Abstract
Objective
To compare the performance of kisspeptin and beta human chorionic gonadotropin (βhCG), both alone and in combination, as biomarkers for miscarriage throughout the first trimester.
Design
Prospective, nested case-control study.
Setting
Tertiary Centre, Queen Charlotte Hospital, London, United Kingdom.
Patient(s)
Adult women who had miscarriages (n = 95, 173 samples) and women with healthy pregnancies (n = 265, 557 samples).
Intervention(s)
The participants underwent serial ultrasound scans and blood sampling for measurement of plasma kisspeptin and βhCG levels during the first trimester.
Main Outcome Measure(s)
The ability of plasma kisspeptin and βhCG levels to distinguish pregnancies complicated by miscarriage from healthy pregnancies unaffected by miscarriage.
Result(s)
Gestation-adjusted levels of circulating kisspeptin and βhCG were lower in samples from women with miscarriages than in women with healthy pregnancies by 79% and 70%, respectively. The area under the receiver-operating characteristic curve for identifying miscarriage during the first trimester was 0.874 (95% confidence interval [CI] 0.844–0.904) for kisspeptin, 0.859 (95% CI 0.820–0.899) for βhCG, and 0.916 (95% CI 0.886–0.946) for the sum of the two markers. The performance of kisspeptin in identifying miscarriage improved with increasing length of gestation, whereas that of βhCG worsened. A decision matrix incorporating kisspeptin, βhCG, and gestational age had 83% to 87% accuracy for the prediction of miscarriage.
Conclusion(s)
Plasma kisspeptin is a promising biomarker for miscarriage and provides additional value to βhCG alone, especially during later gestational weeks of the first trimester.
Key Words: Kisspeptin, miscarriage, pregnancy
Abstract
Interpretación de la kisspeptina plasmática como biomarcador de aborto espontáneo mejora con la edad durante el primer trimestre.
Objetivo
Comparar el rendimiento de la kisspeptina y la betagonadotropina coriónica humana (βhCG), tanto solas como en combinación, como biomarcadores de aborto espontáneo durante el primer trimestre.
Diseño
Estudio prospectivo, de casos y controles anidados.
Entorno
Centro terciario, Queen Charlotte Hospital, London, United Kingdom.
Paciente (s)
Mujeres adultas que tuvieron abortos espontáneos (n=95. 173 muestras) y mujeres con gestaciones evolutivas (n=265. 557 muestras).
Intervención (es)
Las participantes se sometieron a ecografías seriadas y a tomas de muestras de sangre para medir la kisspeptina en plasma y los niveles de βhCG durante el primer trimestre.
Principales medidas de resultado
La capacidad de los niveles plasmáticos de kisspeptina y de la βhCG para distinguir embarazos complicados por aborto espontáneo de embarazos sanos que no se vieron afectados por un aborto espontáneo.
Resultado (s)
Los niveles de kisspeptina y de βhCG circulantes ajustados por edad gestacional fueron más bajos en muestras de mujeres con abortos espontáneos que en mujeres con embarazos evolutivos en un 79% y 70%, respectivamente. El área bajo la curva característica de funcionamiento del receptor para la identificación del aborto espontáneo durante el primer trimestre fue de 0,874 (intervalo de confianza [CI] del 95%: 0.844-0.904) para la kisspeptina, 0.859 (CI del 95% 0.820 a 0.899) para la βhCG y 0,916 (CI del 95%: 0,886 a 0,946) para la suma de los dos marcadores. La actuación de kisspeptina en la identificación del aborto espontáneo mejoró con el aumento de la edad gestacional, mientras que la de βhCG empeoró. Una matriz de decisiones incorporando la kisspeptina, la βhCG y la edad gestacional tuvo una precisión del 83% al 87% para la predicción de aborto espontáneo.
Conclusión (es)
La kisspeptina plasmática es un biomarcador prometedor para el aborto espontáneo y proporciona un valor adicional a la βhCG sola, especialmente durante las últimas semanas de gestación del primer trimestre.
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/32710
Miscarriage (pregnancy loss before 24 weeks of gestation) (1) is the most common complication of pregnancy, affecting one in five pregnancies (2, 3, 4). Miscarriage is most frequently because of cytogenetic abnormality of the developing embryo (5), especially during the first trimester, and is associated with disordered decidualization, implantation, and placentation (6, 7).
The diagnosis of miscarriage is on the basis of strict sonographic criteria (8, 9). Women may experience symptoms that lead them to undergo ultrasound assessment in early pregnancy assessment units. More than one fifth of women have an inconclusive ultrasound scan (pregnancy of unknown location [PUL] or intrauterine pregnancy [IUP] of unknown viability) at the first assessment (10). In addition, women may be falsely reassured by an ultrasound scan that shows a viable pregnancy, because there remains a 12% risk of subsequent miscarriage (11). Currently, no reliable biomarker exists that accurately stratifies the risk of miscarriage (12). Beta human chorionic gonadotropin (βhCG) is the most widely used pregnancy biomarker; however, a single measurement is of limited value for the diagnosis of miscarriage because of the wide variability in βhCG levels during early pregnancy (12). Clinical prediction models for first trimester viability exist, but they have limitations, such as the lack of validation in large cohorts, relatively low specificity, and the use of old criteria for the diagnosis of miscarriage (13, 14). The addition of biomarker information to such models might improve their predictive ability.
Kisspeptin has emerged as a putative marker of placentation, and there has been great interest in evaluating its potential as a novel marker of pregnancy complications (15). Kisspeptins are a family of peptides encoded by the KISS1 gene that bind to the G protein–coupled “kisspeptin receptor” (16, 17, 18). Recent evidence suggests that kisspeptin has an important role in many facets of reproduction, including puberty, ovulation, and placentation (19). Kisspeptin and its receptor are highly expressed in the placenta throughout pregnancy in humans (20, 21, 22, 23). Circulating kisspeptin reaches levels approximately 7,000-fold greater than those in nonpregnant women by the third trimester before rapidly falling postpartum (24, 25). Previous studies suggest that kisspeptin has potential as a biomarker for miscarriage (26, 27). However, a recent editorial advocated that further data are required to establish its utility in the diagnosis of miscarriage (15).
In summary, evidence to date suggests that plasma kisspeptin could potentially be used as a risk-stratification tool for evaluation of miscarriage risk during early pregnancy. In the present study, we aimed to quantify the performance of plasma kisspeptin and βhCG for the identification of miscarriage at different gestational ages during the first trimester. Additionally, we aimed to evaluate whether the combination of the two measurements could improve diagnostic performance over either measurement alone. Finally, we recruited a second cohort of women classified as having a PUL and those with a diagnosis of ectopic pregnancy (EP) to assess the performance of plasma kisspeptin and βhCG in this context.
Methods
Ethical Approval
The study investigating the performance of plasma kisspeptin to assess the risk of miscarriage in women with IUPs was approved by the National Research Ethics Service (NRES) Riverside Committee London (approval no. REC 14/LO/0199) and the North East, Newcastle and North Tyneside Two Research Ethics Committee (approval no. REC 17/NE/0121). A second cohort of women classified as having a PUL or diagnosed with EP was recruited to the “Assessment of Biomarkers in PUL and Ectopic Pregnancy” study, approved by the NRES Committee–North of Scotland (approval no. REC 14/NS/1078).
Study Cohort 1: Women with an Intrauterine Pregnancy at Presentation
The study was hosted at the Early Pregnancy Assessment Unit at Queen Charlotte’s and Chelsea Hospital, London, United Kingdom, between March 2014 and March 2017. Consecutive pregnant women aged between 18 and 49 years with an IUP on ultrasound scan (viable pregnancy or IUP of unknown viability) in the first trimester were invited to participate. Women presented to the unit to seek reassurance after experiencing symptoms such as nausea, vomiting, pelvic pain, or vaginal bleeding, or for their routine dating ultrasound scan in the obstetric ultrasound department. Women were excluded if they had already had a miscarriage at presentation or received a diagnosis of PUL or EP on ultrasonography.
After recruitment, the women were invited for assessments every 2 weeks during the first trimester. Most of the women attended for two to four visits and were followed up until the 14th week of gestation or the day of confirmation of miscarriage. At each visit, the women had an ultrasound scan to assess fetal viability and a blood test. The final outcome was a viable pregnancy or miscarriage occurring by the end of the first trimester (14 weeks of gestation). Miscarriage was diagnosed by ultrasound scan in accordance with national guidelines (1, 8, 9, 28). Women who had a miscarriage received a diagnosis of missed miscarriage on follow-up according to the revised National Institute for Clinical Excellence (NICE) and Royal College of Obstetricians and Gynaecologists guidelines inspired by Abdallah et al. (8). Accordingly, in these pregnancies, the ultrasound scan showed an empty gestational sac with a mean sac diameter >25 mm or a fetal pole with no heartbeat and a crown-rump length (CRL) >7 mm. Complete miscarriage was diagnosed when the follow-up ultrasound showed a thin endometrium. Incomplete miscarriage was diagnosed when the follow-up ultrasound showed retained products of conception, e.g., the gestational sac morphology appeared disrupted.
The control group was selected at random from women who had conceived spontaneously, had no vaginal bleeding, and had no late pregnancy complications (e.g., pre-eclampsia, pregnancy-induced hypertension, intrauterine growth restriction or small for gestational age, gestational diabetes, or preterm birth).
Study Cohort 2: Women with Pregnancy of Unknown Location and Ectopic Pregnancy
A separate cohort of 189 women classified as having a PUL or diagnosed with an EP were prospectively recruited from the early pregnancy unit at Queen Charlotte’s and Chelsea Hospital from July 2018 to January 2020. Transvaginal ultrasonography and plasma blood sampling were performed at flexible intervals until an outcome was confirmed. The outcomes were EP; IUP (viable at 12 weeks’ gestation [VIUP] or nonviable, i.e., evidence of IUP that did not attain or maintain viability by 12 weeks [NVIUP]); failed PUL, i.e., negative pregnancy test 2 weeks from follow-up (FPUL) or persistent PUL (PPUL), i.e., more than three static serial βhCG levels while remaining PUL. Women with VIUP were selected as controls.
Estimation of Gestational Age
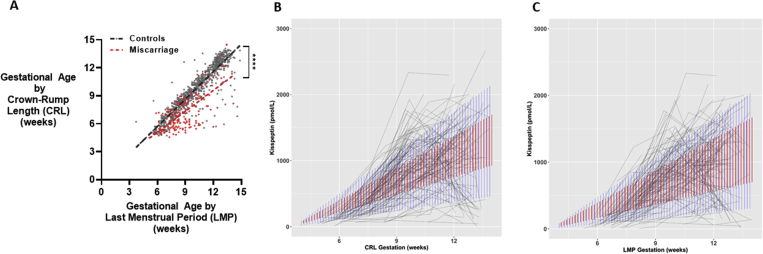
To evaluate putative biomarker levels during pregnancy, it is necessary to accurately determine gestational age at the time of sample collection. During the first trimester, pregnancies were dated according to two measures of gestational age: last menstrual period (LMP) and CRL on ultrasound scan (29). Gestation estimated by LMP can be subject to inaccurate recollection and assumes a regular 28-day cycle (30). In healthy pregnancies, CRL is the most accurate measure of gestational age and is recommended for use by NICE (29, 31). However, CRL may underestimate gestational age in women who miscarry, because embryo size may be reduced in women with failing pregnancies (32, 33). During preliminary analysis, we found that although estimates of gestational age by LMP and CRL did not significantly differ in the control group, CRL underestimated gestational age in women who had a miscarriage by 21% (Supplemental Fig. 1A, available online). Therefore, we used LMP as the measure of gestational age for both groups. The gestational week was used to denote samples taken during that week; e.g., gestational week 7 refers to all samples taken from week 7.0 to 7.99. Gestational weeks 12 to 14 were combined in some analyses because of low numbers of miscarriage samples in these later gestational weeks.
Correction of Plasma Levels for Gestational Age
To correct for gestational age, multiples of median (MoM) values were derived; i.e., the median hormone levels at each week of gestation were determined in healthy controls and then each raw hormone level was expressed as a proportion of the median value for the corresponding week of gestation. Thus, a MoM value of 0.5 denotes a value that is half that of the corresponding median value in healthy controls for that week of gestation.
Hormone Measurements
Plasma βhCG levels were measured by the Roche-E411 electrochemiluminescence analyzer (Rotkreuz, Switzerland). The analyzer has a functional sensitivity of <0.6 IU/L and <8% and <6% interassay and intra-assay coefficients of variation, respectively. The measuring range is from 0.1 to 10,000 IU/L. For values above this range, samples were diluted with the use of the recommended diluent provided by the manufacturer. Plasma kisspeptin levels were measured by a radioimmunoassay developed at Imperial College London (34). The assay has interassay and intra-assay coefficients of variation of 10.2% and 8.2%, respectively. The antibody exhibits <0.01% cross-reactivity with similarly structured RF-amide proteins, such as RF-amide–related peptides RFRP-1, RFRP-2, and RFRP-3. The assay measures all kisspeptin splicing variants, although kisspeptin-54 has been reported to be the dominant circulating form in human pregnancies (34).
Statistical Analysis
Analysis was performed with Prism v8.0 (GraphPad), Stata v14.0 (StataCorp), SPSS v24 (IBM), and R version 3.5.1. Normality was determined by the D’Agostino-Pearson test. The data are presented as mean ± standard deviation (SD) for parametrically distributed data or median and interquartile range (IQR) for nonparametric data. The two groups were compared by an unpaired Student’s t-test if parametric and by the Mann-Whitney U test if nonparametric. Multiple groups were compared by one-way ANOVA if parametrically distributed and by the Kruskal-Wallis test if nonparametrically distributed. Proportions were compared by logistic regression. For multivariable analyses, logistic regression was used for binary outcomes and linear regression for continuous outcomes. Cross-validated multilevel logistic regression models were used to account for repeated sampling and to adjust for relevant confounders. Receiver-operating characteristic (ROC) curves were used to determine the ability of kisspeptin and βhCG levels to differentiate miscarriages from healthy pregnancies. The area under the curve (auROC) was calculated for each gestational week from 6 to 12–14 weeks, and optimal thresholds were determined for βhCG and kisspeptin, both individually and in combination, to identify miscarriage. The optimal cutoff values reported are those that maximize the sum of sensitivity and specificity for the diagnostic case on the basis of the study data. P values <.05 were considered to indicate statistical significance.
Results
Study Population
A total of 1,242 pregnant women were screened, and 1,045 were recruited to the study assessing the risk of miscarriage in women with an IUP. The most common reasons cited for declining to participate were choosing antenatal care at another hospital, personal choice, and inability to attend future visits. Of the 1,045 women recruited, 27 were excluded because of termination of pregnancy (n = 11), loss to follow-up (n = 11), or withdrawal from the study (n = 5). Overall, in study cohort 1, 95 pregnancies (173 plasma samples) ended in miscarriage, and of the eligible healthy patients, 265 asymptomatic pregnancies (557 plasma samples) without any signs or symptoms of pregnancy complications were randomly selected to comprise the control group. There were no significant differences between the control and the miscarriage groups with regards to maternal age or number of previous miscarriages (Supplemental Table 1, available online).
Factors Affecting Kisspeptin Levels in Healthy Control Pregnancies
We performed multivariable linear regression to determine the baseline factors contributing to the variability in plasma kisspeptin levels in healthy control pregnancies. Gestational and maternal age were associated with higher plasma kisspeptin levels, whereas Afro-Caribbean ethnicity, smoking during pregnancy, and higher body mass index (BMI) were associated with lower plasma kisspeptin levels in women with healthy pregnancies (Supplemental Table 2). A mixed-effects linear regression analysis found that only 20% to 30% of the variation in kisspeptin levels was explained by interpersonal differences (Supplemental Fig. 1B: gestational age assessed by CRL, or Supplemental Fig. 1C: gestational age assessed by LMP).
Kisspeptin and βhCG Levels During the First Trimester in Control Pregnancies and Pregnancies Ending in Miscarriage
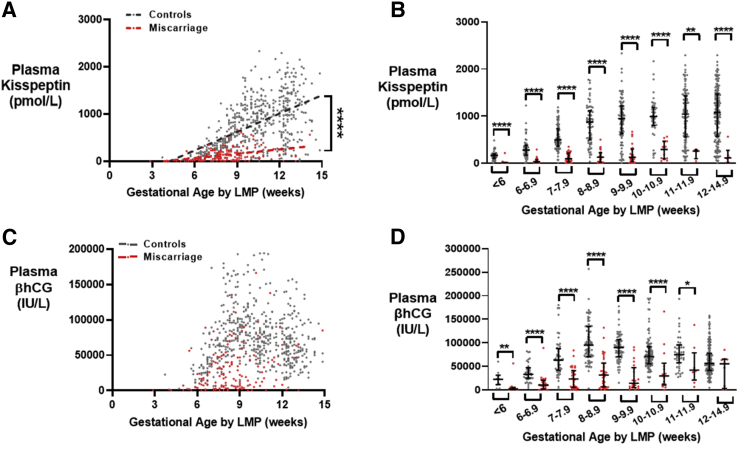
In samples from healthy control pregnancies, plasma kisspeptin levels increased linearly with gestational age during the first trimester (Fig. 1A) and were significantly lower in patients with pregnancies ending in miscarriage at all gestational ages (Fig. 1B). Plasma βhCG levels were highest at 8 weeks of gestation before decreasing (Fig. 1C) and were significantly lower in patients with pregnancies ending in miscarriage at most gestational ages (Fig. 1D).
Figure 1.
Kisspeptin and βhCG levels throughout the first trimester in healthy controls and women with miscarriages. (A) Scatterplot of plasma kisspeptin levels in healthy controls (gray) (n = 265 women providing 545 samples) and women with miscarriages (red) (n = 95 women providing 149 samples) over gestational age (weeks) calculated by LMP, during the first trimester. The data were analyzed by simple linear regression (r2 = 0.31 for controls and 0.14 for women with miscarriages). ∗∗∗∗P<.0001 by analysis of covariance. (B) Median (IQR) plasma kisspeptin levels in healthy controls (gray) (n = 265 women providing 545 samples) and women with miscarriages (red) (n = 95 women providing 149 samples) over gestational age (weeks) calculated by LMP, during the first trimester. ∗∗P<.01, ∗∗∗∗P<.0001. (C) Scatterplot of plasma βhCG levels in healthy controls (gray) (n = 265 women providing 557 samples) and women with miscarriages (red) (n = 95 women providing 173 samples) over gestational age (in weeks) calculated by LMP, during the first trimester. (D) Median (IQR) plasma βhCG levels in healthy controls (gray) (n = 265 women providing 557 samples) and women with miscarriages (red) (n = 95 women providing 173 samples) over gestational age (weeks) calculated by LMP, during the first trimester. ∗P<.5, ∗∗P<.01, ∗∗∗∗P<.0001. βhCG = beta human chorionic gonadotropin; IQR = interquartile range; LMP = last menstrual period.
Multilevel, Multivariable Logistic Regression Model of Kisspeptin Levels to Identify Miscarriage
On univariable analysis, the odds of miscarriage were 38% lower for every 100-pmol/L increase in plasma kisspeptin (P<.0001) (Supplemental Table 3). A multilevel, multivariable logistic regression model was used to adjust for repeated sampling, BMI, age, smoking, and ethnicity. The odds of miscarriage decreased by 35% for every 100-pmol/L increase in plasma kisspeptin during the first trimester (95% CI 32%–38%; P<.0001) (Supplemental Table 3). This effect was not altered significantly after adjustment for gestational age, maternal age, paternal age, ethnicity, smoking status, and BMI (Supplemental Table 3). The multivariable regression model had 75.9% accuracy as a predictor. After fivefold cross-validation. the accuracy was comparable at 80.6%, indicating that the estimated prediction errors are within the standard errors from which the CIs for the model coefficients were derived.
Gestation-Adjusted Plasma Kisspeptin and βhCG Levels in the Prediction of Miscarriage
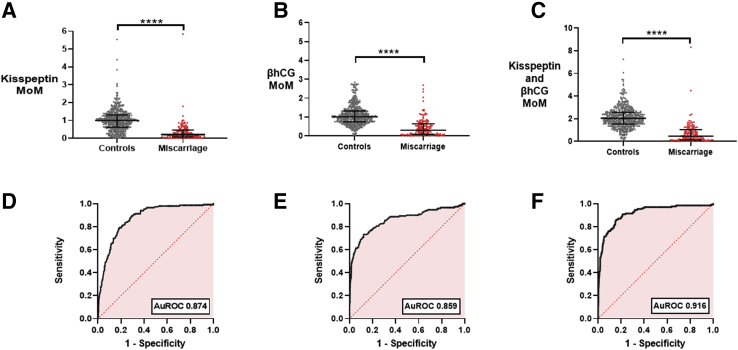
The MoM value of kisspeptin was significantly lower in samples from women who had a miscarriage (median 0.21, IQR 0.08–0.47) than in samples from women with healthy control pregnancies (median 1.00, IQR 0.63–1.31; P <.0001) (Fig. 2A). Similarly, the MoM value of βhCG was lower in samples from women with miscarriages (median 0.30, IQR 0.08–0.64; P <.0001) than in samples from women with healthy control pregnancies (median 1.00, IQR 0.74–1.32; Fig. 2B). The sum of the MoM values of kisspeptin and βhCG was significantly higher in samples from women with healthy control pregnancies (median 2.00, IQR 1.53–2.56) than in samples from women with miscarriages (median 0.46, IQR 0.13–1.03; P<.0001) (Fig. 2C). ROC analysis was performed to quantify the ability of plasma levels to discriminate healthy pregnancies from those affected by miscarriage. The auROC was 0.874 (95% CI 0.844–0.904) for kisspeptin, 0.859 (95% CI 0.820–0.899) for βhCG, and 0.916 (95% CI 0.886–0.946) for the sum of the two markers (Fig. 2D–F). Kisspeptin had more thresholds with high sensitivity (Fig. 2D), whereas βhCG had more thresholds with high specificity (Fig. 2E), and thus the combination of kisspeptin and βhCG could benefit from the complementary sensitivity and specificity in the performance of each measure. A decision tree using both the MoM value of kisspeptin and the MoM value of βhCG had an auROC of 0.90 (95% CI 0.871–0.929) (Supplemental Fig. 3), but this was similar to simply using the sum of the MoM values of kisspeptin and βhCG (auROC 0.916; 95% CI 0.887–0.946) (Fig. 2F).
Figure 2.
MoM values for plasma kisspeptin and βhCG in healthy controls and women with miscarriages and their diagnostic performance. (A) Scatterplot of median (IQR) MoM of gestation-specific kisspeptin values in healthy controls (n = 265 women providing 545 samples) (gray) and women with miscarriages (n = 95 women providing 149 samples) (red). The groups were compared by the Mann-Whitney U test. ∗∗∗∗P<.0001. (B) Scatterplot of median (IQR) MoM of gestation-specific βhCG values in healthy controls (n = 265 women providing 557 samples) (gray) and women with miscarriages (n = 95 providing 173 samples) (red). The groups were compared by the Mann-Whitney U test. ∗∗∗∗P<.0001. (C) Scatterplot of median (IQR) MoM of gestation-specific kisspeptin and βhCG values in healthy controls (n = 265 women providing 538 samples) (gray) and women with miscarriages (n = 95 women providing 130 samples) (red). The groups were compared by the Mann-Whitney U test. ∗∗∗∗P<.0001. (D–F) ROC curves for MoM of kisspeptin (D), βhCG (E), and kisspeptin and βhCG (F). AuROC = area under the receiver-operating characteristic curve; βhCG = beta human chorionic gonadotropin; IQR = interquartile range; MoM = multiple of median. ROC = receiver-operating characteristic curve.
Likelihood of Miscarriage at Different Thresholds of the MoM value of Kisspeptin and the MoM value of βhCG
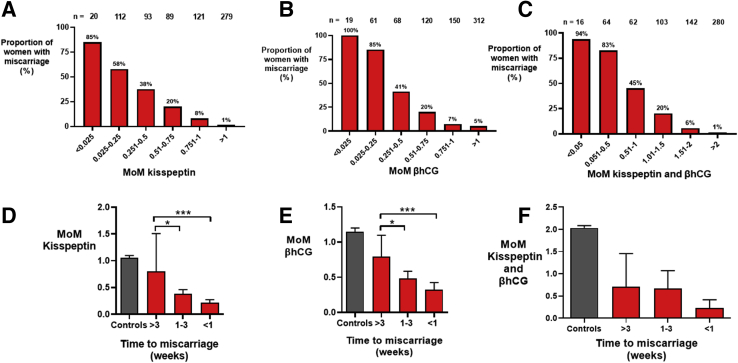
We evaluated the ability of the MoM value of kisspeptin, the MoM value of βhCG, and the sum of the two markers to indicate the likelihood of miscarriage at different threshold values. An above-average level of kisspeptin (MoM value >1.0) was reassuring, with only 1% of 279 samples obtained from women who had a miscarriage, as compared with 85% of those with an MoM value of kisspeptin <0.025 (n = 20) (Fig. 3A), which increased the odds of miscarriage by 24.9-fold (95% CI 7.2–86.0).
Figure 3.
Proportion of women with miscarriages with different MoM cutoffs and time to diagnosis of miscarriage. (A–C) Mean of the proportion of women with miscarriages with different cutoffs of multiples of gestation-specific median for kisspeptin (A), βhCG (B), and the sum of kisspeptin and βhCG) (C). (D–F) Mean (95% CI) of the multiples of gestation-specific median for kisspeptin (D), βhCG (E), and the sum of kisspeptin and βhCG (F) by the time to confirmation of miscarriage (in weeks). The miscarriage groups were compared by the Kruskal-Wallis test. ∗∗P<.01, ∗∗∗P<.001. βhCG = beta human chorionic gonadotropin; CI = confidence interval; MoM = multiple of median.
Similarly, all 19 samples with an MoM value of βhCG <0.025 were from women who had a miscarriage, as compared with 5% of 312 samples with an MoM value of βhCG >1.0 (Fig. 3B). Thus, a sample with a MoM value of βhCG <0.025 was 32.2-fold (95% CI 15.7–65.8) more likely to be from a woman who had a miscarriage than a sample with an MoM value of βhCG >1.0 (Fig. 3B). Only 1% of 280 samples with the sum of the MoM values of kisspeptin and βhCG >2.0 were from women who had a miscarriage, whereas the most (94%) of 16 samples with the sum of the MoM values of kisspeptin and βhCG <0.05 were from women who had a miscarriage (Fig. 3C).
Performance of Plasma Kisspeptin and βhCG at Different First-Trimester Gestational Ages
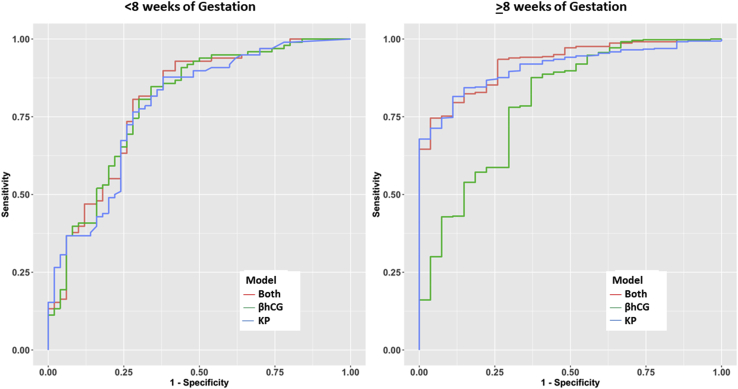
We next examined the auROC of plasma kisspeptin and βhCG by gestational age. The auROC of kisspeptin maintained similar discriminatory performance with increasing first-trimester gestational age, whereas that of βhCG worsened at later gestational ages (Supplemental Table 4). The combination of kisspeptin and βhCG had a marginal diagnostic improvement over either measure alone (Supplemental Table 4). The diagnostic performances of kisspeptin and βhCG were similar at gestational ages under 8 weeks, but there was a trend toward better performance for kisspeptin compared with βhCG at gestational ages of 8 weeks or more (Fig. 4).
Figure 4.
ROC curves for plasma levels of kisspeptin, βhCG, and kisspeptin and βhCG for gestational weeks <eight weeks (left panel) and eight or more weeks (right panel). For the left panel, the AUCs are 78.4% (kisspeptin), 79.5% (βhCG), and 79.7% (both factors). For the right panel, the AUCs are 90.9% (kisspeptin), 79.6% (βhCG), and 92.3% (both factors). Gestational ages were determined by LMP. AUC = area under the curve; βhCG = beta human chorionic gonadotropin; KP = kisspeptin; LMP = last menstrual period; ROC = receiver-operating characteristic.
Effect of Time to Miscarriage and Type of Miscarriage on the MoM Value of Kisspeptin Levels
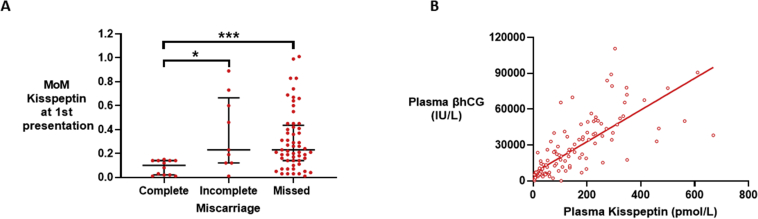
The levels of the MoM value of kisspeptin (Fig. 3D), the MoM value of βhCG (Fig. 3E), and the sum of the MoM values of kisspeptin and βhCG (Fig. 3F) were lower in samples taken in closer proximity to the day of miscarriage confirmation. Furthermore, the MoM value of kisspeptin was lower in samples from women who had completed miscarriages than in those from women with incomplete or missed miscarriages (Supplemental Fig. 3A). Notably, kisspeptin levels were correlated with βhCG levels in women with miscarriages, with an r2 of 0.6 (Supplemental Fig. 3B).
Ability of Kisspeptin and βhCG Levels at Presentation to Predict Miscarriages
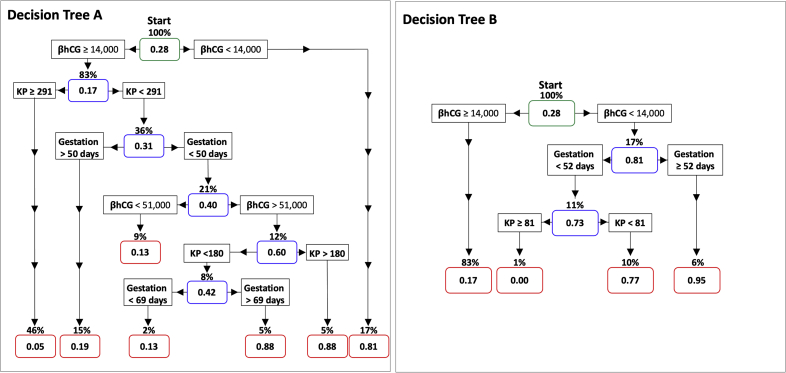
To analyze the ability of kisspeptin and βhCG levels to predict miscarriages, we used only the blood sample taken at the first visit for each participant and devised a decision tree. A decision tree incorporating kisspeptin, βhCG, and gestational age had a diagnostic accuracy of 87.4% (95% CI 83.4%–90.7%) (Supplemental Fig. 4, Decision Tree A). However, this complex model could be susceptible to overfitting, and therefore in addition we produced a more parsimonious (simplified) model that would more likely perform with similar accuracy if applied to other datasets. This model had a diagnostic accuracy of 83.1% (95% CI 78.6%– 86.7%) (Supplemental Fig. 4, Decision Tree B).
Study Cohort 2: Pregnancy of Unknown Location or Ectopic Pregnancy
The mean (± SD) gestational age in study cohort 2 at presentation was 40.4 ± 12.9 days (<6 weeks). For the whole cohort, the mean βhCG level was 1,410 ± 3,903 IU/L, the kisspeptin level was 20.5 ± 24.5 pmol/L, and the progesterone level was 24.6 ± 26.6 nmol/L. There were 68 IUPs (5 IUPs with uncertain viability because of four terminations of pregnancy and one loss to follow-up, 42 VIUPs, and 21 NVIUPs), 31 EPs (15 had samples taken at the time of EP diagnosis), 82 FPULs, and 8 PPULs. The mean (± SD) kisspeptin levels were 21.6 ± 41 pmol/L for VIUPs, 33.5 ± 33.2 pmol/L for NVIUPs, 16.9 ± 12.0 pmol/L for FPULs, 21.5 ± 16.0 pmol/L for PPULs, and 20.1 ± 10.6 pmol/L for EPs. Kisspeptin levels were low in all groups at these early gestational ages, and there were no significant differences in circulating kisspeptin levels between women with VIUPs and women with other PUL outcomes. both independently (P = .07–.99) and after adjustment for maternal age, BMI, gestational age, and ethnicity (P = .16–.95). Other than a borderline difference when non-EP outcomes were combined and compared with EP (P = .043), and between VIUP and NVIUP (P =.046), there were no significant differences in βhCG levels between PUL outcomes after adjustment for maternal age, BMI, gestational age, and ethnicity (P = 0.14–0.49).
Discussion
We have shown that kisspeptin has high diagnostic accuracy for the identification of miscarriage throughout the first trimester, whereas the performance of βhCG declines after 8 weeks of gestation, when 4% to 5% of miscarriages occur (3). Whereas kisspeptin and βhCG had complementary diagnostic performance with regard to sensitivity and specificity, the combination of the two markers outperformed either measure alone. Accordingly, a decision tree incorporating both kisspeptin and βhCG had an accuracy of 83% to 87% for prediction of a subsequent diagnosis of miscarriage. Women with complete miscarriages had even lower levels of kisspeptin than women with other types of miscarriage. Previous data suggest that kisspeptin could be a useful biomarker for miscarriage (26); however, some studies were limited by small sample sizes (27, 35). To our knowledge, the present study includes the largest number of women with miscarriages (n = 95) of any study examining the utility of kisspeptin to date, confirming that kisspeptin has significant potential as a putative marker for pregnancy loss.
Kisspeptin (previously known as “metastin”) was first discovered as a suppressor of metastatic melanoma cell invasiveness (16). Placentation can be regarded as analogous to an invasive oncologic process, but whereas oncologic invasion is uncontrolled (36), placentation is a highly regulated process (37). Kisspeptin is expressed in syncytiotrophoblasts, and its receptor is present in both cytotrophoblasts and syncytiotrophoblasts (21, 38). Placental expression of the KISS1 gene is especially high during the first trimester, which coincides with the time of placentation (21). Placentas from women with recurrent pregnancy loss have significantly lower KISS1 peptide levels in their syncytiotrophoblasts than women with healthy pregnancies (38, 39). In vitro studies of placental explants have shown that kisspeptin decreases trophoblast migration by 70% (21, 40). Kisspeptin accomplishes this by down-regulating the activity of matrix metalloproteinases 2 and 9, which break down the extracellular matrix (21, 41), as well as via the focal adhesion kinase–steroid receptor coactivator pathway (42).
In addition to its putative role in placentation (6 ,21, 38, 43, 44), kisspeptin has been reported to have a role as an immune modulator (45, 46). Maternal immune tolerance is necessary to avoid fetal rejection during pregnancy (45), a process mediated by T-regulatory cells (45). Kisspeptin significantly increased differentiation of human naïve T cells into T-regulatory cells when administered at levels commensurate with those observed during pregnancy (46). Therefore, low kisspeptin levels could hypothetically be associated with reduced maternal immune tolerance and thus an increased risk of miscarriage.
Another possible contributory mechanism for kisspeptin to modify the risk of miscarriage could be through the role of oxidation. The developing embryo needs relative hypoxia to survive, and exposure to premature oxidative stress can increase the risk of miscarriage (47). Kisspeptin has been implicated in the regulation of placental angiogenesis (48). Furthermore, low kisspeptin levels could reflect aberrant decidualization (42) and implantation (49), which are known to be implicated in the mechanism of miscarriage (50). On the other hand, heterozygous female kisspeptin mutant mice do not have abnormal placental function (51), and pregnancies can be carried to term in patients with kisspeptin receptor variants (52), although with poor endometrial maturation (53). Thus, it is in addition possible that kisspeptin could be a biomarker of placental function rather than having a crucial active role in the pathophysiology of miscarriage.
Although miscarriage is often inevitable, it may be amenable to therapy in some instances. A recent large, randomized trial of progesterone supplementation in women at risk for miscarriage suggested that women at highest risk were especially likely to benefit from intervention (54). Furthermore, although it is commonly thought of as a sudden event, miscarriage often presents as a failing pregnancy over a number of weeks (55). Thus, it is plausible that pregnancy biomarkers that identify women at increased risk for miscarriage early in the process could help target interventions to a cohort of women who might benefit. However, even in the absence of a readily available therapy, being able to identify the risk of miscarriage is additionally important to allow couples to prepare psychologically for this eventuality and to allocate resources to those at most risk (10, 29, 56).
The strengths of this study were that it included the largest number of patients with miscarriages to date and that the patients were accurately phenotyped by a specialist early pregnancy unit to have confirmed IUP at presentation. Further, we derived thresholds for identification of miscarriage at different gestational weeks during the first trimester for the first time. This risk stratification could be potentially improved further if it were combined with other clinical, biochemical, or radiologic markers.
The limitations of the study include the need to validate the proposed models in external data sets, and until such time, the auROC and threshold values reported should be regarded as indicative. Additionally, despite the good performance of plasma kisspeptin in this study, kisspeptin can be a labile analyte susceptible to preanalytical factors such as temperature and time to processing before freezing, which could lead to underestimation of its potential performance in the present study as these factors become optimized during further development (57). Furthermore, kisspeptin is expressed in the placenta at very low levels at less than 6 weeks of gestation and thus may not be easily measurable at very early gestational ages, even in healthy pregnancies (58). Indeed, kisspeptin may not be suitable for assessment of PUL and EP, which typically present at early gestational ages when kisspeptin levels are still low. However, despite these limitations, kisspeptin retained high discriminatory performance throughout the first trimester.
In summary, plasma kisspeptin is a promising biomarker for the diagnosis of miscarriage in patients with an IUP at presentation. Furthermore, the combination of kisspeptin and βhCG provided additional diagnostic accuracy over either measure alone. Plasma kisspeptin could thus represent a useful biomarker to further stratify the risk of miscarriage in combination with currently used clinical tools.
Footnotes
A.A. has nothing to disclose. M.A-M. has nothing to disclose. M.P. has nothing to disclose. C.K. has nothing to disclose. P.C.E. has nothing to disclose. R.N. has nothing to disclose. C.I-E. has nothing to disclose. S.A.C. has nothing to disclose. E.G.M. has nothing to disclose. E.D. has nothing to disclose. L.H. has nothing to disclose. E.P. has nothing to disclose. L.Y. has nothing to disclose. B.P. has nothing to disclose. T.T. has nothing to disclose. P.B. has nothing to disclose. A.N.C. has nothing to disclose. H.F. has nothing to disclose. T.W.K. has nothing to disclose. T.B. has nothing to disclose. W.S.D. has nothing to disclose. The research was conducted in the absence of any personal, professional, commercial, or financial relationships that could be construed as a potential conflict of interest.
A.A. and M.A-M. are joint first authors. T.B. and W.S.D. are joint senior authors and co-corresponding authors.
Supported by the National Institute for Health Research (NIHR) Clinical Research Facility and the NIHR Biomedical Research Centre based at Imperial College Healthcare National Health Services (NHS) Trust. The views expressed are those of the investigators and not necessarily those of the NHS, the NIHR, or the Department of Health. The Section of Endocrinology and Investigative Medicine is funded by grants from the Medical Research Council and NIHR. A.A. is supported by an NIHR Clinician Scientist award (CS-2018-18-ST2-002). M.A.M. is supported by Tommy’s National Centre for Miscarriage Research. C.I.-E. is supported by an Imperial College-Biomedical Research Centre Imperial Post-doctoral, Post-CCT Research Fellowship. L.Y. is supported by an Medical Research Council Clinical Training Fellowship (MR/R000484/1). A.N.C. is supported by the NHS and Biomedical Research Centre. T.B. is supported by the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust. W.S.D. is supported by an NIHR Research Professorship (RP-2014-05-001).
All data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.
Supplementary data
Supplementary Figure 1.
Comparison of gestational age and plasma kisspeptin levels by Crown-Rump Length and Last Menstrual Period. A. Scatterplot of gestational age as estimated by Crown-Rump Length (CRL) (in days) over gestational age as estimated by Last Menstrual Period (LMP) (in days) in healthy controls (in grey) (n=101 women) and women with miscarriage (in red) (n=81 women). Data was analysed by simple linear regression (r2=0.82 in controls and r2=0.54 in miscarriage); ∗∗∗∗P<0.0001. B, C. Spaghetti plots of plasma kisspeptin with gestational age (in weeks) as estimated by Crown-Rump Length (CRL) (B) and Last Menstrual Period (LMP) (C). Red bars are 95% confidence limits for a mixed-effects linear regression model fitted to the data; blue bars are 95% prediction limits for the same model. Intraclass Correlation Coefficients (ICC) are calculated from the random effects variance for the models. ICC for panel B is 19.9% and ICC for panel C is 30.3%, indicating that 20% - 30% of the variation in kisspeptin can be explained by subject difference.
Supplementary Figure 2.
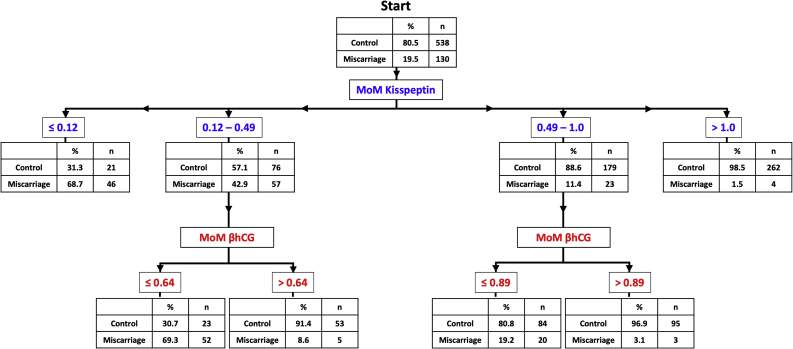
Classification tree presenting the risk of miscarriage on the basis of gestation-adjusted i.e. multiple of median (MoM) of kisspeptin and βhCG levels. The number of samples and the proportion that are from women with control pregnancies, or from women affected by miscarriage is presented in the box. From the ‘start’, the risk of miscarriage can be estimated by following the arrows according to the MoM of plasma kisspeptin, for which the categories are shown in blue. The risk of miscarriage is first categorised according to MoM kisspeptin levels; for example 68.7% of samples with a low MoM kisspeptin level of <0.12 were from women who suffered miscarriage, whereas only 1.5% were if MoM kisspeptin > 1.0. Women with MoM kisspeptin levels between 0.12 and 1.0 were further risk-stratified using MoM βhCG levels (red). The area under receiver operating characteristic curve (auROC) for correct categorization by this decision tree is 0.90 (95% CI: 0.87 – 0.93). MoM, Multiple of gestation-specific median values of women with healthy pregnancies; βhCG, beta chorionic gonadotropin (iU/L).
Supplementary Figure 3.
Plasma kisspeptin by type of miscarriage and relationship with βhCG in women with miscarriage. A. Scatterplot median (IQR) of multiples of gestation specific medians of plasma kisspeptin in samples from women with complete (n=12), incomplete (n=9) and missed (n=57) miscarriage. Groups were compared by Kruskal Wallis test with post hoc Dunn’s multiple comparison test. ∗P<0.05, ∗∗P<0.001. B. Scatterplot of plasma kisspeptin versus plasma βhCG levels in women with miscarriage (n= 95 women providing 132 samples). Values analysed by simple linear regression, r2= 0.6
Supplementary Figure 4.
Decision trees to predict the risk of miscarriage. Only the initial blood sample taken at first presentation was used to generate this decision tree. The risk of miscarriage (between 0 and 1) is presented within each node (rectangles with rounded corners coloured in either green (starting), blue (decision) or red (terminal). The proportion of all women who would reach that node is presented above each node. At the starting node (green), the risk of miscarriage is 0.28. The arrows can then be followed according to the level of unadjusted βhCG (IU/L), kisspeptin levels (pmol/L) and gestational age by last menstrual period (days). The final miscarriage risk is indicated once the terminal nodes (red) have been reached. For example, a pregnant woman that has a βhCG level <14,000 iU/L will have a 0.81 risk of miscarriage and this risk will be present in 17% of the total study population (Decision Tree A). The left panel (Decision Tree A) has an accuracy for correctly classifying women of 87.4% (95% CI: 83.4% - 90.7%), but this complex model could be susceptible to overfitting (i.e. may perform with less accuracy when used in other datasets). The right panel (Decision Tree B) is a parsimonious (simplified) model with an 83.1% accuracy (95% CI: 78.6% - 86.7%) that is more likely to maintain similar performance when applied to other datasets. βhCG, beta chorionic gonadotropin (iU/L); KP, Kisspeptin (pmol/L).
References
- 1.Royal College of Obstetricians and Gynaecologists. The Management of Early Pregnancy Loss—Green Top Guideline 25; 2006.
- 2.Wilcox A.J., Weinberg C.R., O’Connor J.F., Baird D.D., Schlatterer J.P., Canfield R.E. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 3.Ammon Avalos L., Galindo C., Li D.K. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol. 2012;94:417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee S., Velez Edwards D.R., Baird D.D., Savitz D.A., Hartmann K.E. Risk of miscarriage among black women and white women in a US prospective cohort study. Am J Epidemiol. 2013;177:1271–1278. doi: 10.1093/aje/kws393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soler A., Morales C., Mademont-Soler I., Margarit E., Borrell A., Borobio V. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81–89. doi: 10.1159/000477707. [DOI] [PubMed] [Google Scholar]
- 6.Ilekis J.V., Tsilou E., Fisher S., Abrahams V.M., Soares M.J., Cross J.C. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215:S1–S46. doi: 10.1016/j.ajog.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiansen O.B., Steffensen R., Nielsen H.S., Varming K. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol Obstet Investig. 2008;66:257–267. doi: 10.1159/000149575. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah Y., Daemen A., Kirk E., Pexsters A., Naji O., Stalder C. Limitations of current definitions of miscarriage using mean gestational sac diameter and crown-rump length measurements: a multicenter observational study. Ultrasound Obstet Gynecol. 2011;38:497–502. doi: 10.1002/uog.10109. [DOI] [PubMed] [Google Scholar]
- 9.Preisler J., Kopeika J., Ismail L., Vathanan V., Farren J., Abdallah Y. Defining safe criteria to diagnose miscarriage: prospective observational multicentre study. BMJ. 2015;351:h4579. doi: 10.1136/bmj.h4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottomley C., Van Belle V., Mukri F., Kirk E., Van Huffel S., Timmerman D. The optimal timing of an ultrasound scan to assess the location and viability of an early pregnancy. Hum Reprod. 2009;24:1811–1817. doi: 10.1093/humrep/dep084. [DOI] [PubMed] [Google Scholar]
- 11.Hill L.M., Guzick D., Fries J., Hixson J. Fetal loss rate after ultrasonically documented cardiac activity between 6 and 14 weeks, menstrual age. J Clin Ultrasound. 1991;19:221–223. doi: 10.1002/jcu.1870190406. [DOI] [PubMed] [Google Scholar]
- 12.Memtsa M., Jurkovic D., Jauniaux E., Royal College of Obstetricians and Gynaecologists Diagnostic biomarkers for predicting adverse early pregnancy outcomes: Scientific Impact Paper No. 58. BJOG. 2019;126:e107–e113. doi: 10.1111/1471-0528.15468. [DOI] [PubMed] [Google Scholar]
- 13.Bottomley C., Bourne T. Diagnosing miscarriage. Best Pract Res Clin Obstet Gynaecol. 2009;23:463–477. doi: 10.1016/j.bpobgyn.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Elson J., Tailor A., Salim R., Hillaby K., Dew T., Jurkovic D. Expectant management of miscarriage—prediction of outcome using ultrasound and novel biochemical markers. Hum Reprod. 2005;20:2330–2333. doi: 10.1093/humrep/dei038. [DOI] [PubMed] [Google Scholar]
- 15.Savaris R.F. Kisspeptin as a biomarker for miscarriage: let’s wait! Fertil Steril. 2018;109:67. doi: 10.1016/j.fertnstert.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.H., Miele M.E., Hicks D.J., Phillips K.K., Trent J.M., Weissman B.E. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 17.Ohtaki T., Shintani Y., Honda S., Matsumoto H., Hori A., Kanehashi K. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G- protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 18.Kotani M., Detheux M., Vandenbogaerde A., Communi D., Vanderwinden J.M., Le Poul E. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 19.Trevisan C.M., Montagna E., de Oliveira R., Christofolini D.M., Barbosa C.P., Crandall K.A. Kisspeptin/GPR54 system: what do we know about its role in human reproduction? Cell Physiol Biochem. 2018;49:1259–1276. doi: 10.1159/000493406. [DOI] [PubMed] [Google Scholar]
- 20.Janneau J.-L., Maldonado-Estrada J., Tachdjian G., Miran I., Motté N., Saulnier P. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab. 2002;87:5336–5339. doi: 10.1210/jc.2002-021093. [DOI] [PubMed] [Google Scholar]
- 21.Bilban M., Ghaffari-Tabrizi N., Hintermann E., Bauer S., Molzer S., Zoratti C. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- 22.Cartwright J.E., Williams P.J. Altered placental expression of kisspeptin and its receptor in pre-eclampsia. J Endocrinol. 2012;214:79–85. doi: 10.1530/JOE-12-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matjila M., Millar R., van der Spuy Z., Katz A. The differential expression of Kiss1, MMP9 and angiogenic regulators across the feto-maternal interface of healthy human pregnancies: implications for trophoblast invasion and vessel development. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horikoshi Y., Matsumoto H., Takatsu Y., Ohtaki T., Kitada C., Usuki S. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 25.Dhillo W.S., Savage P., Murphy K.G., Chaudhri O.B., Patterson M., Nijher G.M. Plasma kisspeptin is raised in patients with gestational trophoblastic neoplasia and falls during treatment. Am J Physiol Endocrinol Metab. 2006;291:E878–E884. doi: 10.1152/ajpendo.00555.2005. [DOI] [PubMed] [Google Scholar]
- 26.Jayasena C.N., Abbara A., Izzi-Engbeaya C., Comninos A.N., Harvey R.A., Gonzalez Maffe J. Reduced levels of plasma kisspeptin during the antenatal booking visit are associated with increased risk of miscarriage. J Clin Endocrinol Metab. 2014;99:E2652–E2660. doi: 10.1210/jc.2014-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan-Pyke C., Haisenleder D.J., Senapati S., Nicolais O., Eisenberg E., Sammel M.D. Kisspeptin as a new serum biomarker to discriminate miscarriage from viable intrauterine pregnancy. Fertil Steril. 2018;109:137–141.e2. doi: 10.1016/j.fertnstert.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence (NICE) 2019. Ectopic pregnancy and miscarriage: diagnosis and initial management diagnosis and initial management NICE Guideline. [PubMed] [Google Scholar]
- 29.Loughna P., Chitty L., Evans T., Chudleigh T. Fetal size and dating: charts recommended for clinical obstetric practice. Ultrasound. 2009;17:160–166. [Google Scholar]
- 30.Campbell S., Warsof S.L., Little D., Cooper D.J. Routine ultrasound screening for the prediction of gestational age. Obstet Gynecol. 1985;65:613–620. [PubMed] [Google Scholar]
- 31.National Institute for Health and Care Excellence . Clinical Guideline; 2008. Antenatal care for uncomplicated pregnancies. [PubMed] [Google Scholar]
- 32.Reljič M. The significance of crown-rump length measurement for predicting adverse pregnancy outcome of threatened abortion. Ultrasound Obstet Gynecol. 2001;17:510–512. doi: 10.1046/j.1469-0705.2001.00370.x. [DOI] [PubMed] [Google Scholar]
- 33.Mukri F., Bourne T., Bottomley C., Schoeb C., Kirk E., Papageorghiou A.T. Evidence of early first-trimester growth restriction in pregnancies that subsequently end in miscarriage. BJOG. 2008;115:1273–1278. doi: 10.1111/j.1471-0528.2008.01833.x. [DOI] [PubMed] [Google Scholar]
- 34.Dhillo W.S., Chaudhri O.B., Patterson M., Thompson E.L., Murphy K.G., Badman M.K. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 35.Yu H., Liu J., Guo H., Chen C., Han Y., Cui Y. Prognostic value of repeated serum kisspeptin measurements in early first trimester pregnancy: a preliminary study. Reprod Biomed Online. 2019;38:465–471. doi: 10.1016/j.rbmo.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Ciaramella V., Della Corte C.M., Ciardiello F., Morgillo F. Kisspeptin and cancer: molecular interaction, biological functions, and future perspectives. Front Endocrinol (Lausanne) 2018;9:115. doi: 10.3389/fendo.2018.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferretti C., Bruni L., Dangles-Marie V., Pecking A.P., Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 38.Wu S., Zhang H., Tian J., Liu L., Dong Y., Mao T. Expression of kisspeptin/GPR54 and PIBF/PR in the first trimester trophoblast and decidua of women with recurrent spontaneous abortion. Pathol Res Pract. 2014;210:47–54. doi: 10.1016/j.prp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Park D.W., Lee S.K., Hong S.R., Han A.R., Kwak-Kim J., Yang K.M. Expression of kisspeptin and its receptor GPR54 in the first trimester trophoblast of women with recurrent pregnancy loss. Am J Reprod Immunol. 2012;67:132–139. doi: 10.1111/j.1600-0897.2011.01073.x. [DOI] [PubMed] [Google Scholar]
- 40.Roseweir A.K., Katz A.A., Millar R.P. Kisspeptin-10 inhibits cell migration in vitro via a receptor-GSK3 beta-FAK feedback loop in HTR8SVneo cells. Placenta. 2012;33:408–415. doi: 10.1016/j.placenta.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Francis V.A., Abera A.B., Matjila M., Millar R.P., Katz A.A. Kisspeptin regulation of genes involved in cell invasion and angiogenesis in first trimester human trophoblast cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H.M., Huang H.Y., Soong Y.K., Leung P.C.K., Wang H.S. Kisspeptin regulation of human decidual stromal cells motility via FAK–Src intracellular tyrosine kinases. Hum Reprod. 2019;34:1291–1301. doi: 10.1093/humrep/dez061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anin S., Vince G., Quenby S. Trophoblast invasion. Hum Fertil. 2004;7:169–174. doi: 10.1080/14647270400006911. [DOI] [PubMed] [Google Scholar]
- 44.Khong Y., Brosens I. Defective deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25:301–311. doi: 10.1016/j.bpobgyn.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Williams Z. Inducing tolerance to pregnancy. N Engl J Med. 2012;367:1159–1161. doi: 10.1056/NEJMcibr1207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorbunova O.L., Shirshev S.V. Molecular mechanisms of the regulation by kisspeptin of the formation and functional activity of Treg and Th17. Biochem Suppl Ser A Membr Cell Biol. 2016;10:180–187. [Google Scholar]
- 47.Jauniaux E., Poston L., Burton G.J. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramaesh T., Logie J.J., Roseweir A.K., Millar R.P., Walker B.R., Hadoke P.W.F. Kisspeptin-10 inhibits angiogenesis in human placental vessels ex vivo and endothelial cells in vitro. Endocrinology. 2010;151:5927–5934. doi: 10.1210/en.2010-0565. [DOI] [PubMed] [Google Scholar]
- 49.Calder M., Chan Y.M., Raj R., Pampillo M., Elbert A., Noonan M. Implantation failure in female Kiss1-/- mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology. 2014;155:3065–3078. doi: 10.1210/en.2013-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brosens J.J., Pijnenborg R., Brosens I.A. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 51.Herreboudt A.M., Kyle V.R.L., Lawrence J., Doran J., Colledge W.H. Kiss1 mutant placentas show normal structure and function in the mouse. Placenta. 2015;36:52–58. doi: 10.1016/j.placenta.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pallais J.C., Bo-Abbas Y., Pitteloud N., Crowley W.F., Seminara S.B. Neuroendocrine, gonadal, placental, and obstetric phenotypes in patients with IHH and mutations in the G-protein coupled receptor, GPR54. Mol Cell Endocrinol. 2006;254–255:70–77. doi: 10.1016/j.mce.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 53.Hugon-Rodin J., Yoshii K., Lahlou N., Flandrin J., Gompel A., de Roux N. Complete kisspeptin receptor inactivation does not impede exogenous GnRH-induced LH surge in humans. J Clin Endocrinol Metab. 2018;103:4482–4490. doi: 10.1210/jc.2018-00410. [DOI] [PubMed] [Google Scholar]
- 54.Coomarasamy A., Devall A.J., Cheed V., Harb H., Middleton L.J., Gallos I.D. A randomized trial of progesterone in women with bleeding in early pregnancy. N Engl J Med. 2019;380:1815–1824. doi: 10.1056/NEJMoa1813730. [DOI] [PubMed] [Google Scholar]
- 55.Jurkovic D., Overton C., Bender-Atik R. Diagnosis and management of first trimester miscarriage. BMJ. 2013;346:f3676. doi: 10.1136/bmj.f3676. [DOI] [PubMed] [Google Scholar]
- 56.Farren J., Jalmbrant M., Falconieri N., Mitchell-Jones N., Bobdiwala S., Al-Memar M. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. Am J Obstet Gynecol. 2020;222:367.e1–367.e22. doi: 10.1016/j.ajog.2019.10.102. [DOI] [PubMed] [Google Scholar]
- 57.Ramachandran R., Patterson M., Murphy K.G., Dhillo W.S., Patel S., Kazarian A. Preanalytical factors affecting RIA measurement of plasma kisspeptin. Clin Chem. 2008;54:615–617. doi: 10.1373/clinchem.2007.093005. [DOI] [PubMed] [Google Scholar]
- 58.Romero-Ruiz A., Avendaño M.S., Dominguez F., Lozoya T., Molina-Abril H., Sangiao-Alvarellos S. Deregulation of miR-324/KISS1/kisspeptin in early ectopic pregnancy: mechanistic findings with clinical and diagnostic implications. Am J Obstet Gynecol. 2019;220:480.e1–480.e17. doi: 10.1016/j.ajog.2019.01.228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.