ABSTRACT
Synaptic strength is altered during synaptic plasticity by controlling the number of AMPA receptors (AMPARs) at excitatory synapses. During long-term potentiation and synaptic upscaling, AMPARs are accumulated at synapses to increase synaptic strength. Neuronal activity leads to phosphorylation of AMPAR subunit GluA1 (also known as GRIA1) and subsequent elevation of GluA1 surface expression, either by an increase in receptor forward trafficking to the synaptic membrane or a decrease in receptor internalization. However, the molecular pathways underlying GluA1 phosphorylation-induced elevation of surface AMPAR expression are not completely understood. Here, we employ fluorescence recovery after photobleaching (FRAP) to reveal that phosphorylation of GluA1 serine 845 (S845) predominantly plays a role in receptor internalization, rather than forward trafficking, during synaptic plasticity. Notably, internalization of AMPARs depends upon the clathrin adaptor AP2, which recruits cargo proteins into endocytic clathrin-coated pits. In fact, we further reveal that an increase in GluA1 S845 phosphorylation upon two distinct forms of synaptic plasticity diminishes the binding of the AP2 adaptor, reducing internalization and resulting in elevation of GluA1 surface expression. We thus demonstrate a mechanism of GluA1 phosphorylation-regulated clathrin-mediated internalization of AMPARs.
KEY WORDS: AMPA receptor, GluA1, Internalization, Phosphorylation, Synaptic plasticity
Summary: During synaptic plasticity to enhance synaptic strength, phosphorylation of AMPA receptor subunit GluA1 at serine 845 reduces receptor internalization by inhibiting interactions with endocytic proteins.
INTRODUCTION
Tetrameric α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate-type glutamate receptors (AMPARs) mediate the majority of the fast excitatory synaptic transmission in the mammalian central nervous system (Diering and Huganir, 2018). AMPARs are formed by the assembly of subunits GluA1–GluA4 (also known as GRIA1–GRIA4) in different combinations together with several auxiliary proteins that play critical roles in the receptor kinetics and trafficking patterns (Diering and Huganir, 2018; Greger et al., 2017; Henley and Wilkinson, 2016; Pick and Ziff, 2018). Regulation of AMPAR function is highly dynamic in many different forms of synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD), as well as homeostatic synaptic plasticity (Diering and Huganir, 2018). A major part of these forms of synaptic plasticity is the trafficking of AMPARs from or to synapses to decrease or increase the number of AMPARs localized at synapses, which modulates the strength of synaptic activity (Hanley, 2018).
Although a large group of AMPAR auxiliary subunits can provide heterogeneity of AMPAR trafficking (Greger et al., 2017), activity-dependent receptor trafficking has long been known to be regulated by the phosphorylation of GluA1, mainly in a two-step process (Diering and Huganir, 2018; Pick and Ziff, 2018). First, phosphorylation of serine 845 (S845) in GluA1 is mediated by cAMP-dependent protein kinase A (PKA) or cGMP-dependent protein kinase II (cGKII, also known as PRKG2) (Derkach et al., 2007; Roche et al., 1996; Serulle et al., 2007). Importantly, GluA1 S845 phosphorylation promotes GluA1 surface expression, increases channel open-probability, and mediates several forms of synaptic plasticity, including LTP and synaptic upscaling (Banke et al., 2000; Diering et al., 2014; Diering and Huganir, 2018; Ehlers, 2000; Esteban et al., 2003; Kim et al., 2015; Kim and Ziff, 2014; Lee et al., 2000, 2003; Man et al., 2007; Oh et al., 2006). In contrast, calcineurin-mediated dephosphorylation of S845 is involved in receptor internalization during LTD and synaptic downscaling (Banke et al., 2000; Diering et al., 2014; Diering and Huganir, 2018; Ehlers, 2000; Esteban et al., 2003; Kim et al., 2015; Kim and Ziff, 2014; Lee et al., 2000, 2003; Man et al., 2007; Oh et al., 2006). Second, when GluA1 is additionally phosphorylated at serine 831 (S831) by Ca2+/calmodulin-dependent protein kinase II (CaMKII) or protein kinase C (PKC), the single-channel conductance is elevated, contributing to the enhanced synaptic transmission following LTP induction, and GluA1-containing AMPARs are targeted to the postsynaptic density (PSD) (Banke et al., 2000; Barria et al., 1997; Derkach et al., 1999; Kristensen et al., 2011; Lee et al., 2000; Pick and Ziff, 2018). Therefore, cooperative phosphorylation of GluA1 plays important roles in AMPAR trafficking and function during synaptic plasticity.
Extensive studies have yielded inconsistent data on the role of GluA1 phosphorylation in AMPAR trafficking during synaptic plasticity. Nonetheless, the impairment of synaptic plasticity in mice, including by mutations that disrupt GluA1 phosphorylation, strongly supports a requirement for receptor phosphorylation for synaptic plasticity (Lee et al., 2003). However, it has also been proposed that LTP expression does not require the carboxyl tail of GluA1 (Granger et al., 2013). Furthermore, molecular mechanisms concerning the role of GluA1 phosphorylation in regulation of synaptic targeting and stabilization of AMPARs have not been fully understood. Importantly, LTP-inducing stimuli may promote AMPAR surface trafficking via S845 phosphorylation, supporting the idea that S845 phosphorylation has a direct role in GluA1-containing AMPAR forward trafficking to the synaptic membrane (Esteban et al., 2003; Lee et al., 2003). However, other studies reveal that LTD rather than LTP is strongly correlated with S845 dephosphorylation (Kameyama et al., 1998; Lee et al., 2000, 1998), indicating that S845 dephosphorylation is directly involved in LTD-induced internalization of GluA1-containing AMPARs (Lee et al., 2003). The latter idea is further supported by the finding that NMDA-induced AMPAR internalization correlates with the time of maximal dephosphorylation of S845, which is blocked by a calcineurin inhibitor (Ehlers, 2000). Moreover, it has been suggested that S845 phosphorylation stabilizes LTP by inhibiting internalization of recently inserted GluA1-containing receptors (Lee et al., 2003). Thus, GluA1 S845 phosphorylation may directly regulate AMPAR internalization rather than receptor forward trafficking, but the exact mechanisms underlying GluA1 S845 phosphorylation-mediated AMPAR internalization have not been investigated.
Activity-dependent internalization of AMPARs is mediated by the clathrin machinery (Ehlers, 2000; Hanley, 2018; Lee et al., 2002; Man et al., 2000; Parkinson and Hanley, 2018). Clathrin-mediated endocytosis (CME) is a multi-step process that requires several proteins to be recruited to specific membrane domains (Hanley, 2018; McMahon and Boucrot, 2011). The resulting protein complex changes the membrane geometry to create an invagination that leads to pit formation, ultimately resulting in scission of the formed vesicle from the plasma membrane by dynamin proteins (McMahon and Boucrot, 2011). A central player in this process is an adapter protein complex, AP2, which binds to cargo proteins, endocytic accessory proteins and clathrin (Fiuza et al., 2017; Kelly and Owen, 2011; Robinson, 2004; Traub, 2009). Significant progress has been made in identifying adaptor proteins responsible for regulating internalization of AMPARs (Hanley, 2018); however, most of these proteins are known to bind to GluA2 but not GluA1 (Fiuza et al., 2017; Hanley, 2018; Lee et al., 2002). For example, AP2 is known to bind to GluA2 at a KRMK (lysine-arginine-methionine-lysine) motif in the carboxyl terminus, which is located proximal to the transmembrane domain, and promotes AMPAR internalization (Lee et al., 2002). Moreover, protein interacting with C kinase 1 (PICK1), after interacting with the AP2 complex with GluA2, is recruited to clathrin-coated pits (Fiuza et al., 2017). This association is increased during NMDA receptor (NMDAR)-mediated LTD and is able to promote dynamin activation, promoting GluA2 internalization (Fiuza et al., 2017). Interestingly, GluA1 is known to interact with the AP2 complex (Lee et al., 2002). More importantly, GluA1 also contains the KRMK sequence, a candidate site for AP2 complex binding, in the carboxyl terminus (Lee et al., 2002). The KRMK sequence in GluA1 is separated by 14 amino acids from S831 and by 29 amino acids from S845 (Diering and Huganir, 2018). Significantly, S-nitrosylation of cysteine 875 increases the binding of AP2 to GluA1 (Selvakumar et al., 2013), suggesting that changes in structure of distal sites on the carboxyl terminus of GluA1 via posttranslational modifications can affect AP2 complex binding and receptor internalization. Thus, it is possible that S845 phosphorylation regulates the interaction between AP2 and GluA1. However, roles of the interaction between the KRMK sequence in GluA1 and the AP2 complex in AMPAR trafficking have not been completely investigated in depth. In particular, whether phosphorylation of GluA1 S845 can affect this interaction is not yet understood. Therefore, further clarification is needed to understand GluA1 endocytic mechanisms.
Here, we employ fluorescence recovery after photobleaching (FRAP) to show that phosphorylation of GluA1 S845 predominantly plays an important role in internalization rather than receptor forward trafficking. Moreover, we reveal that phosphorylation of GluA1 S845 promoted by two distinct pathways of synaptic plasticity, homeostatic upscaling and chemically induced LTP (cLTP), is sufficient to decrease the internalization rate of GluA1 via the reduction of GluA1 binding to the AP2 complex. This ultimately leads to an increase in GluA1-containing AMPAR surface expression. Our results thus provide a molecular mechanism for how GluA1 S845 phosphorylation regulates GluA1-containing AMPAR trafficking.
RESULTS
Receptor forward trafficking of GluA1 S845A is not disrupted compared with that of wild-type GluA1 during cLTP
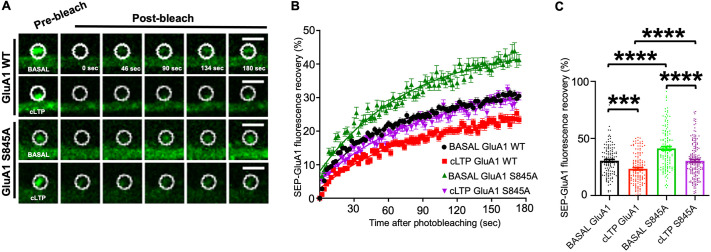
Increased AMPAR surface expression is mediated by either decreased internalization or increased receptor forward trafficking to the synaptic membrane. To distinguish between these mechanisms, we employed FRAP to determine whether GluA1 S845 phosphorylation played a critical role in surface insertion of GluA1-containing AMPARs. To elucidate surface delivery of GluA1-containing AMPARs, we expressed super-ecliptic pHluorin (SEP)-tagged GluA1, which exhibits stronger fluorescence when exposed to pH 7.4 in the extracellular space and is nearly nonfluorescent when exposed to the acidic environment of endosomes (Yudowski et al., 2007). We first examined FRAP of the SEP-tagged wild-type GluA1 (GluA1 WT) on spines in mouse cultured hippocampal neurons at 14 d in vitro (DIV) under basal conditions or following cLTP (Fig. 1A). Under basal conditions, we found ∼30% SEP fluorescence recovery of GluA1 WT at 3 min after photobleaching, whereas significantly lower recovery was found after cLTP (GluA1 WT basal, 30.47±1.13%; GluA1 WT cLTP, 23.39±1.10%; mean±s.e.m; P=0.0002) (Fig. 1B,C). Additionally, FRAP kinetics of SEP–GluA1 WT between these two conditions were significantly different (tau for GluA1 WT basal and GluA1 WT cLTP, 60.61±3.21 s and 79.22±5.88 s, respectively; P=0.006; half-time for GluA1 WT basal and GluA1 WT cLTP, 42.01±2.22 s and 54.91±4.08 s, respectively; P=0.006; mean±s.e.m) (Table S1). A previous study has shown that SEP–GluA1 WT expressed in cultured neurons is largely mobile on spines in the absence of activity (Makino and Malinow, 2009). However, GluA1 stays much longer on spines and becomes immobile following cLTP, thus there is much less space at the synapse for newly trafficked GluA1 (Makino and Malinow, 2009). Therefore, FRAP of SEP–GluA1 in the absence of LTP induction is significantly higher than the SEP–GluA1 recovery after LTP stimulation (Makino and Malinow, 2009). In fact, our data confirmed these findings; SEP–GluA1-containing AMPARs were incorporated into synapses and became immobile after cLTP induction. To further elucidate the direct role of S845 phosphorylation on GluA1 forward trafficking, we generated a mutant GluA1, in which serine (S) was replaced by alanine (A) at the 845 position (S845A) of the carboxyl terminus, which prevented GluA1 S845 from being phosphorylated (Lee, 2006; Lee et al., 2003). We then compared SEP–GluA1 WT and SEP–GluA1 S845A recovery after cLTP induction. SEP–GluA1 S845A showed higher recovery levels when compared to those of SEP–GluA1 WT following cLTP induction (GluA1 WT cLTP, 23.39±1.10%; GluA1 S845A cLTP, 30.42±1.16%; P<0.0001) (Fig. 1B,C). This indicates that a higher percentage of mutant receptors is mobile at synapses when compared to GluA1 WT-containing receptors. However, FRAP kinetics between the two conditions were not significantly different (tau for GluA1 WT cLTP and GluA1 S845A cLTP, 79.22±5.82 s and 89.17±6.81 s, respectively; P=0.27; half-time for GluA1 WT cLTP and GluA1 S845A cLTP, 54.91±4.08 s and 61.81±4.73 s, respectively; P=0.27) (Table S1). This demonstrates that forward trafficking of mutant GluA1 is not altered compared with that of GluA1 WT during cLTP. These results thus suggest that phosphorylation of GluA1 S845 is unlikely to be involved in GluA1 forward trafficking to the synaptic membrane during cLTP.
Fig. 1.
Receptor forward trafficking of GluA1 S845A is not disrupted compared with that of GluA1 WT during cLTP. (A) Representative images of FRAP experiments using SEP–GluA1 WT and SEP–GluA1 S845A under basal and cLTP conditions. Images depicted are before photobleaching; directly after photobleaching; and 44 s, 90 s, 134 s and 180 s after photobleaching for each condition. The photobleached ROI is shown as a white circle. Scale bars: 3 µm. (B) Representative normalized traces of FRAP for each experimental group. Each point represents one image acquired every 2 s. Recovery rates were corrected for internal photobleaching and background and were normalized to pre-photobleaching intensities, followed by one-phase association nonlinear fitting. (C) Representative bar graphs of SEP fluorescence recovery at 3 min after photobleaching for each experimental group. Each ROI represents a spine. Data in B and C are presented as mean±s.e.m. BASAL GluA1, n=128 spines (16 cells); cLTP GluA1, n=142 spines (12 cells); BASAL GluA1 S845A, n=137 spines (15 cells); cLTP GluA1 S845A, n=177 spines (18 cells). ***P<0.01, ****P<0.0001 (one-way ANOVA with uncorrected Fisher's LSD).
The GluA1 carboxyl terminal domain binds to β-adaptin via the KRMK motif
As our FRAP data revealed that GluA1 S845A mutant displayed normal receptor forward trafficking in cLTP conditions (Fig. 1), we examined the direct role of S845 phosphorylation in the binding of the AP2 complex to the GluA1 KRMK motif. This motif is known to play an important role in the binding of GluA2 to β-adaptin (AP2B1), the main subunit of the AP2 adaptor (Lee et al., 2002). We thus generated GST fusion proteins containing the GluA1 carboxyl terminal domain (CTD) and various mutants. We incubated glutathione beads conjugated to GST-tagged CTDs with rat brain cytosolic extracts in a GST pulldown assay. GST and GST–GluA2 CTD served as negative and positive controls, respectively. Both the GluA2 and GluA1 CTDs (GluA2C and GluA1C, respectively) were able to bind to β-adaptin, whereas the GST control was unable to interact with β-adaptin (GluA1C, 1.00; GST, 0.31±0.12, P=0.0011; GluA2, 1.69±0.27, P=0.0021; mean±s.e.m) (Fig. 2), which was consistent with previous reports (Lee et al., 2002). We generated a GluA1C mutant in which serine 845 of GluA1 was replaced with a phosphomimetic aspartate (S845D). Crucially, binding between GluA1C S845D and β-adaptin was significantly reduced (GluA1C, 1.000; GluA1C S845D, 0.33±0.07, P=0.0015) (Fig. 2), confirming that GluA1 S845 phosphorylation was sufficient to reduce binding of the AP2 complex, which would result in a decrease in GluA1 internalization. We generated additional mutants of the KRMK motif in GluA1C (K819A, R820A, M821A and K822A; residue numbers refer to full-length GluA1) to determine whether the KRMK motif in GluA1 was required for β-adaptin binding. The GST pulldown assay revealed that three mutants, K819A, M821A and K822A, bound to β-adaptin significantly less than GluA1C, whereas R820A was able to interact with β-adaptin similarly to GluA1C (GluA1C, 1.00; K819A, 0.48±0.19, P=0.0165; R820A, 0.70±0.20, P=0.1167; M821A, 0.33±0.08, P=0.0014; and K822A, 0.40±0.11, P=0.0039) (Fig. 2), indicating that K819, M821 and K822 in GluA1 are required for interaction with β-adaptin, which is similar to their role in GluA2.
Fig. 2.

Interaction of β-adaptin with GluA1 via the KRMK motif. GST pulldown assay showing binding of β-adaptin in brain cytosolic extracts to GST fusion proteins of the cytoplasmic tails of GluA1 (GluA1C) and the indicated KRMK motif mutants. GST and GluA2C served as negative and positive controls for β-adaptin pulldown, respectively. Coomassie staining shows the amount of GST fusion proteins bound to beads in each lane (KD, kilodalton). Quantification of mean β-adaptin signal intensity is shown below as the mean±s.e.m. of n=4 different experiments (a.u., arbitrary units). *P<0.05, **P<0.01 (one-way ANOVA with uncorrected Fisher's LSD).
cLTP and synaptic upscaling decrease β-adaptin binding to GluA1 via an increase in GluA1 S845 phosphorylation
We addressed whether GluA1 S845 phosphorylation regulates the interaction of GluA1 with β-adaptin, which contributes to receptor internalization. First, co-immunoprecipitation (co-IP) analysis using cell lysates of DIV14 cultured rat cortical neurons confirmed that β-adaptin was able to bind to endogenous GluA1, and this binding was significantly enhanced when we blocked receptor internalization by treating cells with 1 μM Dynole, an inhibitor of dynamin, to inhibit the scission of vesicles from the membrane (control, 1.00; Dynole, 1.46±0.14; mean±s.e.m; P=0.0279) (Fig. 3A). This revealed that GluA1, similar to GluA2, interacted with β-adaptin for receptor internalization. To further examine whether synaptic plasticity that elevated GluA1 S845 phosphorylation and subsequently increased GluA1 surface expression was able to modify the binding of β-adaptin to GluA1, we employed the same cLTP protocol used in the FRAP experiments (Fig. 1) and carried out co-IP experiments in DIV14 cultured neurons. We confirmed that cLTP significantly increased GluA1 S845 phosphorylation (control, 1.00; cLTP, 5.56±0.84; P=0.0002) (Fig. 3B), as seen previously (Diering et al., 2016; Roberts et al., 2021). We observed a significant decrease in the binding of β-adaptin to GluA1 in response to cLTP induction (control, 1.00; cLTP, 0.24±0.08; P<0.0001) (Fig. 3C). We further explored the role of GluA1 phosphorylation and its interaction with β-adaptin in another form of synaptic plasticity. Whereas LTP increases the activity of individual synapses, during homeostatic synaptic plasticity there is a global increase in synaptic strength (Galanis and Vlachos, 2020). We induced synaptic upscaling by chronically inhibiting neuronal activity, a well-established protocol to increase GluA1 S845 phosphorylation and subsequently elevate surface expression (Diering et al., 2014; Kim and Ziff, 2014). For chronic inhibition of neuronal activity, we treated neurons with 2 μM tetrodotoxin (TTX) for 48 h and confirmed that synaptic GluA1 S845 phosphorylation was significantly elevated (control, 1.00; TTX, 1.84±0.23; P=0.008) (Fig. 3D), as seen previously (Diering et al., 2014; Kim and Ziff, 2014). We then examined the effect of TTX treatments on the binding of GluA1 to β-adaptin. We treated neurons with 2 μM TTX for 48 h and measured the interaction of GluA1 with β-adaptin in co-IP experiments. Our results showed that TTX treatment was sufficient to decrease the binding of β-adaptin to GluA1 (control, 1.00; TTX, 0.44±0.12; P=0.009) (Fig. 3E). To further examine whether reduced binding of β-adaptin following synaptic upscaling promoted reduced GluA1 internalization, we treated DIV14 rat cultured hippocampal neurons with 2 μM TTX for 48 h and measured the surface expression of GluA1 and the rate of GluA1 internalization (Fig. 4A). As shown previously (Diering et al., 2014; Kim and Ziff, 2014), TTX treatment was sufficient to increase GluA1 surface expression (control, 1.00; TTX, 1.18±0.04; mean±s.e.m.; P=0.0076) (Fig. 4B). By using an antibody-based live staining and feeding protocol to label the surface GluA1 and internalized GluA1, we revealed that the rate of GluA1 internalization was significantly decreased after TTX treatment (control, 1.00; TTX, 0.63±0.02; P<0.0001) (Fig. 4C). These results confirmed that GluA1 surface accumulation following synaptic upscaling was mediated by a reduced rate of GluA1 internalization. Taken together, our results suggest that during cLTP and synaptic upscaling, an increase in S845 phosphorylation significantly reduces binding of the AP2 adaptor to GluA1, which decreases the internalization rate, ultimately contributing to increased surface expression of GluA1-containing AMPARs, a cellular mechanism to strengthen synaptic activity during synaptic plasticity.
Fig. 3.
cLTP- and TTX-induced up scaling treatments increase phosphorylation of GluA1 S845 and reduce binding of β-adaptin to GluA1. (A) Representative immunoblots and quantitative analysis of co-IP of GluA1 with β-adaptin from cultured cortical neurons in the presence or absence of inhibition of dynamin by Dynole (1 µM for 30 min). Mean±s.e.m. of n=3 different cultures. *P<0.05 (unpaired two-tailed Student’s t-test). (B) Representative immunoblots and quantitative analysis of GluA1 S845 phosphorylation (pGluA1-S845) with or without cLTP induction. Mean±s.e.m. of n=7 different cultures. ***P<0.001 (unpaired two-tailed Student's t-test). (C) Representative immunoblots and quantitative analysis of co-IP of GluA1 with β-adaptin from cultured cortical neurons with and without cLTP induction. Mean±s.e.m. of n=4 different cultures. ****P<0.0001 (unpaired two-tailed Student's t-test). (D) Representative immunoblots and quantitative analysis of GluA1 S845 phosphorylation in control neurons, or neurons treated with either TTX or FK506. Mean±s.e.m. of n=15 western blots using samples from 7 different cultures. **P<0.01, ****P<0.0001 (one-way ANOVA with uncorrected Fisher's LSD). (E) Representative immunoblots and quantitative analysis of co-IP of GluA1 from cultured cortical neurons showing that TTX treatment (2 µM for 48 h) and FK506 treatment (5 µM for 48 h) decrease β-adaptin binding to GluA1. Mean±s.e.m. of n=3 different cultures. **P<0.01 (one-way ANOVA with uncorrected Fisher's LSD). GluA1 (A–C,E) and actin (D) are shown as loading controls used for normalization. Molecular mass markers are indicated in kDa.
Fig. 4.
TTX treatment increases GluA1 surface expression and decreases GluA1 internalization. (A) Neurons were treated with TTX for 48 h (TTX) or left untreated (control) and then incubated with a rabbit anti-GluA1 extracellular domain antibody for 15 min at 37°C to allow the formation and internalization of antibody–GluA1 complexes. Antibody complexes remaining on the surface were removed by a brief acidic wash, neurons were fixed, and surface receptors were relabeled using a mouse anti-GluA1 extracellular domain antibody. Cells were permeabilized, and surface and internalized antibody–GluA1 complexes were differentially stained using fluorescein- and rhodamine-labeled secondary antibodies (raised in donkey), respectively. Green, surface receptors; red, internalized receptors; blue, MAP2-positive dendrites (MAPII). Scale bars: 100 μm (upper panels), 20 μm (bottom panels). Quantification of (B) relative surface and (C) internalized GluA1-containing AMPAR levels in the presence or absence of TTX treatment. Mean±s.e.m. of n=4 different cultures, average of 10 neurons imaged per culture. **P<0.01, ****P<0.0001 (unpaired two-tailed Student’s t-tests).
Kinase and phosphatase activity-mediated regulation of GluA1 S845 phosphorylation plays important roles in the interaction between β-adaptin and GluA1
We directly compared the interaction of β-adaptin with GluA1 WT and GluA1 S845A in neurons. DIV14–17 cultured rat cortical neurons were infected with Sindbis virus expressing HA–GluA1 WT or HA–GluA1 S845A for 16 h, and we performed co-IP with an antibody against the HA tag. We observed that the β-adaptin interaction with HA–GluA1 S845A was significantly higher than that with HA–GluA1 WT (HA–GluA1 WT, 1.00; HA–GluA1 S845A, 4.39±1.48; mean±s.e.m; P=0.0298) (Fig. 5A), further implicating GluA1 S845 phosphorylation in the regulation of AP2 binding to GluA1. To further explore the role of GluA1 S845 phosphorylation on the binding of AP2 to GluA1, we treated DIV14–17 cortical cultures with an activator of PKA, 8-bromo-cAMP (8-Br-cAMP). We found that PKA activation with 500 μM 8-Br-cAMP, a condition that has been previously shown to significantly increase GluA1 S845 phosphorylation (Serulle et al., 2007), decreased the binding of β-adaptin to GluA1 (control, 1.00; 8-Br-cAMP 0.78±0.07; P=0.0218) (Fig. 5B). Additionally, we employed another protocol to elevate GluA1 S845 phosphorylation and surface expression by inhibiting calcineurin, a phosphatase that is known to dephosphorylate GluA1 and reduce surface GluA1 expression by promoting receptor internalization (D'Amelio et al., 2011; Kim and Ziff, 2014). We treated neurons with the calcineurin inhibitor FK506 at 5 μM for 12 h, a condition that increases GluA1 S845 phosphorylation and surface expression in cultured neurons (Kim and Ziff, 2014), and confirmed that FK506 treatment was sufficient to increase GluA1 S845 phosphorylation (control, 1.00; FK506 2.63±0.311; P<0.0001) (Fig. 3D), as seen previously (Diering et al., 2014; Kim and Ziff, 2014). We then measured the interaction of GluA1 with β-adaptin using co-IP. As expected, reduced calcineurin activity was capable of significantly decreasing β-adaptin binding to GluA1 (control, 1.00; FK506, 0.48±0.09; P=0.004) (Fig. 3E). Taken together, our results suggest that kinase and phosphatase activity-mediated regulation of GluA1 S845 phosphorylation plays important roles in the interaction between β-adaptin and GluA1, ultimately contributing to the level of GluA1-containing AMPAR surface expression.
Fig. 5.

GluA1 S845A shows reduced β-adaptin binding. (A) Representative immunoblots and quantitative analysis of co-IP of HA–GluA1 WT and HA–GluA1 S845A with β-adaptin virally expressed in cultured cortical neurons. Mean±s.e.m. of n=6 different cultures. (B) Representative immunoblots and quantitative analysis of co-IP of GluA1 with β-adaptin from cultured cortical neurons in the presence or absence of PKA activation by 8-Br-cAMP. Mean±s.e.m. of n=3 different cultures. GluA1 is shown as a loading control used for normalization. Molecular mass markers are indicated in kDa. *P<0.05 (unpaired two-tailed Student's t-test).
GluA1 S831 phosphorylation might not be involved in the interaction between β-adaptin and GluA1
GluA1 S831 is known to be phosphorylated by both PKC and CaMKII, which promotes GluA1 targeting to the PSD; thus, GluA1 S831 phosphorylation is also important for AMPAR surface expression and synaptic plasticity (Diering and Huganir, 2018). We therefore examined whether GluA1 S831 phosphorylation regulated the interaction of GluA1 with β-adaptin in cultured rat cortical neurons. Similar to GluA1 S845 phosphorylation, cLTP induction was sufficient to significantly increase GluA1 S831 phosphorylation (control, 1.00; cLTP, 4.65±0.96; mean±s.e.m; P=0.0016) (Fig. S1A). Interestingly, synaptic upscaling by TTX treatment was unable to alter GluA1 S831 phosphorylation (control, 1.00; TTX, 0.63±0.08; P=0.2606) (Fig. S1B), which was consistent with previous findings (Diering et al., 2014). In contrast, pharmacological inhibition of calcineurin activity by treating neurons with FK506 significantly elevated GluA1 S831 phosphorylation (control, 1.00; FK506, 1.75±0.26; P=0.007) (Fig. S1B). These results suggest that GluA1 S831 phosphorylation is important for cLTP, but it is unlikely to play critical roles in synaptic upscaling. To determine whether GluA1 S831 phosphorylation regulated β-adaptin binding to GluA1, we generated a GluA1 S831A mutant, infected cortical cells with Sindbis virus expressing HA–GluA1 S831A, and assayed β-adaptin binding to this mutant using co-IP. We observed that β-adaptin interaction with HA–GluA1 S831A was unaltered when compared to the interaction with HA–GluA1 WT (HA–GluA1 WT, 1.00; HA–GluA1 S831A, 0.79±0.14; P=0.2276) (Fig. S1C), suggesting that GluA1 S831 phosphorylation has no impact on GluA1 internalization under basal conditions. To further explore the role of GluA1 S831 phosphorylation on the binding of β-adaptin to GluA1, we treated DIV14–17 cortical cultures with an activator of PKC, phorbol ester (TPA). First, we treated neurons with 5 μM TPA for 10 min, which has been shown to significantly increase GluA1 S831 phosphorylation (Lee et al., 2007), and carried out co-IP. In contrast to the effect of the GluA1 S831A mutation, TPA treatment significantly decreased the binding of β-adaptin to GluA1 (control, 1.00; TPA, 0.41±0.07; P=0.0013) (Fig. S1D). This suggests that PKC activity can regulate the interaction between β-adaptin and GluA1, but this regulation is unlikely to be mediated by GluA1 S831 phosphorylation.
DISCUSSION
Synaptic plasticity is an activity-dependent alteration in synaptic strength by means of control of the number of AMPARs at synapses (Park, 2018). In particular, hippocampal synaptic plasticity has long been considered a synaptic correlate for learning and memory (Park, 2018). GluA1-containing AMPARs have been suggested to play an important role in hippocampal synaptic plasticity, including LTP and synaptic scaling (Díaz-Alonso et al., 2017; Diering et al., 2014; Hayashi et al., 2000; Jia et al., 1996; Kim and Ziff, 2014; Shi et al., 2001; Zamanillo et al., 1999). Importantly, the interplay between synaptic phosphorylation and dephosphorylation of GluA1 is central to regulation of AMPAR synaptic expression and synaptic plasticity (Diering and Huganir, 2018; Purkey and Dell'Acqua, 2020). During synaptic plasticity, synaptic strength can be enhanced by an increase in GluA1-containing AMPAR surface expression, which can be promoted by either increased receptor forward trafficking to the synaptic membrane or decreased internalization of the receptors. However, the molecular mechanisms of how AMPAR trafficking is regulated by GluA1 phosphorylation are not completely understood. In this study, our FRAP experiments demonstrate that the kinetics of cLTP-induced forward trafficking of both GluA1 WT- and mutant GluA1 S845A-containing AMPARs are similar, whereas the recovery of mutant GluA1 S845A-containing receptors is higher than that of GluA1 WT-containing receptors (Fig. 1; Table S1). Interestingly, under basal conditions, FRAP kinetics of GluA1 WT- and mutant GluA1 S845A-containing AMPARs are significantly different (Fig. 1; Table S1). This suggests that mutant GluA1 is largely mobile on spines compared with GluA1 WT under both conditions. Given that retention of GluA1 at the surface is likely due to protein interactions and clustering of the receptor by phosphorylation (Purkey and Dell'Acqua, 2020), retention of mutant GluA1 at the surface could be decreased, which would contribute to increased mobility of mutant GluA1-containing AMPARs. Taken together, our data suggest that GluA1 S845 phosphorylation is unlikely to have a major role in receptor forward trafficking, but in fact affects receptor internalization.
The requirement for the GluA1 CTD for LTP has been challenged by a report showing that LTP requires AMPAR trafficking, independent of subunit type (Granger et al., 2013). In addition, recent studies have demonstrated that the extracellular amino-terminal domains of AMPARs govern their trafficking for synaptic plasticity in a manner that is dependent on the AMPAR subunit type (Díaz-Alonso et al., 2017; Watson et al., 2017). Moreover, a study has reported that the levels of GluA1 phosphorylation are too low to regulate GluA1-dependent synaptic plasticity (Hosokawa et al., 2015). However, another study has demonstrated that significantly higher levels of GluA1-containing AMPARs are phosphorylated at either S845 or S831 under basal conditions (Diering et al., 2016). Nonetheless, LTP expression has been thought to be dependent on the rapid synaptic insertion of GluA1-containing AMPARs (Hayashi et al., 2000; Shi et al., 2001), whereas internalization of AMPARs is important for LTD expression (Beattie et al., 2002; Carroll et al., 1999; Ehlers, 2000; Lee et al., 2003; Man et al., 2000). A study using phosphomutant (S831A and S845A) GluA1 mice has demonstrated that LTP is not completely absent, but LTD is completely dependent on the capacity for GluA1 phosphorylation (Lee et al., 2003), supporting our idea that that GluA1 phosphorylation primarily regulates receptor internalization and is less important for receptor forward trafficking at excitatory synapses. Interestingly, PKA and calcineurin are targeted to GluA1 via the interaction with A-kinase anchoring protein 150 (AKAP150, also known as AKAP5) in the PSD of excitatory synapses, where they regulate GluA1 phosphorylation (Sanderson et al., 2012). A study using mutant AKAP150 lacking the calcineurin-anchoring motif also shows an increase in GluA1 S845 phosphorylation and the inhibition of LTD (Sanderson et al., 2012), emphasizing the role of S845 phosphorylation in receptor internalization. Additionally, AMPAR trafficking in neurons can be constitutive or activity-dependent during synaptic plasticity (Anggono and Huganir, 2012). However, roles in AMPAR internalization during LTP have not been fully investigated. A previous study suggests that AMPAR internalization in dendrites provides receptor pools for forward trafficking during LTP (Zheng et al., 2015). This thus suggests that both internalization and forward trafficking of AMPARs are associated with LTP, but the detailed mechanisms need to be investigated in further studies.
Our findings reveal that synaptic plasticity that increases synaptic strength, such as cLTP and synaptic upscaling, significantly increases GluA1 S845 phosphorylation by either increasing kinase activity or decreasing phosphatase activity (Fig. 3B,D), which in turn reduces the interaction between GluA1-containing AMPARs and β-adaptin (Fig. 3C,E), contributing to a decrease in receptor internalization (Fig. 4C). Ultimately, AMPAR surface expression is increased (Fig. 4B), thus synaptic strength is enhanced during this synaptic plasticity. Interestingly, we find that GluA1 S831 phosphorylation is unlikely to be involved in the interaction between GluA1 and β-adaptin (Fig. S1C); however, PKC activity may play another important role in GluA1-containing receptor internalization (Fig. S1D). In fact, PKC can phosphorylate serine 818 (S818), S831, and threonine 840 (T840) of the GluA1 CTD (Diering and Huganir, 2018). Importantly, PKC-mediated S818 phosphorylation increases GluA1 interaction with 4.1N (also known as EBP41L1), a cytoskeletal scaffold protein, which indirectly affects AMPAR surface retention and/or forward trafficking (Diering and Huganir, 2018; Lin et al., 2009). Therefore, it is possible that PKC can regulate GluA1 internalization by phosphorylating S818, which is located near to the KRMK motif, but not S831. Moreover, GluA1 internalization has been shown to be regulated by other forms of GluA1 CTD posttranslational modification, including nitrosylation and palmitoylation (Diering and Huganir, 2018; Lin et al., 2009; Man et al., 2000; Selvakumar et al., 2013; Widagdo et al., 2015). Therefore, our current report and previous studies demonstrate that internalization of GluA1-containing AMPARs is likely mediated by not only S845 phosphorylation but also additional posttranslational modifications of the GluA1 CTD.
Bidirectional changes in synaptic function are related to the reversible regulation of AMPAR phosphorylation (Diering et al., 2014; Lee et al., 2000). For example, high-frequency stimulation in hippocampal CA1 neurons increases phosphorylation of GluA1 S831, resulting in LTP, whereas low-frequency stimulation activates a phosphatase that dephosphorylates GluA1 S845, promoting LTD (Lee et al., 2000). Another study has demonstrated that the GluA1 S845A mutant is unable to display TTX-induced synaptic upscaling, but not bicuculline-induced synaptic downscaling (Diering et al., 2014). This suggests that the requirement for GluA1 S845 phosphorylation in regulating AMPAR trafficking is dependent on the type of synaptic plasticity.
GluA1 homomeric AMPARs are Ca2+ permeable, which plays important roles in several different types of synaptic plasticity, including homeostatic synaptic plasticity (Kim and Ziff, 2014), drug-related incubation of craving (Conrad et al., 2008) and NMDA receptor-mediated LTP (Barria et al., 1997; Diering and Huganir, 2018; Purkey and Dell'Acqua, 2020). However, the mechanism that enables the minority population of GluA2-lacking AMPA receptors to be accumulated at synapses during synaptic plasticity is not known. Interestingly, a study using mice specifically lacking phosphorylation of the GluA1 S845 site (GluA1 S845A mutant) has demonstrated that this phosphorylation is required for GluA1 homomeric AMPAR surface retention (He et al., 2009). Specifically, in GluA1 S845A mutant neurons, homomeric receptors are removed from synapses mainly due to clathrin-mediated internalization and subsequent lysosomal degradation (He et al., 2009). This could be explained by the inability of PKA to re-phosphorylate S845, an essential step for promoting the recycling of GluA1 back to the plasma membrane and maintaining the pool of surface AMPARs (Fernandez-Monreal et al., 2012). Therefore, our findings further provide a mechanism that explains how GluA1 homomers can accumulate at synapses during synaptic plasticity.
GluA1 S845 phosphorylation is also implicated in several brain disorders (Zhang and Abdullah, 2013). β-amyloid peptide (Aβ) has been recognized as a causative factor for the cognitive impairments in Alzheimer's disease (AD), and several studies have shown that Aβ leads to an increase in intracellular Ca2+ activity in cortical and hippocampal neurons (Brown et al., 2011; Busche et al., 2012, 2008; Harris et al., 2010; Hartley et al., 1999; Kuchibhotla et al., 2008; Liu et al., 2013; Minkeviciene et al., 2009; Palop et al., 2007; Palop and Mucke, 2009; Roberson et al., 2011; Sun et al., 2019; Verret et al., 2012). In particular, Aβ-induced Ca2+ hyperexcitation in hippocampal neurons stimulates the Ca2+/calmodulin-dependent protein phosphatase calcineurin, which dephosphorylates GluA1 S845 phosphorylation, enabling GluA1-containing AMPARs to be internalized from the plasma membrane (Lee et al., 1998; Roberts et al., 2021; Sanderson et al., 2012; Sun et al., 2019). This leads to a reduction of synaptic strength and ultimately disrupts synaptic plasticity and cognitive function in AD (Forner et al., 2017). Another example is cocaine craving, a cue-induced cocaine-seeking behavior that is intensified after withdrawal from chronic exposure to cocaine. The expression of incubated craving is mediated by GluA1 homomeric AMPARs in the nucleus accumbens (Conrad et al., 2008). In fact, elevated synaptic levels of GluA1 homomeric receptors result in part from an increase in GluA1 S845 phosphorylation (Ferrario et al., 2011). Furthermore, enhanced GluA1 S845 phosphorylation and surface expression play important roles in the antidepressant effects of ketamine (Zhang et al., 2016). Taken together, these findings indicate that GluA1 S845 phosphorylation has a unique role in various brain disorders and may serve a novel therapeutic target for these diseases.
Finally, we would like to note the limitations of our study. First, our study uses overexpression of various exogenous forms of GluA1, such as SEP–GluA1, GluA1 WT, GluA1 S845A and GluA1 S831A, in several experiments in addition to endogenous GluA1. Thus, it is still unknown how much endogenous GluA1 is involved in our experimental readouts. This limitation could be overcome by expressing exogenous GluA1 in GluA1-knockout neurons. Second, without electrophysiological data, our conclusions based on biochemical and cell biological data are limited.
MATERIALS AND METHODS
Expression vectors
Plasmids and viral vectors expressing HA–GluA1, GST–GluA2C and GST–GluA1C have been described previously (Greger et al., 2003; Osten et al., 2000; Srivastava et al., 1998). Rat sequences were used for GluA1 and GluA2. GST–GluA1C mutants were cloned by PCR and ligated into pGEX-4T1 (Pharmacia). The HA–GluA1 S831A and S845A mutants were generated using a QuikChange Site-Directed Mutagenesis kit (Agilent). SEP–GluA1 WT plasmid was a gift from Dr Thomas Blanpied at University of Maryland School of Medicine, Baltimore, MD, USA, and has been described previously (Kerr and Blanpied, 2012). SEP–GluA1 S845A was generated using a PCR-based QuikChange Site-Directed Mutagenesis kit (Agilent) according to the manufacturer's protocol. All mutants were confirmed by sequencing.
Antibodies
The following antibodies were used: purified mouse anti-β-adaptin clone 74/β-adaptin (RUO) (BD Biosciences, 610381); anti-glutamate receptor 1 antibody (Millipore, AB1504); anti-GluR1-NT antibody, clone RH95 (Millipore, MAB2263); anti-glutamate receptor 1 antibody, phosphoSer 845 (1:1000; Millipore, AB5849); anti-phospho-GluR1 (Ser831) antibody, clone N453, rabbit monoclonal (1:1000; Millipore, 04-823); anti-GluR1 antibody (Calbiochem, PC246); purified anti-MAP2 antibody (Biolegend, 822501; previously Covance, PCK-554P); HA-probe antibody (Y11) (Santa Cruz, sc-805); anti-actin antibody [ACTN05(C4)] (1:2000; Abcam, ab3280).
Viral production and neuronal infection
Baby hamster kidney (BHK) cells (Invitrogen) were electroporated with RNA of pSinRep5-HA–GluA1 or the C-terminal mutants and the helper DH (26S), according to the Sindbis Expression System manual (Invitrogen) and as previously described (Osten et al., 2000). The pseudovirion-containing medium was collected after 24 h, and the titer for each construct was tested empirically in neuronal cultures. For experimental expression, neurons were infected at DIV14–21 with a titer resulting in infection of 5–10% of neurons (typically 5–20 ml of a-MEM virus stock diluted in 600 ml conditioned NB-B27 medium per well of a 6-well dish). The infectious medium was applied for 16 h. Expression with no obvious adverse effects on morphology of the infected neurons was observed the next day. For immunoprecipitation experiments, neurons were cultured at 1 million cells per 6 cm dish and infected at DIV13 with 60–100 μl of virus stock in 3 ml of conditioned NB-B27 medium for 16 h.
Primary hippocampal and cortical neuronal culture
Primary rat hippocampal and cortical neuron cultures were prepared by a previously described protocol (Lu et al., 2014; Osten et al., 1998; Restituito et al., 2011). Animal experiments were conducted in compliance with the Institutional Animal Care and Use Committee at the New York University School of Medicine. The day before dissection, coverslips or 6 cm Petri dishes were coated with poly-L-lysine in boric acid buffer at 37°C overnight. Before dissection, coverslips or dishes were washed twice with phosphate-buffered saline (PBS) and stored in the incubator ready for plating neurons. Primary hippocampal and cortical neuron cultures were obtained from Sprague Dawley rat embryos at embryonic day (E)18–19. Pregnant rats were anesthetized with CO2, and embryos were then removed. Dissection was carried out in ice-cold PHG buffer (10 mM HEPES and 0.6% glucose in PBS, pH 7.35). After decapitation of the head, cortices and hippocampus were isolated under a dissection microscope in a sterile hood. Hippocampi and cortices were separately trypsinized for 15 min at 37°C, washed three times in PHG buffer, and then resuspended in 5 ml of plating medium [minimal essential medium (Invitrogen), 10% horse serum (Invitrogen), 0.45% glucose, 1 mM pyruvate, 1% penicillin-streptomycin (Invitrogen)] warmed to 37°C. Hippocampi and cortices were triturated with a 5 ml sterile pipette until the cell suspension appeared homogeneous, and cells were then counted with a hemocytometer. Cells were plated at a density of 120,000 per coverslip or 1,000,000 per 6 cm Petri dish in plating medium. 2–4 h after plating, all media were removed and replaced with Neurobasal medium supplemented with B27 supplement (Invitrogen), glutamine (500 μM) and antibiotics. Every 4 d, half of the volume of medium remaining on the cells was removed and replaced with fresh Neurobasal medium. Anti-glia growth drug (3 μM cytosine β-D-arabinofuranoside; Millipore Sigma) was usually added to coverslips into growth medium after DIV8.
For FRAP experiments, mouse hippocampal neuron cultures were prepared as described previously (Roberts et al., 2021; Sun et al., 2019; Sztukowski et al., 2018). Hippocampi were isolated from postnatal day 0 (P0) CD-1 mouse (Charles River) brain tissues and digested with 10 U/mL papain (Worthington Biochemical Corp., Lakewood, NJ). Mouse hippocampal neurons were plated on poly-lysine-coated glass-bottom dishes (500,000 cells), transfected using Lipofectamine 2000 (Invitrogen) with 2 µg DNA of SEP–GluA1 WT or SEP–GluA1 S845A on DIV4, and imaged on DIV14. Cells were grown in Neurobasal Medium (Life Technologies, Carlsbad, CA) with B27 supplement (Life Technologies, Carlsbad, CA), 0.5 mM Glutamax (Life Technologies) and 1% penicillin-streptomycin (Life Technologies). Colorado State University's Institutional Animal Care and Use Committee reviewed and approved the animal care and protocol (16-6779A).
Neuronal immunocytochemistry
Cells were initially treated with 2 µM TTX for 48 h before the experiment, as previously described (Pick et al., 2017). After drug treatment, DIV14–17 hippocampi cultures were stained live with an anti-GluA1 antibody (GluA1 Calbiochem PC246; rabbit; 1:20) for 15 min at 37°C in a humidified chamber, to allow for antibody binding and antibody–receptor complex internalization (endocytosed receptors). Cells were then washed with an acidic buffer (0.5 M NaCl and 0.2 M acetic acid in PBS) for stripping of remaining antibodies at the cell surface, followed by three quick washes in PBS and fixation for 10 min at room temperature using 4% paraformaldehyde (PFA) and 0.12 M sucrose in PBS. Cells were then incubated with a different anti-GluA1 antibody (Millipore, MAB2263; mouse; 1:500) to allow for staining of the remaining surface GluA1 (not endocytosed receptor). Cells were permeabilized for 5 min in 0.2% Triton X–100 and washed three times with PBS for 5 min at room temperature. Cells were blocked in 10% BSA in PBS for 1 h at room temperature and then incubated with an anti-MAP2 antibody diluted in 3% BSA (MAP2, chicken; 1:10,000; Biolegend) for 60 min at room temperature. Cells were washed three times in PBS with 5 min washes and incubated with secondary antibody diluted in 3% BSA for 60 min at room temperature. Secondary antibodies were conjugated to fluorophores (Alexa Fluor 488, Alexa Fluor 568 or Alexa Fluor 647) either from Molecular Probes (1:1000) or from Jackson ImmunoResearch (1:300). Cells were then washed three times with PBS for 5 min, mounted on coverslips, and stored. Immunofluorescence images were acquired on a Nikon PCM 2000 confocal microscope using a 60× objective. All images were acquired using the same settings for one experiment.
Fluorescence recovery after photobleaching
FRAP experiments were performed as previously described (Kim et al., 2016; Sole et al., 2019) using a spinning-disk confocal microscope based on a Yokogawa CSUX1 system built on an Olympus IX83 inverted stand coupled to an Andor laser launch containing 405, 488, 561 and 637 nm diode lasers, 100–150 mW each. Images were collected using a 60× Plan Apo N 1.4 NA objective and two iXon EMCCD cameras (DU-897, Andor). The system was equipped with the ZDC constant focus system and a Tokai Hit chamber and objective heater. The stage and objective were heated to 37°C. Photobleaching was performed as previously described (Kim et al., 2016; Sole et al., 2019) using the FRAPPA system (Andor). Images were acquired every 2 s during 3 min, and the recovery of SEP–GluA1 WT and SEP–GluA1 S845A fluorescence within the bleached region (elliptical, 10×10 pixels) was quantitated as described below.
Image analysis and quantitation
Images were analyzed using ImageJ software (NIH, Bethesda, MD). For immunocytochemistry, the areas of three dendrites on each image were manually defined by outlining the MAP2 signal, and the intensity of the fluorescence of surface GluA1 or endocytosed GluA1 was divided by total GluA1 intensity (the sum of surface GluA1 and endocytosed GluA1 fluorescence intensity) within the outlined dendrites. For FRAP, three regions of interest (ROIs) – the bleach region (BL), which was used to test for recovery; the background region (BG), which was used to correct for noise; and the reference region (REF), which was used to show internal photobleaching during acquisition – were measured. To eliminate noisy signals from the data and normalize corrected bleach to corrected reference,we used the following formula that has been used previously (Webster et al., 2015): FRAP=(BLt−BGt)/(REFt−BGt). All data was plotted in GraphPad Prism 9, and a one-phase association nonlinear fit was performed.
Co-immunoprecipitation
Cells were initially treated with drugs as described for each figure (TTX, Tocris Biosciences; FK506, Abcam; Dynole, Abcam; 8-Br-cAMP, Tocris Biosciences; and phorbol ester TPA, Millipore Sigma). Co-immunoprecipitation was carried out as described previously (Sathler et al., 2016), with some modifications. Briefly, DIV14–17 cortical cultures were collected, homogenized in 1 ml of radioimmune precipitation assay buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 10 mM EGTA, 10 mM EDTA, phosphatase inhibitor cocktails I and II (Sigma) and Protease Inhibitor Complete (Roche Applied Science, Indianapolis, IN, USA). For the input sample, 50 μl of protein lysate was removed, rocked for 60 min at 4°C, and boiled in an equal volume of 2× loading buffer. The immunoprecipitation was performed by incubating the protein lysate with 1 μg of anti-GluA1 antibody (Millipore, AB1504) or 1 μg anti-HA antibody (Santa Cruz, sc-805) for 16 h at 4°C followed by binding to Protein A Plus agarose beads (Santa Cruz) at 4°C for 60 min. Beads were then pelleted by centrifugation and washed three times in wash buffer (50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 5 mM EGTA and 0.1% Triton X-100), suspended in 1× loading buffer (30 μl) and boiled (immunoprecipitated sample). Equal volumes were loaded for western blotting, and all membranes were probed with anti-β-adaptin antibody (1:1000, BD Biosciences), and anti-GluA1 or anti-HA (1:1000) antibody to control for amount precipitated.
GST pulldown
GST fusion proteins were expressed and purified as described (Osten et al., 1998). GST, GST–GluA2C, GST–GluA1C or GST–GluA1C mutants (10 μg each) bound on Glutathione Sepharose (Pharmacia) beads were incubated with rat whole brain cytosolic fraction for 3 h at 4°C, as described previously with modifications (Osten et al., 1998). Beads were then pelleted by centrifugation and washed three times in wash buffer (20 mM HEPES, 150 mM NaCl and 0.1% Triton X-100), suspended in 1× loading buffer (30 μl), boiled and equal volumes were loaded for western blotting. The bottom half of the acrylamide gel was stained with Coomassie Blue, and the bands were used as loading control.
Chemically induced LTP protocol
cLTP protocol was followed as previously described (Roberts et al., 2021). Briefly, DIV14–17 cortical cultured neurons were washed three times in Mg2+-free buffer (150 mM NaCl, 2 mM CaCl2, 5 mM KCl, 10 mM HEPES, 30 mM glucose, 1 μM strychnine and 20 μM bicuculline) and incubated in glycine buffer (Mg2+-free buffer with 0.2 mM glycine) at 37°C for 5 min. Then, Mg2+ buffer (Mg2+-free buffer with 2 mM MgCl2) was added to block NMDARs, and cells were incubated at 37°C for 30 min before being processed for co-immunoprecipitation or FRAP.
Synaptosome fraction preparation
Synaptosomal preparation was carried out as described previously (Kim and Ziff, 2014). Cortical cells in 150 mm dishes were rinsed with PBS, collected to 15 ml tubes and spun at 700 g for 5 min at 4°C. The pellet was resuspended in solution A (320 mM sucrose, 1 mM NaHCO3, 1 mM MgCl2 and 0.5 mM CaCl2) and centrifuged at 1,400 g for 10 min at 4°C. Then the pellet (P1) was discarded, and the supernatant was centrifuged at 13,800 g for 20 min at 4°C. The pellet (P2) was homogenized in solution B (320 mM sucrose, 1 mM NaHCO3), placed on top of a 1 M sucrose and 1.2 M sucrose gradient, and centrifuged at 82,500 g for 2 h at 4°C. The resulting interface between the gradient was centrifuged at 200,000 g for 45 min at 4°C after a six-fold dilution with solution B. The pellet corresponds to the synaptosome fraction, which was resuspended in 2% SDS and 25 mM Tris. Protein content was measured by the BCA method (Thermo Scientific).
Statistics
All statistical comparisons were analyzed using GraphPad Prism 9. Unpaired two-tailed Student's t-tests were used in single comparisons. For multiple comparisons, we used one-way ANOVA followed by Fisher's Least Significant Difference (LSD) test to determine statistical significance. Results are represented as a mean±s.e.m., and P<0.05 is considered statistically significant.
Supplementary Material
Acknowledgements
We first thank Dr Thomas Blanpied at University of Maryland School of Medicine for the SEP–GluA1 WT plasmid. We thank Drs Joseph Pick and Danielle Ferreira, and the rest of the Ziff laboratory and Kim laboratory, for their helpful discussion and comments. We also thank Dr Michael Tamkun and the Tamkun laboratory for their help with the FRAP experiments.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: M.F.S., E.B.Z., S.K.; Methodology: M.F.S., L.K., S.K.; Formal analysis: M.F.S.; Investigation: M.F.S., L.K., J.P.R., I.G.S., A.Z.; Resources: E.B.Z., S.K.; Data curation: M.F.S., S.K.; Writing - original draft: M.F.S., E.B.Z., S.K.; Writing - review & editing: M.F.S., E.B.Z., S.K.; Visualization: M.F.S.; Supervision: R.C.C.K., E.B.Z., S.K.; Project administration: M.F.S., E.B.Z., S.K.; Funding acquisition: M.F.S., J.P.R., I.G.S., E.B.Z., S.K.
Funding
This work was supported by the Brazilian agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; to M.F.S.) and the National Institutes of Health (grant R37AG013620 to E.B.Z.). J.P.R. was supported by The Barry Goldwater Scholarship and Excellence in Education Foundation, the Astronaut Scholarship Foundation, and Student Experiential Learning Grants from Colorado State University. I.G.S. was supported by the COVID-19 Teaching & Research Student Employment Initiative from Colorado State University. This research was supported by funds from the College Research Council Shared Research Program of Colorado State University, National Institutes of Health National Center for Advancing Translational Sciences Colorado CTSA grant (UL1 TR002535) and the Boettcher Foundation’s Webb–Waring Biomedical Research Program (to S.K.). Deposited in PMC for release after 12 months.
References
- Anggono, V. and Huganir, R. L. (2012). Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 22, 461-469. 10.1016/j.conb.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke, T. G., Bowie, D., Lee, H.-K., Huganir, R. L., Schousboe, A. and Traynelis, S. F. (2000). Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 20, 89-102. 10.1523/JNEUROSCI.20-01-00089.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria, A., Muller, D., Derkach, V., Griffith, L. C. and Soderling, T. R. (1997). Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276, 2042-2045. 10.1126/science.276.5321.2042 [DOI] [PubMed] [Google Scholar]
- Beattie, E. C., Stellwagen, D., Morishita, W., Bresnahan, J. C., Ha, B. K., Von Zastrow, M., Beattie, M. S. and Malenka, R. C. (2002). Control of synaptic strength by glial TNFalpha. Science 295, 2282-2285. 10.1126/science.1067859 [DOI] [PubMed] [Google Scholar]
- Brown, J. T., Chin, J., Leiser, S. C., Pangalos, M. N. and Randall, A. D. (2011). Altered intrinsic neuronal excitability and reduced Na+ currents in a mouse model of Alzheimer's disease. Neurobiol. Aging 32, 2109.e1-2109.e14. 10.1016/j.neurobiolaging.2011.05.025 [DOI] [PubMed] [Google Scholar]
- Busche, M. A., Eichhoff, G., Adelsberger, H., Abramowski, D., Wiederhold, K.-H., Haass, C., Staufenbiel, M., Konnerth, A. and Garaschuk, O. (2008). Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science 321, 1686-1689. 10.1126/science.1162844 [DOI] [PubMed] [Google Scholar]
- Busche, M. A., Chen, X., Henning, H. A., Reichwald, J., Staufenbiel, M., Sakmann, B. and Konnerth, A. (2012). Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 109, 8740-8745. 10.1073/pnas.1206171109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, R. C., Beattie, E. C., Xia, H., Luscher, C., Altschuler, Y., Nicoll, R. A., Malenka, R. C. and von Zastrow, M. (1999). Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 96, 14112-14117. 10.1073/pnas.96.24.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, K. L., Tseng, K. Y., Uejima, J. L., Reimers, J. M., Heng, L.-J., Shaham, Y., Marinelli, M. and Wolf, M. E. (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454, 118-121. 10.1038/nature06995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amelio, M., Cavallucci, V., Middei, S., Marchetti, C., Pacioni, S., Ferri, A., Diamantini, A., De Zio, D., Carrara, P., Battistini, L.et al. (2011). Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat. Neurosci. 14, 69-76. 10.1038/nn.2709 [DOI] [PubMed] [Google Scholar]
- Derkach, V., Barria, A. and Soderling, T. R. (1999). Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. USA 96, 3269-3274. 10.1073/pnas.96.6.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach, V. A., Oh, M. C., Guire, E. S. and Soderling, T. R. (2007). Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 8, 101-113. 10.1038/nrn2055 [DOI] [PubMed] [Google Scholar]
- Díaz-Alonso, J., Sun, Y. J., Granger, A. J., Levy, J. M., Blankenship, S. M. and Nicoll, R. A. (2017). Subunit-specific role for the amino-terminal domain of AMPA receptors in synaptic targeting. Proc. Natl. Acad. Sci. USA 114, 7136-7141. 10.1073/pnas.1707472114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering, G. H. and Huganir, R. L. (2018). The AMPA receptor code of synaptic plasticity. Neuron 100, 314-329. 10.1016/j.neuron.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering, G. H., Gustina, A. S. and Huganir, R. L. (2014). PKA-GluA1 coupling via AKAP5 controls AMPA receptor phosphorylation and cell-surface targeting during bidirectional homeostatic plasticity. Neuron 84, 790-805. 10.1016/j.neuron.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering, G. H., Heo, S., Hussain, N. K., Liu, B. and Huganir, R. L. (2016). Extensive phosphorylation of AMPA receptors in neurons. Proc. Natl. Acad. Sci. USA 113, E4920-E4927. 10.1073/pnas.1610631113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers, M. D. (2000). Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511-525. 10.1016/S0896-6273(00)00129-X [DOI] [PubMed] [Google Scholar]
- Esteban, J. A., Shi, S.-H., Wilson, C., Nuriya, M., Huganir, R. L. and Malinow, R. (2003). PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 6, 136-143. 10.1038/nn997 [DOI] [PubMed] [Google Scholar]
- Fernandez-Monreal, M., Brown, T. C., Royo, M. and Esteban, J. A. (2012). The balance between receptor recycling and trafficking toward lysosomes determines synaptic strength during long-term depression. J. Neurosci. 32, 13200-13205. 10.1523/JNEUROSCI.0061-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario, C. R., Loweth, J. A., Milovanovic, M., Ford, K. A., Galiñanes, G. L., Heng, L.-J., Tseng, K. Y. and Wolf, M. E. (2011). Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology 61, 1141-1151. 10.1016/j.neuropharm.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza, M., Rostosky, C. M., Parkinson, G. T., Bygrave, A. M., Halemani, N., Baptista, M., Milosevic, I. and Hanley, J. G. (2017). PICK1 regulates AMPA receptor endocytosis via direct interactions with AP2 alpha-appendage and dynamin. J. Cell Biol. 216, 3323-3338. 10.1083/jcb.201701034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner, S., Baglietto-Vargas, D., Martini, A. C., Trujillo-Estrada, L. and LaFerla, F. M. (2017). Synaptic impairment in Alzheimer's disease: a dysregulated symphony. Trends Neurosci. 40, 347-357. 10.1016/j.tins.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Galanis, C. and Vlachos, A. (2020). Hebbian and homeostatic synaptic plasticity-do alterations of one reflect enhancement of the other? Front. Cell Neurosci. 14, 50. 10.3389/fncel.2020.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger, A. J., Shi, Y., Lu, W., Cerpas, M. and Nicoll, R. A. (2013). LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493, 495-500. 10.1038/nature11775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger, I. H., Khatri, L., Kong, X. and Ziff, E. B. (2003). AMPA receptor tetramerization is mediated by Q/R editing. Neuron 40, 763-774. 10.1016/S0896-6273(03)00668-8 [DOI] [PubMed] [Google Scholar]
- Greger, I. H., Watson, J. F. and Cull-Candy, S. G. (2017). Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron 94, 713-730. 10.1016/j.neuron.2017.04.009 [DOI] [PubMed] [Google Scholar]
- Hanley, J. G. (2018). The regulation of AMPA receptor endocytosis by dynamic protein-protein interactions. Front. Cell Neurosci. 12, 362. 10.3389/fncel.2018.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, J. A., Devidze, N., Verret, L., Ho, K., Halabisky, B., Thwin, M. T., Kim, D., Hamto, P., Lo, I., Yu, G.-Q.et al. (2010). Transsynaptic progression of amyloid-beta-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron 68, 428-441. 10.1016/j.neuron.2010.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, D. M., Walsh, D. M., Ye, C. P., Diehl, T., Vasquez, S., Vassilev, P. M., Teplow, D. B. and Selkoe, D. J. (1999). Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 19, 8876-8884. 10.1523/JNEUROSCI.19-20-08876.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, Y., Shi, S. H., Esteban, J. A., Piccini, A., Poncer, J. C. and Malinow, R. (2000). Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262-2267. 10.1126/science.287.5461.2262 [DOI] [PubMed] [Google Scholar]
- He, K., Song, L., Cummings, L. W., Goldman, J., Huganir, R. L. and Lee, H.-K. (2009). Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc. Natl. Acad. Sci. USA 106, 20033-20038. 10.1073/pnas.0910338106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley, J. M. and Wilkinson, K. A. (2016). Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci. 17, 337-350. 10.1038/nrn.2016.37 [DOI] [PubMed] [Google Scholar]
- Hosokawa, T., Mitsushima, D., Kaneko, R. and Hayashi, Y. (2015). Stoichiometry and phosphoisotypes of hippocampal AMPA-type glutamate receptor phosphorylation. Neuron 85, 60-67. 10.1016/j.neuron.2014.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Z., Agopyan, N., Miu, P., Xiong, Z., Henderson, J., Gerlai, R., Taverna, F. A., Velumian, A., MacDonald, J., Carlen, P.et al. (1996). Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 17, 945-956. 10.1016/S0896-6273(00)80225-1 [DOI] [PubMed] [Google Scholar]
- Kameyama, K., Lee, H.-K., Bear, M. F. and Huganir, R. L. (1998). Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron 21, 1163-1175. 10.1016/S0896-6273(00)80633-9 [DOI] [PubMed] [Google Scholar]
- Kelly, B. T. and Owen, D. J. (2011). Endocytic sorting of transmembrane protein cargo. Curr. Opin. Cell Biol. 23, 404-412. 10.1016/j.ceb.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Kerr, J. M. and Blanpied, T. A. (2012). Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J. Neurosci. 32, 658-673. 10.1523/JNEUROSCI.2927-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. and Ziff, E. B. (2014). Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLoS Biol. 12, e1001900. 10.1371/journal.pbio.1001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S., Titcombe, R. F., Zhang, H., Khatri, L., Girma, H. K., Hofmann, F., Arancio, O. and Ziff, E. B. (2015). Network compensation of cyclic GMP-dependent protein kinase II knockout in the hippocampus by Ca2+-permeable AMPA receptors. Proc. Natl. Acad. Sci. USA 112, 3122-3127. 10.1073/pnas.1417498112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S., Sato, Y., Mohan, P. S., Peterhoff, C., Pensalfini, A., Rigoglioso, A., Jiang, Y. and Nixon, R. A. (2016). Evidence that the rab5 effector APPL1 mediates APP-betaCTF-induced dysfunction of endosomes in Down syndrome and Alzheimer's disease. Mol. Psychiatry 21, 707-716. 10.1038/mp.2015.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen, A. S., Jenkins, M. A., Banke, T. G., Schousboe, A., Makino, Y., Johnson, R. C., Huganir, R. and Traynelis, S. F. (2011). Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat. Neurosci. 14, 727-735. 10.1038/nn.2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla, K. V., Goldman, S. T., Lattarulo, C. R., Wu, H.-Y., Hyman, B. T. and Bacskai, B. J. (2008). Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59, 214-225. 10.1016/j.neuron.2008.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. K. (2006). AMPA receptor phosphorylation in synaptic plasticity: insights from knockin mice. In The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology (ed. Kittler J. T. and Moss S. J.). Boca Raton, FL: CRC Press/Taylor & Francis; [PubMed] [Google Scholar]
- Lee, H.-K., Kameyama, K., Huganir, R. L. and Bear, M. F. (1998). NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21, 1151-1162. 10.1016/S0896-6273(00)80632-7 [DOI] [PubMed] [Google Scholar]
- Lee, H.-K., Barbarosie, M., Kameyama, K., Bear, M. F. and Huganir, R. L. (2000). Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955-959. 10.1038/35016089 [DOI] [PubMed] [Google Scholar]
- Lee, S. H., Liu, L., Wang, Y. T. and Sheng, M. (2002). Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36, 661-674. 10.1016/S0896-6273(02)01024-3 [DOI] [PubMed] [Google Scholar]
- Lee, H.-K., Takamiya, K., Han, J.-S., Man, H., Kim, C.-H., Rumbaugh, G., Yu, S., Ding, L., He, C., Petralia, R. S.et al. (2003). Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631-643. 10.1016/S0092-8674(03)00122-3 [DOI] [PubMed] [Google Scholar]
- Lee, H.-K., Takamiya, K., Kameyama, K., He, K., Yu, S., Rossetti, L., Wilen, D. and Huganir, R. L. (2007). Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol. Cell. Neurosci. 36, 86-94. 10.1016/j.mcn.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, D.-T., Makino, Y., Sharma, K., Hayashi, T., Neve, R., Takamiya, K. and Huganir, R. L. (2009). Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat. Neurosci. 12, 879-887. 10.1038/nn.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q., Xie, X., Lukas, R. J., St John, P. A. and Wu, J. (2013). A novel nicotinic mechanism underlies beta-amyloid-induced neuronal hyperexcitation. J. Neurosci. 33, 7253-7263. 10.1523/JNEUROSCI.3235-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, W., Khatri, L. and Ziff, E. B. (2014). Trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptor subunit GluA2 from the endoplasmic reticulum is stimulated by a complex containing Ca2+/calmodulin-activated kinase II (CaMKII) and PICK1 protein and by release of Ca2+ from internal stores. J. Biol. Chem. 289, 19218-19230. 10.1074/jbc.M113.511246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino, H. and Malinow, R. (2009). AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64, 381-390. 10.1016/j.neuron.2009.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man, H.-Y., Lin, J. W., Ju, W. H., Ahmadian, G., Liu, L., Becker, L. E., Sheng, M. and Wang, Y. T. (2000). Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 25, 649-662. 10.1016/S0896-6273(00)81067-3 [DOI] [PubMed] [Google Scholar]
- Man, H.-Y., Sekine-Aizawa, Y. and Huganir, R. L. (2007). Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl. Acad. Sci. USA 104, 3579-3584. 10.1073/pnas.0611698104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, H. T. and Boucrot, E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517-533. 10.1038/nrm3151 [DOI] [PubMed] [Google Scholar]
- Minkeviciene, R., Rheims, S., Dobszay, M. B., Zilberter, M., Hartikainen, J., Fulop, L., Penke, B., Zilberter, Y., Harkany, T., Pitkanen, A.et al. (2009). Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 29, 3453-3462. 10.1523/JNEUROSCI.5215-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, M. C., Derkach, V. A., Guire, E. S. and Soderling, T. R. (2006). Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 281, 752-758. 10.1074/jbc.M509677200 [DOI] [PubMed] [Google Scholar]
- Osten, P., Srivastava, S., Inman, G. J., Vilim, F. S., Khatri, L., Lee, L. M., States, B. A., Einheber, S., Milner, T. A., Hanson, P. I.et al. (1998). The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron 21, 99-110. 10.1016/S0896-6273(00)80518-8 [DOI] [PubMed] [Google Scholar]
- Osten, P., Khatri, L., Perez, J. L., Köhr, G., Giese, G., Daly, C., Schulz, T. W., Wensky, A., Lee, L. M. and Ziff, E. B. (2000). Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron 27, 313-325. 10.1016/S0896-6273(00)00039-8 [DOI] [PubMed] [Google Scholar]
- Palop, J. J. and Mucke, L. (2009). Epilepsy and cognitive impairments in Alzheimer disease. Arch. Neurol. 66, 435-440. 10.1001/archneurol.2009.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop, J. J., Chin, J., Roberson, E. D., Wang, J., Thwin, M. T., Bien-Ly, N., Yoo, J., Ho, K. O., Yu, G.-Q., Kreitzer, A.et al. (2007). Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron 55, 697-711. 10.1016/j.neuron.2007.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. (2018). AMPA receptor trafficking for postsynaptic potentiation. Front. Cell Neurosci. 12, 361. 10.3389/fncel.2018.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, G. T. and Hanley, J. G. (2018). Mechanisms of AMPA receptor endosomal sorting. Front. Mol. Neurosci. 11, 440. 10.3389/fnmol.2018.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick, J. E. and Ziff, E. B. (2018). Regulation of AMPA receptor trafficking and exit from the endoplasmic reticulum. Mol. Cell. Neurosci. 91, 3-9. 10.1016/j.mcn.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick, J. E., Khatri, L., Sathler, M. F. and Ziff, E. B. (2017). mGluR long-term depression regulates GluA2 association with COPII vesicles and exit from the endoplasmic reticulum. EMBO J. 36, 232-244. 10.15252/embj.201694526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkey, A. M. and Dell'Acqua, M. L. (2020). Phosphorylation-dependent regulation of Ca(2+)-permeable AMPA receptors during hippocampal synaptic plasticity. Front. Synaptic Neurosci. 12, 8. 10.3389/fnsyn.2020.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restituito, S., Khatri, L., Ninan, I., Mathews, P. M., Liu, X., Weinberg, R. J. and Ziff, E. B. (2011). Synaptic autoregulation by metalloproteases and gamma-secretase. J. Neurosci. 31, 12083-12093. 10.1523/JNEUROSCI.2513-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson, E. D., Halabisky, B., Yoo, J. W., Yao, J., Chin, J., Yan, F., Wu, T., Hamto, P., Devidze, N., Yu, G.-Q.et al. (2011). Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J. Neurosci. 31, 700-711. 10.1523/JNEUROSCI.4152-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, J. P., Stokoe, S. A., Sathler, M. F., Nichols, R. A. and Kim, S. (2021). Selective co-activation of alpha7- and alpha4beta2-nicotinic acetylcholine receptors reverses beta-amyloid-induced synaptic dysfunction. J. Biol. Chem. 296, 100402. 10.1016/j.jbc.2021.100402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, M. S. (2004). Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167-174. 10.1016/j.tcb.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Roche, K. W., O'Brien, R. J., Mammen, A. L., Bernhardt, J. and Huganir, R. L. (1996). Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16, 1179-1188. 10.1016/S0896-6273(00)80144-0 [DOI] [PubMed] [Google Scholar]
- Sanderson, J. L., Gorski, J. A., Gibson, E. S., Lam, P., Freund, R. K., Chick, W. S. and Dell'Acqua, M. L. (2012). AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J. Neurosci. 32, 15036-15052. 10.1523/JNEUROSCI.3326-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathler, M. F., Stutz, B., Martins, R. S., Dos Santos Pereira, M., Pecinalli, N. R., Santos, L. E., Taveira-da-Silva, R., Lowe, J., de Freitas, I. G., de Melo Reis, R. A.et al. (2016). Single exposure to cocaine impairs aspartate uptake in the pre-frontal cortex via dopamine D1-receptor dependent mechanisms. Neuroscience 329, 326-336. 10.1016/j.neuroscience.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Selvakumar, B., Jenkins, M. A., Hussain, N. K., Huganir, R. L., Traynelis, S. F. and Snyder, S. H. (2013). S-nitrosylation of AMPA receptor GluA1 regulates phosphorylation, single-channel conductance, and endocytosis. Proc. Natl. Acad. Sci. USA 110, 1077-1082. 10.1073/pnas.1221295110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle, Y., Zhang, S., Ninan, I., Puzzo, D., McCarthy, M., Khatri, L., Arancio, O. and Ziff, E. B. (2007). A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron 56, 670-688. 10.1016/j.neuron.2007.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, S.-H., Hayashi, Y., Esteban, J. A. and Malinow, R. (2001). Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331-343. 10.1016/S0092-8674(01)00321-X [DOI] [PubMed] [Google Scholar]
- Sole, L., Wagnon, J. L., Akin, E. J., Meisler, M. H. and Tamkun, M. M. (2019). The MAP1B binding domain of Nav1.6 is required for stable expression at the axon initial segment. J. Neurosci. 39, 4238-4251. 10.1523/JNEUROSCI.2771-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, S., Osten, P., Vilim, F. S., Khatri, L., Inman, G., States, B., Daly, C., DeSouza, S., Abagyan, R., Valtschanoff, J. G.et al. (1998). Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron 21, 581-591. 10.1016/S0896-6273(00)80568-1 [DOI] [PubMed] [Google Scholar]
- Sun, J. L., Stokoe, S. A., Roberts, J. P., Sathler, M. F., Nip, K. A., Shou, J., Ko, K., Tsunoda, S. and Kim, S. (2019). Co-activation of selective nicotinic acetylcholine receptors is required to reverse beta amyloid-induced Ca(2+) hyperexcitation. Neurobiol. Aging 84, 166-177. 10.1016/j.neurobiolaging.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztukowski, K., Nip, K., Ostwald, P. N., Sathler, M. F., Sun, J. L., Shou, J., Jorgensen, E. T., Brown, T. E., Elder, J. H., Miller, C.et al. (2018). HIV induces synaptic hyperexcitation via cGMP-dependent protein kinase II activation in the FIV infection model. PLoS Biol. 16, e2005315. 10.1371/journal.pbio.2005315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub, L. M. (2009). Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10, 583-596. 10.1038/nrm2751 [DOI] [PubMed] [Google Scholar]
- Verret, L., Mann, E. O., Hang, G. B., Barth, A. M. I., Cobos, I., Ho, K., Devidze, N., Masliah, E., Kreitzer, A. C., Mody, I.et al. (2012). Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708-721. 10.1016/j.cell.2012.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, J. F., Ho, H. and Greger, I. H. (2017). Synaptic transmission and plasticity require AMPA receptor anchoring via its N-terminal domain. eLife 6, e23024. 10.7554/eLife.23024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, A., Li, S., Hur, J. K., Wachsmuth, M., Bois, J. S., Perkins, E. M., Patel, D. J. and Aravin, A. A. (2015). Aub and Ago3 are recruited to Nuage through two mechanisms to form a ping-pong complex assembled by Krimper. Mol. Cell 59, 564-575. 10.1016/j.molcel.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widagdo, J., Chai, Y. J., Ridder, M. C., Chau, Y. Q., Johnson, R. C., Sah, P., Huganir, R. L. and Anggono, V. (2015). Activity-dependent ubiquitination of GluA1 and GluA2 regulates AMPA receptor intracellular sorting and degradation. Cell Rep. 10, 783-795. 10.1016/j.celrep.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski, G. A., Puthenveedu, M. A., Leonoudakis, D., Panicker, S., Thorn, K. S., Beattie, E. C. and von Zastrow, M. (2007). Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J. Neurosci. 27, 11112-11121. 10.1523/JNEUROSCI.2465-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo, D., Sprengel, R., Hvalby, O., Jensen, V., Burnashev, N., Rozov, A., Kaiser, K. M. M., Köster, H. J., Borchardt, T., Worley, P.et al. (1999). Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805-1811. 10.1126/science.284.5421.1805 [DOI] [PubMed] [Google Scholar]
- Zhang, J. and Abdullah, J. M. (2013). The role of GluA1 in central nervous system disorders. Rev. Neurosci. 24, 499-505. 10.1515/revneuro-2013-0021 [DOI] [PubMed] [Google Scholar]
- Zhang, K., Xu, T., Yuan, Z., Wei, Z., Yamaki, V. N., Huang, M., Huganir, R. L. and Cai, X. (2016). Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci. Signal. 9, ra123. 10.1126/scisignal.aai7884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, N., Jeyifous, O., Munro, C., Montgomery, J. M. and Green, W. N. (2015). Synaptic activity regulates AMPA receptor trafficking through different recycling pathways. eLife 4, e06878. 10.7554/eLife.06878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.