Abstract
Ureaplasma urealyticum is a causative agent of nongonococcal urethritis and is implicated in the pathogenesis of several other diseases. The species is divided into 14 serovars and two biovars, of which biovar 1 is most commonly isolated from clinical specimens. Reported associations between individual serovars and diseases have been difficult to confirm because of practical difficulties with serotyping. The multiple-banded antigen (MBA) is the predominant U. urealyticum antigen recognized during infections in humans and probably has a significant role in virulence. The 5′ end of the MBA gene is relatively conserved but contains biovar, and possibly serovar, specificity. The 5′ ends of the MBA genes of standard strains of U. urealyticum biovar 1, consisting of serovars 1, 3, 6, and 14, were amplified, cloned into pUC19, and sequenced to identify serovar-specific differences. The 5′ end of the MBA gene sequence of serovar 3 was identical with the previously published sequence and differed by only three bases from that of serovar 14. Significant differences between the MBA gene sequences allowed biovar 1 to be divided into two subgroups, containing serovars 3/14 and serovars 1 and 6, respectively, using primers UMS-125–UMA269 and UMS-125–UMA269′. Serovars 1 and 6 were distinguished by restriction enzyme analysis of the amplicon and/or by PCR specific for serovar 6. These methods were used to identify and type U. urealyticum in 185 (46.3%) of 400 genital specimens from women. Biovar 1 was detected in 89.2% and biovar 2 in 18.3% of positive specimens. Of 165 specimens containing U. urealyticum biovar 1, 22.2% contained more than one serovar and 46.7, 46.1, and 25.5% contained serovars 1, 3/14, and 6, respectively. U. urealyticum was found in a significantly higher proportion of pregnant women than in sex workers and other women attending a sexually transmissible diseases clinic (P < 0.01). The methods described are relatively rapid, practicable, and specific for serotyping isolates and for direct detection and identification of individual serovars in clinical specimens containing U. urealyticum biovar 1.
Ureaplasma urealyticum is a recognized cause of urethritis and disseminated infection in immunocompromised patients (8, 15–17). It has also been implicated in other genitourinary syndromes (10) and a number of complications of pregnancy, including chorioamnionitis, preterm birth, and chronic neonatal lung disease (1–3). However, it is found commonly among the genital tract flora of healthy women, and its pathogenic role in a small proportion of individuals is difficult to prove (4, 23).
There are two biovars and 14 serovars of U. urealyticum. The majority of human isolates of U. urealyticum belong to biovar 1 (1, 6, 7, 9, 23), which includes serovars 1, 3, 6, and 14. Some serovars have been associated with disease syndromes more often than they are found in normal flora (4, 9, 10). However, these findings are difficult to confirm because of problems with conventional serotyping methods (4, 9, 12). A rapid molecular method for identification of individual serovars would be of great value in studies of epidemiology and pathogenesis of infections with U. urealyticum (11, 13, 18, 23).
The MBA (multiple-banded antigen) is the predominant antigen recognized during U. urealyticum infections and is probably an important virulence determinant. It is species specific and contains both serovar-specific and cross-reactive epitopes (19, 21–23). The 5′ end of the MBA gene is relatively conserved, but it also includes biovar- and serovar-specific regions (22). The aim of this study was to identify sequence differences among the 5′ ends of the MBA genes of U. urealyticum serovars 1, 3, 6, and 14 of biovar 1 which could be used as the basis of a practical molecular-serotyping method.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The reference strains used were U. urealyticum serovar 1 (ATCC 27813), serovar 2 (ATCC 27814), serovar 3 (ATCC 27815), serovar 4 (ATCC 27816), serovar 5 (ATCC 27817), serovar 6 (ATCC 27818), serovar 7 (ATCC 27819), serovar 8 (ATCC 27618), serovar 9 (ATCC 33175), serovar 10 (ATCC 33699), serovar 11 (ATCC 33695), serovar 12 (ATCC 33696), serovar 13 (ATCC 33698), and serovar 14 (ATCC 33697). Clinical isolates of serovars 1 to 14, which had been serotyped by conventional methods, were kindly provided by H. L. Watson (Department of Microbiology, University of Alabama at Birmingham, Birmingham) Escherichia coli DH5α was the host for plasmid pUC19, which was obtained from the Institute of Genetics, Chinese Academy of Science.
Clinical specimens.
Cervical swabs from 200 female sex workers and 100 other female clients attending the Sexually Transmissible Diseases (STD) Clinic and 100 pregnant women attending the Antenatal Clinic of the First Hospital of Beijing Medical University were collected to test the practical usefulness of the direct detection, biotyping, and serotyping methods. DNA was prepared for PCR (see below) as soon as possible after receipt of specimens in the laboratory. Cultures were not performed for these specimens.
Oligonucleotide primers.
Oligonucleotide primers UMS-125, UMA226, UMS51, and UMA427, based on the previously published sequence of the serovar 3 MBA gene (19, 22), were used for amplification of the 5′ ends of the U. urealyticum serovar 1, 3, 6, and 14 MBA genes. UMS-125 (GTA TTT GCA ATC TTT ATA TGT TTT CG) and UMA226 (CAG CTG ATG TAA GTG CAG CAT TAA ATT C) were used without modification. UMS51 and UMA427 were modified as follows. (i) Primers UMS51 and UMA427 were modified by adding BamHI and EcoRI restriction enzyme sites, respectively (underlined in the sequences below), to facilitate cloning of the coding DNA sequences of the 5′ ends of the serovar 1, 3, and 14 MBA genes. (ii) In ureaplasmas, the sequence TGA encodes tryptophan (amino acid 13), but in E. coli it represents a stop codon. Therefore, a point mutation was introduced into UMS51 (at base 38) to change the sequence to TGG, which also encodes tryptophan. The modified primer sequences used were UMS51, TGT GGA TCC TTC TGG GCT ATG ACA TTA GGT GTT ACC (BamHI site underlined) and UMA427, CTC GAA TTC ACC TGG TTG TGT AGT TTC AAA GTT CAC (EcoRI site underlined).
The additional primers UMA269 (serovar 3/14 specific), UMA269′ (serovar 1 and 6 specific), and UMS-54 (serovar 6 specific) were designed, using the sequence data generated by this study, and were used for PCR identification (see below). The sequences of these primers are as follows: UMA269 (serovar 3/14), CTA AAT GAC CTT TTT CAA GTG TAC; UMA269′ (serovars 1 and 6), CCA AAT GAC CTT TTG TAA CTA GAT; and UMS-54 (serovar 6), CTT AGT GTT CAT ATT TTT TAC TAG.
DNA preparations.
Cells from 0.5 ml of ureaplasma broth (10B) cultures of each U. urealyticum serovar were harvested in late logarithmic growth phase by centrifugation at 14,000 × g for 20 min; clinical specimens were processed directly. DNA was isolated from both cultures and clinical specimens by treatment with 500 μl of digestion buffer (10 mM Tris-HCl, pH 8.0, 0.45% Triton X-100, and 0.45% Tween 20) and proteinase K (100 μg/ml) at 55°C for 1 h and then extraction with phenol-chloroform-isoamyl alcohol (25:24:1) and chloroform-isoamyl alcohol (24:1). DNA was precipitated with 1/10 volume of 3 M sodium acetate (pH 5.2) and 2 volumes of ethanol. The washed and dried pellets were hydrated in 200 μl of ultrapure sterile water.
PCR.
The 25-μl amplification reaction mixtures contained 2.5 μl of 10× PCR buffer (1× PCR buffer is 10 mM Tris-HCl [pH 8.8 at 25°C], 1.5 mM MgCl2, 50 mM KCl, and 0.1% Triton X-100), 0.5 U of Taq polymerase (Finnzymes OY, Espoo, Finland), 200 μM (each) deoxynucleoside triphosphate (dATP, dCTP, dGTP, and dTTP) (Boehringer, Mannheim, Germany), 10 pmol of each primer, 5 μl of sample DNA, and ultrapure sterile water added to 25 μl. In each reaction, positive and negative controls were processed in parallel with the tested samples to detect possible inhibition or contamination.
The denaturation, annealing, and elongation temperatures and times used were 95°C for 30 s, 58°C for 30 s, and 72°C for 1 min, respectively, for 40 cycles with a Perkin-Elmer thermocycler. Eight microliters of PCR product was analyzed by electrophoresis on 2.0% agarose gels which were stained with 0.5 μg of ethidium bromide/ml. A visible band of the appropriate size on UV translumination was considered a positive result.
Cloning and sequencing.
The amplified fragments of the 5′ ends of U. urealyticum MBA genes (amplified with UMS-125–UMA226 for serovar 6 and UMS51-UMA427 for serovars 1, 3, and 14) were ligated to plasmid vector pUC19, which had been digested by SmaI. The ligation products were transformed into E. coli DH5α, and positive transformants were identified by PCR, using the primers UMS51-UMA427 for serovars 1, 3, and 14 and UMS-125–UMA226 for serovar 6. The positive clones were sequenced in two directions with the ABI 373A DNA-sequencing system.
To confirm the results of cloning experiments, the PCR products of both sets of primers for ATCC reference strains and clinical isolates of all four serovars, 1, 3, 6, and 14, were directly sequenced in two directions with the ABI 373A DNA-sequencing system.
Identification of serovars by PCR with restriction enzyme analysis and serovar-specific PCR.
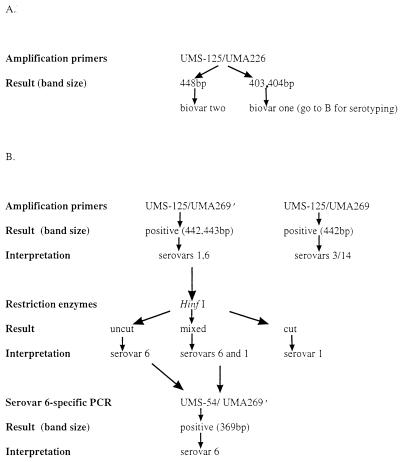
Primers UMS-125, UMA226, UMA269, and UMA269′ were used to amplify DNA from reference (ATCC) strains, from clinical isolates that had been serotyped previously by conventional methods, and directly from clinical specimens. Primers UMS-125 and UMA226 were used to detect and biotype U. urealyticum. Primers UMS-125 and UMA269 were specific for serovars 3/14; primers UMS-125 and UMA269′ were specific for serovars 1 and 6. Two different methods were used to further identify U. urealyticum serovars 1 and 6 (Fig. 1).
FIG. 1.
Algorithm for identification of serovars of U. urealyticum biovar 1 in clinical specimens or cultures. (A) Biotyping of U. urealyticum; (B) serotyping of U. urealyticum biovar 1.
(i) Restriction enzyme analysis.
Ten microliters of the PCR product of primers UMS-125 and UMA269′ was treated with the restriction enzyme HinfI (New England Biolabs, Beverly, Mass.) and analyzed by electrophoresis. Those that were cut by the enzyme were identified as serovar 1, those not cut were identified as serovar 6, and those that were partially digested were assumed to be mixed serovars 1 and 6.
(ii) Confirmatory PCR for serovar 6.
Primers UMS-54 (specific for serovar 6) and UMA269′ were used in a separate PCR to confirm the identity of serovar 6-positive isolates or specimens.
Nucleotide sequence accession numbers.
The sequence data obtained have been accepted by the GenBank nucleotide sequence database and have been assigned accession no. AF056982 (serovar 14), AF056983 (serovar 1), and AF056984 (serovar 6). They will also appear in EMBL in Europe and the DNA database Bank of Japan.
RESULTS
PCR.
The amplified fragments of the 5′ ends of the MBA genes of all 14 U. urealyticum serovars obtained by PCR with primers UMS-125 and UMA226 were 403 bp (404 bp for serovar 6) for biovar 1 and 448 bp for biovar 2; those obtained with primers UMS51 and UMA427 were 447 bp for biovar 1 only. Both primer sets allowed a clear distinction between biovar 1 (serovars 1, 3, 6, and 14) and biovar 2 (serovars 2, 4, 5, 7, 8, 9, 10, 11, 12, and 13).
Cloning and sequencing.
At least two positive clones were identified for each of the amplified fragments of the 5′ ends of the MBA genes of all four serovars of U. urealyticum biovar 1, and at least one was chosen for sequencing. The other amplified fragments were directly sequenced from two directions. The sequences of amplicons from clinical isolates of serovars 1, 6, and 14 were identical to those of the ATCC reference strains.
Comparison of the 5′ end sequences of MBA genes and differentiation among serovars by PCR and restriction enzyme analysis.
The sequences of the MBA genes of U. urealyticum serovars 1, 3, 6, and 14 were compared with the published sequence for serovar 3 by using multiple sequence alignment, as shown in Fig. 2. The primer sequences and the restriction sites for relevant enzymes are shown. The sequence of the 5′ end of the U. urealyticum serovar 3 MBA gene was identical with the previously published sequence (22). The sequence of the serovar 14 MBA gene was different from that of serovar 3 at only three sites. Differences between the sequences of the 5′ ends of the serovar 3, 14, 1, and 6 MBA genes occurred at 37 sites, as shown in Table 1.
FIG. 2.
Multiple sequence alignment of the 5′ ends of MBA gene sequences of U. urealyticum serovars 1, 3, 6, and 14 (ATCC strains). Relevant primers (arrows) and restriction enzyme sites (bars) are shown.
TABLE 1.
Comparative analysis of the 5′ end sequences of MBA genes among four serovars of U. urealyticum biovar 1
| Site | Base
|
|||
|---|---|---|---|---|
| Serovar 3 | Serovar 14 | Serovar 1 | Serovar 6 | |
| −103 | G | G | G | A |
| −100 | G | G | G | A |
| −84 | A | A | G | A |
| −83 | C | A | C | A |
| −82 | C | T | T | T |
| −56 | A | A | A | T |
| −55 | T | T | C | A |
| −54 | A | A | A | G |
| −46 | T | |||
| −41 | A | A | G | A |
| −6 | T | T | T | C |
| 29 | G | T | G | G |
| 45 | T | T | A | A |
| 81 | A | A | A | G |
| 102 | T | T | C | T |
| 114 | G | G | A | G |
| 122 | A | A | A | G |
| 131 | C | C | C | T |
| 144 | C | C | C | T |
| 146 | G | G | A | A |
| 162 | G | G | G | A |
| 196 | A | A | G | G |
| 199 | G | G | A | A |
| 218 | G | G | A | A |
| 220 | A | A | G | G |
| 251 | C | C | T | T |
| 269 | G | G | A | A |
| 271 | A | A | C | C |
| 272 | C | C | T | T |
| 274 | C | C | G | G |
| 277 | G | G | A | A |
| 278 | A | A | C | C |
| 291 | A | A | G | G |
| 359 | G | G | A | A |
| 370 | T | T | C | T |
| 387 | T | T | C | C |
| 390 | G | G | G | A |
Individual differences that could be used for restriction enzyme analysis were identified. Serovar 6 differed from serovar 1 and from serovars 3/14 in having a base change from T to C at position −6, with loss of the HinfI restriction enzyme site. This difference was used to distinguish serovars 6 and 1 after amplification with primers UMS-125 and UMA269′ and treatment with HinfI. The PCR products of serovar 1, but not those of serovar 6, were cut by HinfI (Fig. 3).
FIG. 3.
Results of PCR amplification of MBA genes with primers UMS-125 and UMA269′ followed by restriction enzyme analysis with HinfI. Lanes M, molecular weight markers ΦX174 DNA/HaeIII; lanes 1 and 2, U. urealyticum serovar 1 ATCC strain PCR products before and after digestion by HinfI; lanes 3 and 4, U. urealyticum serovar 1 clinical isolate PCR products before and after digestion by HinfI; lanes 5 and 6, U. urealyticum serovar 1 positive clinical specimen PCR products after digestion by HinfI; lanes 7 and 8, U. urealyticum serovar 6 ATCC strain PCR product before and after digestion by HinfI; lane 9, U. urealyticum serovar 6 clinical isolate PCR product after digestion by HinfI; lanes 10 to 13, PCR products of four clinical specimens digested by HinfI. Lanes 10 and 12 were identified as serovar 1, and lanes 11 and 13 were identified as serovar 6.
The 5′ ends of the MBA gene sequences of serovars 1 and 6 differed from those of serovars 3/14 at two restriction endonuclease sites. (i) At base 251, C in serovars 3/14 is replaced by T in serovars 1 and 6, with loss of the PvuII restriction enzyme site. DNA from serovars 1, 3, 6, and 14 was amplified with primers UMS51 and UMA427, and the products were compared before and after digestion with PvuII. The PCR products of serovars 3 and 14, but not those of serovars 1 and 6, were cut with PvuII. (ii) At bases 269 to 272, GTAC in serotypes 3/14 is replaced by ATCT in serovars 1 and 6, with loss of the RsaI restriction enzyme site. The presence of several differences in the sequences between bases 269 and 282 allowed us to design new primers, UMA269 and UMA269′; which were specific for serovars 3/14 and serovars 1 and 6, respectively, and which could be used with UMS-125 to distinguish these two serovar pairs by PCR. The sequence differences at −54, −55, and −56 also allowed us to design primer UMS-54, which was specific for serovar 6 and can be used with UMA269′ to differentiate serovar 6 from serovar 1.
The specificity of primers UMA269–UMA269′ and UMS-54 were tested with all 14 serovars of U. urealyticum, and the results are shown in Fig. 4, 5, and 6.
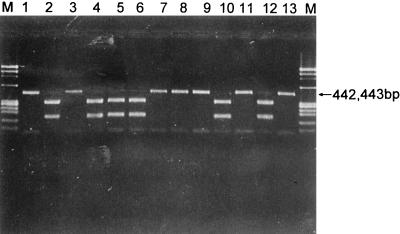
FIG. 4.
Results of PCR amplification of MBA genes of all 14 serovars of U. urealyticum with primers UMS-125 and UMA269 (specific for serovars 3/14). A positive result is shown by a 442-bp band. Lanes M, molecular weight markers ΦX174 DNA/HaeIII; lanes 1 and 2, U. urealyticum serovars 1 and 2 ATCC strains; lanes 3 and 4, U. urealyticum serovar 3 ATCC strain and clinical isolate; lanes 5 to 14, U. urealyticum serovars 4 to 13 ATCC strains; lanes 15 and 16, U. urealyticum serovar 14 ATCC strain and clinical isolate.
FIG. 5.
Results of PCR amplification of MBA genes of all 14 serovars of U. urealyticum with primers UMS-125 and UMA269′ (specific for serovars 1 and 6). A positive result is shown by a 442- or 443-bp band. Lanes M, molecular weight markers ΦX174 DNA/HaeIII; lanes 1 and 2, U. urealyticum serovar 1 ATCC strain and clinical isolate; lanes 3 to 6, U. urealyticum serovars 2 to 5 ATCC strains; lanes 7 and 8, U. urealyticum serovar 6 ATCC strain and clinical isolate; lanes 9 to 16, U. urealyticum serovars 7 to 14 ATCC strains.
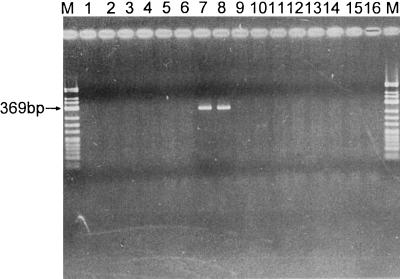
FIG. 6.
Results of PCR amplification of MBA genes of all 14 serovars of U. urealyticum with primers UMS-54 (specific for serovar 6) and UMA269′. A positive result is shown by a 369-bp band. Lanes M, molecular weight markers ΦX174 DNA/HinfI; lanes 1 and 2, U. urealyticum serovar 1 ATCC strain and clinical isolate; lanes 3 to 6, U. urealyticum serovars 2 to 5 ATCC strains; lanes 7 and 8, U. urealyticum serovar 6 ATCC strain and clinical isolate; lanes 9 to 16, U. urealyticum serovars 7 to 14 ATCC strains.
Detection, biotyping and serotyping of U. urealyticum biovar 1 from clinical specimens.
The results of direct PCR for detection and biotyping of U. urealyticum in clinical specimens are shown in Table 2. The U. urealyticum MBA gene was identified by PCR in 185 (46.3%) of 400 cervical swabs. Serovars belonging to biovar 1 only were detected in 151 swabs (37.8%), biovar 2 strains only were detected in 20 swabs (5.0%), and strains belonging to both biovars were detected in 14 swabs (3.5%). U. urealyticum was detected significantly more frequently in cervical swabs of pregnant women (58.0%) than in those of sex workers and other women attending the STD clinic (42.3%) (P < 0.01; odds ratio, 1.9; 95% confidence interval, 1.2 to 3.0).
TABLE 2.
Detection of U. urealyticum biovars 1 and 2 in cervical swabsa
| Subjects | No. (%) of swabs containing:
|
||||
|---|---|---|---|---|---|
| Total no. of swabs | U. urealyticum | Biovar 1 | Biovar 2 | Both biovars | |
| Sex workers | 200 | 87 (43.5) | 68 (34.0) | 8 (4.0) | 11 (5.5) |
| STD clinic clients | 100 | 40 | 31 | 7 | 2 |
| Antenatal clinic clients | 100 | 58 | 52 | 5 | 1 |
| Total | 400 | 185 (46.3) | 151 (37.8) | 20 (5.0) | 14 (3.5) |
Cervical swabs from women in three different groups were tested by PCR and restriction endonuclease analysis as outlined in Fig. 1. The proportion of women attending an antenatal clinic in whom any U. urealyticum strain was detected (58.0%) was significantly higher than that in the two groups of women attending the STD clinic (40.0%) (P < 0.01; odds ratio, 1.88; 95% confidence interval, 1.19 to 2.98). The difference remained significant for biovar 1 only (52.0% vs 31.0%; P < 0.01).
The serovars identified in 165 cervical swabs in which U. urealyticum biovar 1 was detected are shown in Table 3. Serovar 1 and serovars 3/14 were found most commonly (and in approximately equal numbers of specimens) in 77 (46.7%) and 76 (46.1%) specimens, respectively; serovar 6 was found in 42 (25.5%) positive specimens. A high proportion of specimens (22.2% overall) contained more than one serovar.
TABLE 3.
Serovars of U. urealyticum detected in 165 cervical swabs containing biovar 1
| Subjects | No. of swabs containing serovar(s):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3/14 | 6 | 1 and 3/14 | 1 and 6 | 3/14 and 6 | 1, 3/14, and 6 | Total | |
| Sex workers | 29 | 25 | 9 | 6 | 5 | 2 | 3 | 79 |
| STD clinic clients | 11 | 12 | 6 | 2 | 2 | 33 | ||
| Antenatal clinic clients | 13 | 22 | 11 | 3 | 3 | 1 | 53 | |
| Total (biovar 2)a | 53 (5) | 59 (3) | 26 (1) | 11 (2) | 10 (1) | 3 (1) | 3 (1) | 165 (14) |
Data in parentheses are specimens in which biovar 2 was found, in addition to one or more serovars of biovar 1.
DISCUSSION
The two biovars of U. urealyticum can be distinguished by various molecular techniques (5, 13, 14, 18), while serotyping by conventional methods remains difficult. Currently, there are no commercially available antisera, and cross-reactions were common even when monoclonal antisera were used (4, 9, 12). Faster and more specific serotyping methods are required to confirm the reported associations of particular serovars with diseases in which U. urealyticum has been implicated.
In this study we amplified the 5′ ends of the MBA genes of the four U. urealyticum serovars belonging to biovar 1. The amplified DNA was cloned into E. coli by using the plasmid pUC19. The clones were used to provide DNA for sequencing and have been used to express biovar-specific MBA genes for use in serological tests to measure biovar-specific antibody responses of patients with suspected U. urealyticum infections (to be published separately). We have shown that the 5′ ends of the MBA genes in serovars 3 and 14 are very similar, with base differences occurring only at three sites. We have demonstrated significant differences between serovars 3/14, 1, and 6 of biovar 1 and, based on these differences, we have used a combination of biovar-specific PCR, restriction enzyme analysis, and serovar-specific PCR to identify individual serovars of biovar 1. This allows rapid biotyping and serotyping of isolates and direct detection and serovar identification of U. urealyticum in clinical specimens, the majority of which belong to biovar 1.
To test the practical usefulness of our methods, genital specimens from three groups of women were tested. U. urealyticum was detected in nearly half (46.3%) of the cervical swabs tested, and of these, 89.2% contained biovar 1 and 18.3% contained biovar 2. Serovars 3/14 and 1 were each identified, alone or with other serovars, in nearly half of the specimens containing biovar 1, 22.2% of which contained more than one serovar. Pregnant women were significantly more likely to be colonized with U. urealyticum, and specifically, with serovar 1. This appeared to be attributable to a higher proportion of pregnant women in whom serovars 3/14 alone were found. Otherwise, there appeared to be no difference in the distribution of serovars between the different groups of subjects. The reason for this difference is uncertain, but it could be due to hormonal effects, which could increase ureaplasma counts, and thus the likelihood of detection, during pregnancy. The distribution of serovars is consistent with that found in other studies in which U. urealyticum isolates have been serotyped, using indirect-immunofluorescence tests, by antisera or monoclonal antibodies (4, 9, 23). For example, Naessens et al. showed that more than 90% of colonized women had isolates belonging to biovar 1, of which 52.2% were serovar 3, 30.3% were serovar 6, and 9.5% were serovar 1 (9).
Further investigation is needed to distinguish serovar 3 from serovar 14. It has recently been shown that there are differences in the 3′ ends of the MBA gene repetitive units of these serovars, demonstrating potential alternative sites of difference. In the future, biotyping and serotyping by direct use of biovar-specific and even serovar-specific primers will assist in the study of the pathogenesis and epidemiology of U. urealyticum infections, which until now has been restricted by the practical difficulties of conventional methods.
ACKNOWLEDGMENTS
We thank Xueqian Gong and Shouyi Chen for their precious help in cloning and sequencing and Greg James, Peter Jelfs, and Zhenfang Ma for their assistance with this project.
REFERENCES
- 1.Abele-Horn M, Peters J, Genzel-Boroviczeny O, Wolff C, Zimmermann A, Gottschling W. Vaginal Ureaplasma urealyticum colonization: influence on pregnancy outcome and neonatal morbidity. Infection. 1997;25:286–291. doi: 10.1007/BF01720398. [DOI] [PubMed] [Google Scholar]
- 2.Abele-Horn M, Wolff C, Dressel P, Pfaff F, Zimmerman A. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J Clin Microbiol. 1997;35:1199–1202. doi: 10.1128/jcm.35.5.1199-1202.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassell G H, Crouse D T, Waites K B, Rudd P T, Davis J K. Does Ureaplasma urealyticum cause respiratory disease in newborns? Pediatr Infect Dis J. 1988;7:535–541. [PubMed] [Google Scholar]
- 4.Cheng X, Naessens A, Lauwers S. Identification of serotype 1-, 3-, and 6-specific antigens of Ureaplasma urealyticum by using monoclonal antibodies. J Clin Microbiol. 1994;32:1060–1062. doi: 10.1128/jcm.32.4.1060-1062.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grattard F, Pozzetto B, de Barbeyrac B, Renaudin H, Clerc M, Gaudin O G, Bebear C. Arbitrarily-primed PCR confirms the differentiation of strains of Ureaplasma urealyticum to two biovars. Mol Cell Probes. 1995;9:383–389. doi: 10.1006/mcpr.1995.0060. [DOI] [PubMed] [Google Scholar]
- 6.Grattard F, Soleihac B, de Barbeyrac B, Bebear C, Seffert P, Pozzetto B. Epidemiologic and molecular investigations of genital mycoplasmas from women and neonates at delivery. Pediatr Infect Dis J. 1995;14:853–858. doi: 10.1097/00006454-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kong F, Zhu X, Zhou J. Grouping and typing of Ureaplasma urealyticum. Chung-Hua I Hsueh Tsa Chih. 1996;76:138–140. [PubMed] [Google Scholar]
- 8.Krieger J N, Boatman E S, Kenny G E. Ureaplasma urealyticum upper urinary tract infection: persistence and pathogenicity in a canine model. J Urol. 1989;141:1437–1443. doi: 10.1016/s0022-5347(17)41341-3. [DOI] [PubMed] [Google Scholar]
- 9.Naessens A, Foulon W, Breynaert J, Lauwers S. Serotype of Ureaplasma urealyticum isolated from normal pregnant women and patients with pregnancy complications. J Clin Microbiol. 1988;26:319–322. doi: 10.1128/jcm.26.2.319-322.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkawa M, Yamaguchi K, Tokunaga S, Nakashima T, Fujita S. Ureaplasma urealyticum in the urogenital tract of patients with chronic prostatitis or related symptomatology. Br J Urol. 1993;72:918–921. doi: 10.1111/j.1464-410x.1993.tb16297.x. [DOI] [PubMed] [Google Scholar]
- 11.Razin S. DNA probes and PCR in diagnosis of mycoplasma infections. Mol Cell Probes. 1994;8:497–511. doi: 10.1006/mcpr.1994.1071. [DOI] [PubMed] [Google Scholar]
- 12.Robertson J A, Stemke G W. Expanded serotyping scheme for Ureaplasma urealyticum strains isolated from humans. J Clin Microbiol. 1982;15:873–878. doi: 10.1128/jcm.15.5.873-878.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson J A, Vekris A, Bebear C, Stemke G W. Polymerase chain reaction using 16S rRNA gene sequences distinguishes the two biovars of Ureaplasma urealyticum. J Clin Microbiol. 1993;31:824–830. doi: 10.1128/jcm.31.4.824-830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruifu Y, Minli Z, Guo Z, Wang X. Biovar diversity is reflected by variations of genes encoding urease of Ureaplasma urealyticum. Microbiol Immunol. 1997;41:625–627. doi: 10.1111/j.1348-0421.1997.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor-Robinson D, Furr P M. Genital mycoplasma infections. Wien Klin Wochenschr. 1997;109:578–583. [PubMed] [Google Scholar]
- 16.Taylor-Robinson D, Furr P M, Webster A D. Ureaplasma urealyticum causing persistent urethritis in a patient with hypogammaglobulinaemia. Genitourin Med. 1985;61:404–408. doi: 10.1136/sti.61.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng K, Li M, Yu W, Li H, Shen D, Liu D. Comparison of PCR with culture for detection of Ureaplasma urealyticum in clinical samples from patients with urogenital infections. J Clin Microbiol. 1994;32:2232–2234. doi: 10.1128/jcm.32.9.2232-2234.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng L J, Ho S W, Ho H N, Liaw S J, Lai H C, Luh K T. Rapid detection and biovar differentiation of Ureaplasma urealyticum in clinical specimens by PCR. J Formos Med Assoc. 1995;94:396–400. [PubMed] [Google Scholar]
- 19.Teng L J, Zheng X, Glass J I, Watson H L, Tsai J, Cassell G H. Ureaplasma urealyticum biovar specificity and diversity are encoded in multiple-banded antigen gene. J Clin Microbiol. 1994;32:1464–1469. doi: 10.1128/jcm.32.6.1464-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thirkell D, Myles A D, Taylor-Robinson D. A comparison of four major antigens in five human and several animal strains of ureaplasmas. J Med Microbiol. 1990;32:163–168. doi: 10.1099/00222615-32-3-163. [DOI] [PubMed] [Google Scholar]
- 21.Watson H L, Blalock D K, Cassell G H. Variable antigens of Ureaplasma urealyticum containing both serovar-specific and serovar-cross-reactive epitopes. Infect Immun. 1990;58:3679–3688. doi: 10.1128/iai.58.11.3679-3688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Teng L J, Watson H L, Glass J I, Blanchard A, Cassell G H. Small repeating units within the Ureaplasma urealyticum MB antigen gene encode serovar specificity and are associated with antigen size variation. Infect Immun. 1995;63:891–898. doi: 10.1128/iai.63.3.891-898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X, Watson H L, Waites K B, Cassell G H. Serotype diversity and antigen variation among invasive isolates of Ureaplasma urealyticum from neonates. Infect Immun. 1992;60:3472–3474. doi: 10.1128/iai.60.8.3472-3474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]