1080/87565640903133509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlin-Jones B, Tang K, Liu J, & Anders TF (2009). Sleep problems, sleepiness and daytime behavior in preschool-age children. Journal of Child Psychology and Psychiatry, 50(12), 1532–1540. doi: 10.1111/j.1469-7610.2009.02110.x [DOI] [PubMed] [Google Scholar]
- Gregory AM, & Sadeh A (2012). Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Medicine Reviews, 16(2), 129–136. doi: 10.1016/j.smrv.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Gruber R, Fontil L, Bergmame L, Wiebe ST, Amsel R, Frenette S, & Carrier J (2012). Contributions of circadian tendencies and behavioral problems to sleep onset problems of children with ADHD. BMC Psychiatry, 12(1), 1–11. doi: 10.1186/1471-244X-12-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzadzinski R, Lord C, Sanders SJ, Werling D, & Bal VH (2018). Children with autism spectrum disorder who improve with fever: Insights from the Simons Simplex Collection. Autism Research, 11(1), 175–184. doi: 10.1002/aur.1856 [DOI] [PubMed] [Google Scholar]
- Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J, … Van der Aa N (2014). A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nature Genetics, 46(4), 380–384. doi: 10.1038/ng.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst SC, Bersagliere A, Bachmann V, Berger W, Achermann P, & Landolt HP (2014). Dopaminergic role in regulating neurophysiological markers of sleep homeostasis in humans. Journal of Neuroscience, 34(2), 566–573. doi: 10.1523/JNEUROSCI.4128-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, & Rutter M (2004). Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, 45(2), 212–229. doi: 10.1111/j.1469-7610.2004.00215.x [DOI] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Humphreys JS, Gringras P, Blair PS, Scott N, Henderson J, Fleming PJ, & Emond AM (2014). Sleep patterns in children with autistic spectrum disorders: A prospective cohort study. Archives of Disease in Childhood, 99(2), 114–118. doi: 10.1136/archdischild-2013-304083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, … Wigler M (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature, 515(7526), 216–221. doi: 10.1038/nature13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson AE, Dorman JS, Chasens ER, Feeley CA, & Devlin B (2019). Variations in genes related to sleep patterns in children with autism spectrum disorder. Biological Research for Nursing, 21(3), 335–342. doi: 10.1177/1099800419843604 [DOI] [PubMed] [Google Scholar]
- Johansson AE, Rohay JM, & Chasens ER (2018). Psychometric properties of the Simons simplex collection sleep interview. Journal of Nursing Measurement, 26(3), 453–469. doi: 10.1891/1061-3749.26.3.453 [DOI] [PubMed] [Google Scholar]
- Kline RB (2011). Principles and practice of structural equation modeling. New York City, NY: Guilford Press. [Google Scholar]
- Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, & Hansen RL (2008). Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. Journal of Sleep Research, 17(2), 197–206. doi: 10.1111/j.1365-2869.2008.00650.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurabayashi N, Hirota T, Sakai M, Sanada K, & Fukada Y (2010). DYRK1A and glycogen synthase kinase 3β, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Molecular and Cellular Biology, 30(7), 1757–1768. doi: 10.1128/MCB.01047-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoges E, Mottron L, Bolduc C, Berthiaume C, & Godbout R (2005). Atypical sleep architecture and the autism phenotype. Brain, 128(5), 1049–1061. doi: 10.1093/brain/awh425 [DOI] [PubMed] [Google Scholar]
- Liu X, Hubbard JA, Fabes RA, & Adam JB (2006). Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry and Human Development, 37(2), 179–191. doi: 10.1007/s10578-006-0028-3 [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, … Rutter M (2000). The Autism Diagnostic observation schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. doi: 10.1023/A:1005592401947 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. doi: 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Malishkevich A, Amram N, Hacohen-Kleiman G, Magen I, Giladi E, & Gozes I (2015). Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer’s pathologies. Translational Psychiatry, 5(2), e501. doi: 10.1038/tp.2014.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, & Stone WL (2006). Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep, 29(12), 1563–1571. doi: 10.1093/sleep/29.12.1563 [DOI] [PubMed] [Google Scholar]
- Malow B, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, & Burnette C (2012). Melatonin for sleep in children with autism: A controlled trial examining dose, tolerability, and outcomes. Journal of Autism and Developmental Disorders, 42(8), 1729–1737. doi: 10.1007/s10803-011-1418-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, & Petroski GF (2015). Sleep problems in children with autism spectrum disorder: Examining the contributions of sensory over-responsivity and anxiety. Sleep Medicine, 16(2), 270–279. doi: 10.1016/j.sleep.2014.11.006 [DOI] [PubMed] [Google Scholar]
- McMakin DL, & Alfano CA (2015). Sleep and anxiety in late childhood and early adolescence. Current Opinion in Psychiatry, 28(6), 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning (pp. 58–64). Circle Pines, MN: AGS. [Google Scholar]
- O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, … Shendure J (2012). Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science, 338(6114), 1619–1622. doi: 10.1126/science.1227764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Cho S-C, Cho IH, Kim B-N, Kim J-W, Shin M-S, … Yoo HJ (2012). Sleep problems and their correlates and comorbid psychopathology of children with autism spectrum disorders. Research in Autism Spectrum Disorders, 6(3), 1068–1072. doi: 10.1016/j.rasd.2012.02.004 [DOI] [Google Scholar]
- Piazza CC, Fisher WW, & Kahng SW (2008). Sleep patterns in children and young adults with mental retardation and severe behavior disorders. Developmental Medicine & Child Neurology, 38(4), 335–344. doi: 10.1111/j.1469-8749.1996.tb12099.x [DOI] [PubMed] [Google Scholar]
- Posar A, & Visconti P (2020). Sleep problems in children with autism spectrum disorder. Pediatric Annals, 49(6), e278–e282. doi: 10.3928/19382359-20200511-01 [DOI] [PubMed] [Google Scholar]
- Revelle W (1978). ICLUST: A cluster analytic approach to exploratory and confirmatory scale construction. Behavior Research Methods & Instrumentation, 10(5), 739–742. doi: 10.3758/BF03205389 [DOI] [Google Scholar]
- Revelle W (2011). An overview of the psych package. Department Psychology at Northwestern University, 3, 1–25. [Google Scholar]
- Richdale AL (1999). Sleep problems in autism: Prevalence, cause, and intervention. Developmental Medicine & Child Neurology, 41(1), 60–66. doi: 10.1017/S0012162299000122 [DOI] [PubMed] [Google Scholar]
- Richdale AL, & Schreck KA (2009). Sleep problems in autism spectrum disorders: Prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews, 13(6), 403–411. doi: 10.1016/j.smrv.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Rosseel Y (2012). Lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48(2), 1–36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Sethi M, Joshi SS, Webb RL, Beckett TL, Donohue KD, Murphy MP, … Duncan MJ (2015). Increased fragmentation of sleep-wake cycles in the 5XFAD mouse model of Alzheimer’s disease. Neuroscience, 290, 80–89. doi: 10.1016/j.neuroscience.2015.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora DM, Johnson K, Clemons T, & Katz T (2012). The relationship between sleep problems and daytime behavior in children of different ages with autism spectrum disorders. Pediatrics, 130(Supplement 2), S83–S90. doi: 10.1542/peds.2012-0900F [DOI] [PubMed] [Google Scholar]
- Souders MC, Mason TBA, Valladares O, Bucan M, Levy SE, Mandell DS, … Pinto-Martin J (2009). Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep, 32(12), 1566–1578. doi: 10.1093/sleep/32.12.1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessman HA, Bernier R, & Eichler EE (2014). A genotype-first approach to defining the subtypes of a complex disease. Cell, 156(5), 872–877. doi: 10.1016/j.cell.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MA, Schreck KA, & Mulick JA (2012). Sleep disruption as a correlate to cognitive and adaptive behavior problems in autism spectrum disorders. Research in Developmental Disabilities, 33(5), 1408–1417. doi: 10.1016/j.ridd.2012.03.013 [DOI] [PubMed] [Google Scholar]
- Traylor RN, Dobyns WB, Rosenfeld JA, Wheeler P, Spence JE, Bandholz AM, … Ballif BC (2012). Investigation of TBR1 Hemizygosity: Four individuals with 2q24 microdeletions. Molecular Syndromology, 3(3), 102–112. doi: 10.1159/000342008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon BW, Coe BP, Bernier R, Green C, Gerdts J, Witherspoon K, … Gecz J (2015). Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Molecular Psychiatry, 21, 126–132. doi: 10.1038/mp.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeweyer G, Helsmoortel C, Van Dijck A, Vulto-van Silfhout AT, Coe BP, Bernier R, … Kooy RF (2014). The transcriptional regulator ADNP links the BAF (SWI/SNF) complexes with autism. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 166(3), 315–326. doi: 10.1002/ajmg.c.31413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch OJ, Keenan BT, Gehrman PR, Malow BA, & Pack AI (2017). Pleiotropic genetic effects influencing sleep and neurological disorders. The Lancet Neurology, 16(2), 158–170. doi: 10.1016/S1474-4422(16)30339-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch OJ, Maxwell-Horn AC, & Malow BA (2015). Sleep in Autism Spectrum Disorders. Current Sleep Medicine Reports, 1(2), 131–140. doi: 10.1007/s40675-015-0012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch OJ, Pendergast JS, Allen MJ, Leu RM, Johnson CH, Elsea SH, & Malow BA (2015). Genetic variation in melatonin pathway enzymes in children with autism spectrum disorder and comorbid sleep onset delay. Journal of Autism and Developmental Disorders, 45(1), 100–110. doi: 10.1007/s10803-014-2197-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, … Blakely RD (2012). Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proceedings of the National Academy of Sciences, 109(14), 5469–5474. doi: 10.1073/pnas.1112345109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler intelligence scale for children–Fourth Edition (WISC- [Database] IV). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weiner CL, Meredith Elkins R, Pincus D, & Comer J (2015). Anxiety sensitivity and sleep-related problems in anxious youth. Journal of Anxiety Disorders, 32, 66–72. doi: 10.1016/j.janxdis.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs L, & Stores G (2004). Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Developmental Medicine & Child Neurology, 46(6), 372–380. doi: 10.1017/S0012162204000611 [DOI] [PubMed] [Google Scholar]
- Williamson AA, Mindell JA, Hiscock H, & Quach J (2020). Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. Journal of Child Psychology and Psychiatry, 61(10), 1092–1103. doi: 10.1111/jcpp.13303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing L (1981). Language, social, and cognitive impairments in autism and severe mental retardation. Journal of Autism and Developmental Disorders, 11(1), 31–44. doi: 10.1007/BF01531339 [DOI] [PubMed] [Google Scholar]
- Wirth RJ, & Edwards MC (2007). Item factor analysis: Current approaches and future directions. Psychological Methods, 12(1), 58–79. doi: 10.1037/1082-989X.12.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. y., Huang S. d., Zou J. j., Wang Q. x., Naveed M, Bao H. n., … Han F (2020). Autism spectrum disorder (ASD): Disturbance of the melatonin system and its implications. Biomedicine & Pharmacotherapy, 130, 110496. doi: 10.1016/j.biopha.2020.110496 [DOI] [PubMed] [Google Scholar]
- Xu S, Poidevin M, Han E, Bi J, & Jin P (2012). Circadian rhythm-dependent alterations of gene expression in Drosophila brain lacking fragile X mental retardation protein. PLoS One, 7(5), e37937. doi: 10.1371/journal.pone.0037937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fang Z, Jud C, Vansteensel MJ, Kaasik K, Lee CC, … Nelson DL (2008). Fragile X-related proteins regulate mammalian circadian behavioral rhythms. The American Journal of Human Genetics, 83(1), 43–52. doi: 10.1016/j.ajhg.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are in part available from SFARI Base. Approved researchers can obtain the SSC population dataset described in this study by applying at https://base.sfari.org. The remaining data collected via the TIGER study are available from the corresponding author, C.M.H., upon reasonable request.


