Abstract
The oxazolidinone antibiotic linezolid has demonstrated potent antimicrobial activity against Gram-positive bacterial pathogens, including methicillin-resistant staphylococci. This article systematically reviews the published literature for reports of linezolid-resistant Staphylococcus (LRS) infections to identify epidemiological, microbiological and clinical features for these infections. Linezolid remains active against >98% of Staphylococcus, with resistance identified in 0.05% of Staphylococcus aureus and 1.4% of coagulase-negative Staphylococcus (CoNS). In all reported cases, patients were treated with linezolid prior to isolation of LRS, with mean times of 20.0 ± 47.0 months for S. aureus and 11.0 ± 8.0 days for CoNS. The most common mechanisms for linezolid resistance were mutation (G2576T) to the 23S rRNA (63.5% of LRSA and 60.2% of LRCoNS) or the presence of a transmissible cfr ribosomal methyltransferase (54.5% of LRSA and 15.9% of LRCoNS). The emergence of linezolid resistance in Staphylococcus poses significant challenges to the clinical treatment of infections caused by these organisms, and in particular CoNS.
Keywords: antimicrobial resistance, staphylococci, MRSA, coagulase-negative Staphylococcus , healthcare-associated infection
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative Staphylococcus (MRCoNS) are major causes of both healthcare- and community-associated infections.1–3 Linezolid, with both oral and parenteral formulations, is one of the few therapeutic options shown to be effective against MRSA, including treatment of complicated skin and soft tissue infections (SSTIs), osteomyelitis, and pneumonia.4 Little is known regarding the efficacy of linezolid for the treatment of serious infections caused by MRCoNS. In particular, linezolid is not approved for the treatment of patients with catheter-site or catheter-related bloodstream infections or infections where coagulase-negative Staphylococcus (CoNS) are commonly implicated.
Data from the USA and global surveillance studies report <1% of S. aureus and 2% of CoNS5–9 are linezolid resistant. Nonetheless, multifocal outbreaks of linezolid-resistant Staphylococcus (LRS) have been reported,10–13 and both vertical and horizontal transmission of linezolid resistance determinants may occur. Very little data exist regarding treatment and clinical outcomes for LRS infections. A better understanding of the epidemiology and mechanisms of linezolid resistance are important to mandate judicious use of linezolid, both to preserve its clinical utility and prevent nosocomial transmission of LRS. Herein we systematically review the literature for all reported cases of LRS infection to document the current epidemiological, microbiological and clinical features of LRS infection.
Methods
A literature search was performed in PubMed and EMBASE through April 2012 using the National Library of Medicine's medical subject heading (MeSH) terms ‘linezolid’, ‘staphylococcus’, and ‘resistance’ for articles that reported linezolid-resistant Staphylococcus. The search was not restricted by language. The references cited in these articles were examined to identify additional reports. Linezolid resistance in Staphylococcus is defined by both the Clinical Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) as a linezolid MIC of ≥8 mg/L, and this breakpoint was used throughout interpretation of the literature.
Publications identified from the literature search were checked by title and abstract. If an article appeared relevant, the full text was reviewed. Articles included original articles, short communications, correspondences, letters or case reports that documented clinical isolates of linezolid-resistant Staphylococcus. Exclusion criteria were as follows: review articles, basic research on the mechanism of linezolid resistance, reports that described isolates with linezolid MICs <8 mg/L and duplicate isolates reported in multiple studies.
Statistical analysis was performed with χ2 and Fisher's exact test when appropriate to compare rates of linezolid resistance among S. aureus and CoNS.
Results
Incidence and epidemiology of linezolid resistance
Linezolid susceptibility among clinically significant isolates is monitored through two surveillance programmes, the global Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) and the USA Linezolid Experience and Accurate Determination of Resistance (LEADER). In these programmes, non-duplicate isolates from bacterial pneumonia and acute bacterial skin and skin-structure infections (ABSSSIs) are submitted by participating clinical laboratories and linezolid susceptibility is confirmed centrally using CLSI reference broth microdilution (BMD) MIC methods. Staphylococcus tested between 2002 and 2010 by both LEADER and ZAAPS were almost universally susceptible to linezolid (Table 1).5,8,9,14–16 LEADER documented linezolid resistance in 0.05% of S. aureus (n = 13/23 077 isolates), and 1.4% of CoNS (n = 73/5202 isolates).5,7,15–18 The increased incidence of linezolid resistance in CoNS is significant (χ2 = 249.6, P < 0.0001). In contrast, ZAAPS identified only one LRSA8,9,14 and 10 LRCoNS9 between 2002 and 2010, yielding an overall 0.14% rate of linezolid resistance among 8122 Staphylococcus tested. Denominator data on the number of S. aureus and CoNS isolates tested by ZAAPS have not been published.
Table 1.
Incidence of linezolid resistance among Staphylococcus from ZAAPS global (2002–2010) and LEADER USA (2004–2010) surveillance programs
| Number of isolates tested |
|||||||
|---|---|---|---|---|---|---|---|
| LEADER |
Number of isolates resistant (S. aureus) |
Number of isolates resistant (CoNS) |
|||||
| Year | ZAAPSa | S. aureus | CoNS | ZAAPS | LEADER | ZAAPS | LEADER |
| 2002 | 502 | ND | ND | 0 | ND | 0 | ND |
| 2003 | 373 | ND | ND | 0 | ND | 0 | ND |
| 2004 | 419 | 2872 | 496 | 0 | 0 | 0 | 1 |
| 2005 | 465 | 3021 | 530 | 0 | 1 | 0 | 6 |
| 2006 | 657 | 2913 | 808 | 1 | 1 | 1 | 13 |
| 2007 | 1138 | 3318 | 1020 | 0 | 2 | 2 | 18 |
| 2008 | 1214 | 3156 | 856 | 0 | 3 | 3 | 15 |
| 2009 | 1184 | 3257 | 816 | 0 | 5 | 1 | 12 |
| 2010 | 3045 | 4540 | 676 | 0 | 1 | 3 | 8 |
ND, no data.
aNumbers of S. aureus or CoNS tested not separately noted.
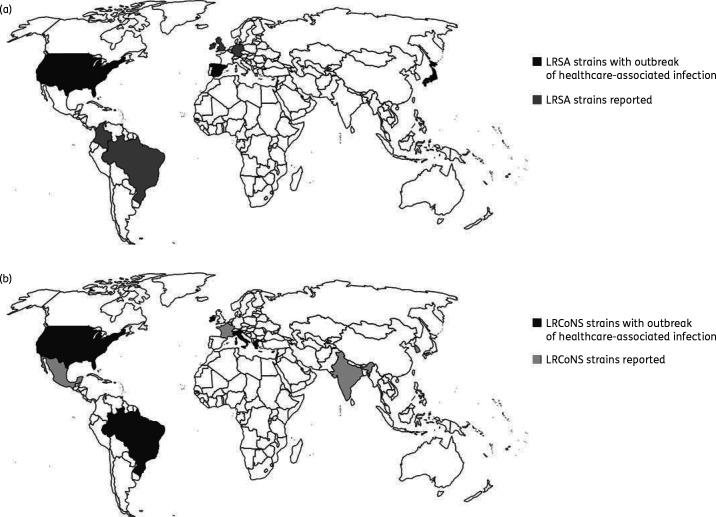
Including those isolates documented above, a total of 812 citations were identified from PubMed and 2757 citations from EMBASE searches. A further five articles were identified through manual review of the references found in these publications. Following exclusion as defined in the methods, 22 publications describing clinical isolates of LRSA (n = 65 cases; Table 2) and 28 of LRCoNS (n = 351 cases) were included in this review. The majority of LRS were isolated from patients in North America and Europe. Overall, 46.2% (30/65) of LRSA were reported in North America, 30.8% (20/65) in Europe, 20.0% (13/65) in Asia, and 3.1% (2/65) in South America (Table 2 and Figure 1). LRCoNS were reported in Europe (53.6% of 351 isolates), North America (42.5%), South America (2.8%) and Asia (1.1%; Figure 1). LRCoNS comprised nine different species, among which 76.4% (268/351) were Staphylococcus epidermidis, 9.1% (32/351) were Staphylococcus hominis and 8.8% (31/351) were Staphylococcus haemolyticus.
Table 2.
Clinical information for linezolid-resistant S. aureus
| Author (Reference) | Number of patients | Sample type(s) | Year(s) isolated | Location | Treatment | Outcome |
|---|---|---|---|---|---|---|
| North America | ||||||
| Endimiani A, 201119 | 8 | sputum, throat swab | 2000–2009 | USA | TMP/SXT + DOX (2/6, 33.3%); TMP/SXT + DOX + VAN (2/6, 33.3%); TMP/SXT + CAZ + VAN (1/6, 16.7%) | survived: 5/6 (83.3%) |
| MEM + CLI (1/6, 16.7%) | died: 1/6 (16.7%) | |||||
| ND: 2 cases | ND: 2 cases | |||||
| Farrell DJ, 201118 | 5 | ND | 2009 | USA | ND | ND |
| Farrell DJ, 20097 | 3 | ND | 2008 | USA | ND | ND |
| Jones RN, 20085 | 2 | ND | 2007 | USA | ND | ND |
| Jones RN, 200717 | 1 | ND | 2006 | USA | ND | ND |
| Zhu W, 200722 | 5 | ND | ND | USA | ND | ND |
| Roberts SM, 200633 | 2 | nares, drainage | Mar 2005 | USA | CLI + LNZ | survived |
| Peeters MJ, 200534 | 1 | wound | ND | USA | VAN | infection resolved but died of ventricular tachycardia |
| Meka VG, 200435 | 1 | ND | ND | USA | VAN | survived |
| Meka VG, 200423 | 1 | blood | ND | USA | ND | |
| Tsiodras S, 200110 | 1 | peritoneal fluid | ND | USA | AZM + GEN + LVX + Q/D (with Enterococcus faecalis and Pseudomonas aeruginosa infection) | infection resolved but died of underlying disease |
| South America | ||||||
| Gales AC, 200636 | 1 | sputum | Jul 2002 | Brazil | ND | ND |
| Toh SM, 200737 | 1 | sputum | 2005 | Colombia | ND | ND |
| Europe | ||||||
| Sánchez García M, 201029 | 12a | ND | Apr 2008–Jun 2008 | Spain | TGC (5/10, 50.0%); VAN (4/10, 40.0%); VAN + TGC (1/10, 10.0%); ND: 2 cases | survived: 7/10 (70.0%); died: 3/10 (30.0%); ND: 2 cases |
| Morales G, 201013 | 15 | ND | Apr 2008–Jun 2008 | Spain | TGC (6/12, 50.0%); VAN (5/12, 41.7%); VAN + TGC (1/12, 8.3%); ND: 3 cases | survived: 9/12 (75.0%); died: 3/12 (25.0%); ND: 3 cases |
| Hill RL, 201038 | 2 | cystic fibrosis, sputum | ND | UK | ND | ND |
| Wilson P, 200339 | 1 | wound swab, empyema fluid | ND | UK | TEC + RIF | survived |
| Hentschke M, 200840 | 1 | stool | Jun 2005 | Germany | ND | ND |
| Ross JE, 20119 | 1 | ND | 2006 | Ireland | ND | ND |
| Asia | ||||||
| An D, 201141 | 1 | blood and pleural fluid | ND | Korea | TMP/SXT + MIN + TGC | died |
| Ikeda-Dantsuji Y, 201120 | 11 | ND | 2006–2008 | Japan | ND | ND |
| Yoshida K, 200942 | 1 | blood, catheter, stool | Sep 2008 | Japan | ND | ND |
Abbreviations: +, positive; −, negative; ND, not done; TMP/SXT, trimethoprim/sulfamethoxazole; DOX, doxycycline; VAN, vancomycin; CLI, clindamycin; MEM, meropenem; CAZ, ceftazidime; MIN, minocycline; TGC, tigecycline; CIP, ciprofloxacin; GEN, gentamicin; DAP, daptomycin; Q/D, quinupristin/dalfopristin; TEC, teicoplanin; AZM, azithromycin; RIF, rifampicin; LVX, levofloxacin.
aThe 12 isolates in reference 29 were also included in reference 13, therefore the 12 isolates were not included in the total number of LRSA strains.
Figure 1.
Distribution of linezolid resistance in S. aureus (a) and CoNS (b) worldwide. (a) Linezolid-resistant S. aureus (LRSA) strains reported in North America (USA), South America (Brazil, Colombia), Europe (Spain, UK, Germany, and Ireland), and Asia (Korea and Japan). (b) Linezolid-resistant CoNS (LRCoNS) reported in North America (USA, Mexico), South America (Brazil), Europe (Greece, Spain, Italy, France, and Ireland), and Asia (India.
Clonal spread of LRS
Three reports of LRSA (13.6% of 22 studies) and 14 reports of LRCoNS (50% of 28 studies) documented clonal dissemination of LRS within or across healthcare settings (Table 2). Two LRSA outbreaks were described in Spain, each involving a single hospital, and 15 or 12 patients, respectively.13,19 The third LRSA outbreak was reported from Japan, and involved seven patients in six different hospitals.20 Both outbreaks in Spain were caused by LRSA harbouring the mobile cfr resistance gene, whereas the Japanese study did not test for cfr. Five linezolid-resistant S. epidermidis with identical PFGE types were recovered from patients at two geographically disparate institutions in the USA between 2006 and 2008.7 In Greece, two clones of LRCoNS were identified among 26 patients in four hospitals.21 Neither the US nor the Greek isolates harboured cfr. These publications did not distinguish LRCoNS colonization versus infection.
Mechanisms of linezolid resistance
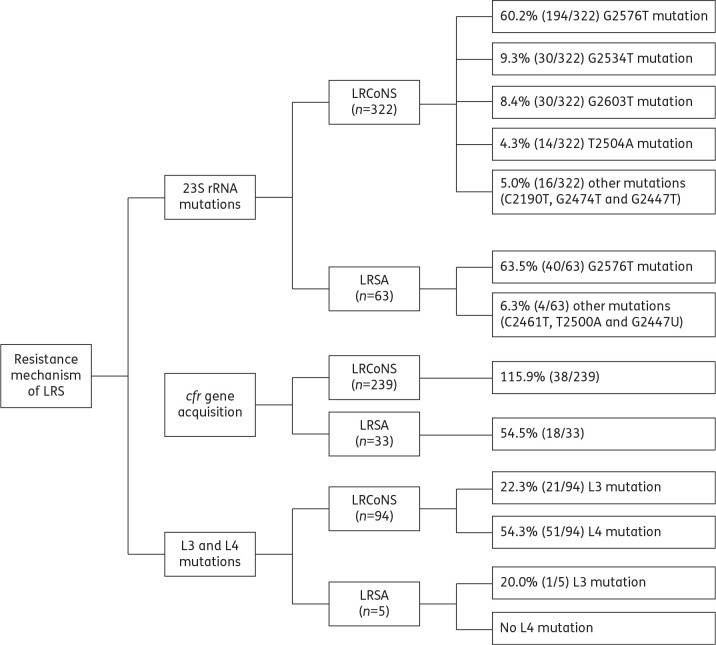
Linezolid resistance occurs by mutations in the linezolid 23S rRNA binding site, the ribosomal proteins L3 and/or L4 of the peptide translocation centre of the ribosome or by acquisition of a plasmid-borne ribosomal methyltransferase gene, cfr.22,23 All three mechanisms have been documented in LRSA and LRCoNS.24 Sixty-three LRSA (Figure 2) and 322 LRCoNS (Figure 2) were investigated for mechanisms of linezolid resistance. While every molecular study evaluated the presence of the 23S rRNA G2576T mutation, a significant portion of the studies did not investigate cfr or L3/L4. More specifically, 52.4% of LRSA (n = 33/63 isolates tested) and 74.2% of LRCoNS (n = 239/322 isolates tested) were tested for the presence of the cfr gene, and 7.9% of LRSA (5/63 isolates tested) and 29.2% of LRCoNS (n = 94/322 isolates) were tested for the L3 and/or L4 mutation (Figure 2). G2576T was found among the majority of LRSA (63.5%, n = 63 isolates tested; Figure 2) and LRCoNS (60.2%, n = 322 isolates tested; Figure 2). cfr was detected in 18 (54.5%) of the LRSA tested (Figure 2) and in only 38 (15.9%) of the LRCoNS tested (Figure 2). When bias imposed by testing of clonally related strains was removed, cfr was found in 10/25 (40%) unique LRSA and 10/54 (18.5%) unique LRCoNS.
Figure 2.
Resistance mechanisms of linezolid-resistant Staphylococcus. The percentage of isolates that harbour each mechanism of linezolid resistance among the number of isolates tested for each mechanism are shown.
In all cases (21/21) with available information, LRSA was isolated following linezolid treatment, the mean duration of which was 20.0 ± 47.0 months. All cases of CoNS infection (74/74 cases) with available information were also in patients previously treated with linezolid, with a mean duration of therapy of 11.0 ± 8.0 days. The difference in exposure times to linezolid prior to isolation of LRSA and LRCoNS was significant (P < 0.0001, Student's t-test).
Susceptibility testing and in vitro susceptibility data
Nineteen of 22 (86.4%) studies reported the susceptibility testing method used to determine linezolid resistance in S. aureus and 27 of 28 (96.4%) in CoNS. The majority of studies used Etest (31/46, 67.4%), BMD (28/46, 60.9%), and disc diffusion (DD) (19/46, 41.3%). However, 19.6% (9/46) of studies used Vitek® or Vitek2® (bioMerieux, Durham, NC, USA), 10.9% (5/46) agar dilution, 8.7% (4/46) MicroScan (Siemens), and 2.2% (1/46) broth macrodilution. Most studies (32/46, 69.6%) used two or more methods to detect and confirm linezolid resistance.
In vitro susceptibility data for antimicrobial agents in addition to linezolid were reported for 56.3% (234/416) of LRS strains reviewed (Table 3). All LRSA tested were resistant to oxacillin (n = 17), chloramphenicol (n = 15), and minocycline (n = 11) and susceptible to vancomycin (n = 33), daptomycin (n = 18), teicoplanin (n = 33), tigecycline (n = 15), amikacin (n = 12) and quinupristin/dalfopristin (n = 4). Variable resistance to clindamycin (20/21, 95.2%), gentamicin (17/19, 89.5%), ciprofloxacin (15/17, 88.2%), erythromycin (17/20, 85.0%), trimethoprim/sulfamethoxazole (5/17, 29.4%) and rifampicin (3/5, 60.0%) was reported (Table 3).
Table 3.
Summary of antimicrobial susceptibility in linezolid-resistant Staphylococcus
| % Susceptible (number tested) |
||
|---|---|---|
| Antimicrobial | LRSA | LRCoNS |
| Oxacillin | 0.0 (17) | 0.0 (94) |
| Erythromycin | 15.0 (20) | 25.0 (88) |
| Clindamycin | 4.8 (21) | 1.4 (71) |
| Tetracycline | ND | 10.7 (56) |
| Minocycline | 0.0 (11) | ND |
| Tigecycline | 100.0 (15) | 100.0 (80) |
| Amikacin | 100.0 (12) | 18.2 (11) |
| Gentamicin | 10.5 (19) | 5.3 (57) |
| Tobramycin | ND | 0.0 (46) |
| Ciprofloxacin | 11.8 (17) | 4.3 (69) |
| Levofloxacin | ND | 0.0 (30) |
| Trimethoprim/sulfamethoxazole | 70.6 (17) | ND |
| Rifampicin | 40.0 (5) | 58.7 (46) |
| Quinupristin/dalfopristin | 100.0 (4) | 91.2 (57) |
| Vancomycin | 100.0 (33) | 99.5 (191) |
| Teicoplanin | 100.0 (33) | 70.9 (141) |
| Daptomycin | 100.0 (18) | 100.0 (176) |
ND, no data.
All LRCoNS isolates tested were resistant to oxacillin (n = 94), levofloxacin (n = 30), and tobramycin (n = 46). LRCoNS strains also exhibited resistance to clindamycin (70/71, 98.6%), ciprofloxacin (66/69, 95.7%), gentamicin (54/57, 94.7%), amikacin (9/11, 81.8%), erythromycin (66/88, 75.0%) and tetracycline (50/56, 89.3%). Variable resistance rates were noted for rifampicin (19/46, 41.3%), teicoplanin (41/141, 29.1%) and quinupristin/dalfopristin (5/57, 8.8%). All but one LRCoNS tested (n = 190) were susceptible to vancomycin; this isolate was vancomycin intermediate and emerged during treatment with vancomycin.25 All LRCoNS tested were susceptible to daptomycin (n = 176) and tigecycline (n = 80).
Sites of infection
The type of infection was documented in 20 (30.8%) LRSA cases and 269 (76.6%) LRCoNS cases. For LRSA, 60.0% (12/20) were respiratory tract infections, 10.0% (2/20) were bloodstream infections (BSIs), 10.0% (2/20) were surgical site infections (SSIs) and 20.0% (4/20) were other sites. BSI was the most common infection documented for LRCoNS, with 98.6% (265/269) of the reported cases; 2 (0.7%) cases were reported each for SSI and other infections.
Discussion
In 2001, 1 year after linezolid was approved for clinical use, the first LRSA was reported in a US patient who had received a 1 month linezolid treatment for dialysis-associated peritonitis.10 Since then, several cases of LRS have been reported in North and South America, Europe and Asia. While the incidence of linezolid resistance remains exceedingly low for S. aureus, more worrisome is the incidence of LRCoNS, which is roughly 28 times that of LRSA. One factor contributing to this increased incidence is the ability of CoNS to more readily develop resistance following linezolid exposure, although this has not been proven in vitro to our knowledge. The mean time of linezolid therapy reported prior to isolation of LRS was significantly shorter (11 days versus 20 months) for cases of LRCoNS. A second factor associated with selection for LRS is over-prescription of linezolid for staphylococcal bacteraemia, and in particular CoNS infections, as identified by the preponderance of LRCoNS isolated from the blood. However, this finding is likely biased by the fact that most clinical laboratories do not test antimicrobial susceptibility of CoNS unless isolated from a normally sterile site such as blood. Finally, significantly more LRCoNS were associated with outbreaks; 50% of the studies identified herein that investigated LRCoNS involved clonal LRCoNS, across one or more patients and facilities.
It is important to note that resistance rates among patients treated with linezolid for extended periods may be significantly elevated as compared with data reported in surveillance studies. For example, cystic fibrosis patients with respiratory tract infections caused by S. aureus have LRSA rates of up to 11%, directly related to the number and length of linezolid treatments.19 Linezolid is the only antibiotic with good activity against MRSA available as an oral formulation, making it desirable for outpatient treatment. However, up to a quarter of patients prescribed the oral formulation of linezolid are non-adherent with therapy.26 While all cases of LRS with available clinical data indicated prior exposure to linezolid, the formulation was only reported in three studies: two studies described oral10,19 and one parenteral dosing.27 The relationship between compliance with linezolid therapy and linezolid resistance has not been formally evaluated, but may also be a factor driving linezolid resistance rates.
We identified a surprisingly high incidence of cfr among LRSA (40% of unique LRSA clones; Figure 2), a factor that strongly suggests horizontal gene transfer may be more common than previously appreciated. This finding raises significant concern about the possibility that isolates harbouring cfr may act as reservoirs for resistance.28 Clonal spread of LRS with cfr has been documented in both institutional-level and multi-institutional outbreaks.13,20,29,30 Infection control practices targeted to halt the spread of MRSA should be effective at preventing the dissemination of LRSA; in contrast, CoNS are rarely considered true pathogens and LRCoNS may go unrecognized. These isolates then have the potential to transfer cfr to more pathogenic organisms, such as S. aureus. This concern is more than theoretical; Mendes and colleagues documented transmission of a mobile cfr onto two plasmids that were then acquired by Staphylococcus cohnii and Staphylococcus epidermidis isolated from the blood of two patients with sepsis.28
Linezolid resistance may be under-reported based on technical hurdles in laboratory interpretation of both MIC and disc diffusion results. Compared with the standard CLSI BMD reference method, one study demonstrated 8/15 (53.3%) LRS were falsely reported susceptible by disc diffusion and 6/15 (40.0%) by Etest.31 Errors in interpreting linezolid disc diffusion zones may be minimized by using endpoint reading recommendations published in the CLSI standards.32 However, in our own unpublished observations, inter-user interpretation of the linezolid disc diffusion zones and MIC endpoints for the staphylococci varied significantly, even among seasoned technologists. To address this concern, it is advisable that clinical laboratories confirm any LRS, preferably by a second method, prior to reporting,32 something that was done in only 72% of the studies evaluated.
Treatment options for LRS are limited, but based on current in vitro susceptibility data, LRS remain universally susceptible to vancomycin, daptomycin and tigecycline. It is clear that the preservation of these antimicrobials for the treatment of infections cause by highly resistant organisms such as LRS is critical.
The results reported herein may suffer from publication bias and other biases inherent in single studies. Furthermore, the spread of clonally related isolates may overestimate the prevalence of LRS infections in healthcare settings.
Conclusions
Linezolid remains highly active against most staphylococci, and its value in treating serious infections caused by MRSA has been well documented. Clinicians should remain cognizant that linezolid resistance may arise following prolonged treatment with linezolid and of the possibility of LRS in patients that have not been previously treated with linezolid, given the high incidence of LRSA carrying cfr. Susceptibility testing for linezolid resistance should be considered prior to using linezolid for serious infections. Further, judicious use of linezolid, accurate identification of resistance and application of strict infection control measures are essential to the preservation of linezolid as a therapeutic agent. To date, LRS remain susceptible to vancomycin, daptomycin and tigecycline. Further studies are needed to investigate the clinical outcome of LRS infections in order to optimize treatment of these infections.
Transparency declarations
None to declare.
Acknowledgements
The authors thank Jim Ross for offering the latest linezolid resistance data from ZAAPS and LEADER programmes in 2010.
References
- 1.MacKenzie FM. Bruce J. Struelens MJ. et al. Antimicrobial drug use and infection control practices associated with the prevalence of methicillin-resistant Staphylococcus aureus in European hospitals. Clin Microbiol Infect. 2007;13:269–76. doi: 10.1111/j.1469-0691.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 2.Widerstrom M. Wistrom J. Sjostedt A. et al. Coagulase-negative staphylococci: update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus . Eur J Clin Microbiol Infect Dis. 2012;31:7–20. doi: 10.1007/s10096-011-1270-6. [DOI] [PubMed] [Google Scholar]
- 3.Chua K. Laurent F. Coombs G. et al. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician's guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis. 2011;52:99–114. doi: 10.1093/cid/ciq067. [DOI] [PubMed] [Google Scholar]
- 4.Diekema DJ. Jones RN. Oxazolidinone antibiotics. Lancet. 2001;358:1975–82. doi: 10.1016/S0140-6736(01)06964-1. [DOI] [PubMed] [Google Scholar]
- 5.Jones RN. Ross JE. Castanheira M. et al. United States resistance surveillance results for linezolid (LEADER Program for 2007) Diagn Microbiol Infect Dis. 2008;62:416–26. doi: 10.1016/j.diagmicrobio.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Sader HS. Jones RN. Antimicrobial susceptibility of Gram-positive bacteria isolated from US medical centers: results of the Daptomycin Surveillance Program (2007–2008) Diagn Microbiol Infect Dis. 2009;65:158–62. doi: 10.1016/j.diagmicrobio.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Farrell DJ. Mendes RE. Ross JE. et al. Linezolid surveillance program results for 2008 (LEADER Program for 2008) Diagn Microbiol Infect Dis. 2009;65:392–403. doi: 10.1016/j.diagmicrobio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Jones RN. Kohno S. Ono Y. et al. ZAAPS International Surveillance Program (2007) for linezolid resistance: results from 5591 Gram-positive clinical isolates in 23 countries. Diagn Microbiol Infect Dis. 2009;64:191–201. doi: 10.1016/j.diagmicrobio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Ross JE. Farrell DJ. Mendes RE. et al. Eight-year (2002–2009) summary of the linezolid (Zyvox(R) Annual Appraisal of Potency and Spectrum; ZAAPS) program in European countries. J Chemother. 2011;23:71–6. doi: 10.1179/joc.2011.23.2.71. [DOI] [PubMed] [Google Scholar]
- 10.Tsiodras S. Gold HS. Sakoulas G. et al. Linezolid resistance in a clinical isolate of Staphylococcus aureus . Lancet. 2001;358:207–8. doi: 10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- 11.Peer MA. Nasir RA. Kakru DK. et al. Sepsis due to linezolid resistant Staphylococcus cohnii and Staphylococcus kloosii: first reports of linezolid resistance in coagulase negative staphylococci from India. Indian J Med Microbiol. 2011;29:60–2. doi: 10.4103/0255-0857.76527. [DOI] [PubMed] [Google Scholar]
- 12.Mendes RE. Deshpande LM. Farrell DJ. et al. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J Antimicrob Chemother. 2010;65:2329–35. doi: 10.1093/jac/dkq331. [DOI] [PubMed] [Google Scholar]
- 13.Morales G. Picazo JJ. Baos E. et al. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus . Clin Infect Dis. 2010;50:821–5. doi: 10.1086/650574. [DOI] [PubMed] [Google Scholar]
- 14.Jones RN. Ross JE. Fritsche TR. et al. Oxazolidinone susceptibility patterns in 2004: report from the Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) Program assessing isolates from 16 nations. J Antimicrob Chemother. 2006;57:279–87. doi: 10.1093/jac/dki437. [DOI] [PubMed] [Google Scholar]
- 15.Draghi DC. Sheehan DJ. Hogan P. et al. In vitro activity of linezolid against key gram-positive organisms isolated in the united states: results of the LEADER 2004 surveillance program. Antimicrob Agents Chemother. 2005;49:5024–32. doi: 10.1128/AAC.49.12.5024-5032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillar CM. Draghi DC. Sheehan DJ. et al. Prevalence of multidrug-resistant, methicillin-resistant Staphylococcus aureus in the United States: findings of the stratified analysis of the 2004 to 2005 LEADER Surveillance Programs. Diagn Microbiol Infect Dis. 2008;60:221–4. doi: 10.1016/j.diagmicrobio.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Jones RN. Fritsche TR. Sader HS. et al. LEADER surveillance program results for 2006: an activity and spectrum analysis of linezolid using clinical isolates from the United States (50 medical centers) Diagn Microbiol Infect Dis. 2007;59:309–17. doi: 10.1016/j.diagmicrobio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Farrell DJ. Mendes RE. Ross JE. et al. LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States. Antimicrob Agents Chemother. 2011;55:3684–90. doi: 10.1128/AAC.01729-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endimiani A. Blackford M. Dasenbrook EC. et al. Emergence of linezolid-resistant Staphylococcus aureus after prolonged treatment of cystic fibrosis patients in Cleveland, Ohio. Antimicrob Agents Chemother. 2011;55:1684–92. doi: 10.1128/AAC.01308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda-Dantsuji Y. Hanaki H. Sakai F. et al. Linezolid-resistant Staphylococcus aureus isolated from 2006 through 2008 at six hospitals in Japan. J Infect Chemother. 2011;17:45–51. doi: 10.1007/s10156-010-0085-1. [DOI] [PubMed] [Google Scholar]
- 21.Liakopoulos A. Spiliopoulou I. Damani A. et al. Dissemination of two international linezolid-resistant Staphylococcus epidermidis clones in Greek hospitals. J Antimicrob Chemother. 2010;65:1070–1. doi: 10.1093/jac/dkq065. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W. Tenover FC. Limor J. et al. Use of pyrosequencing to identify point mutations in domain V of 23S rRNA genes of linezolid-resistant Staphylococcus aureus and Staphylococcus epidermidis . Eur J Clin Microbiol Infect Dis. 2007;26:161–5. doi: 10.1007/s10096-007-0261-0. [DOI] [PubMed] [Google Scholar]
- 23.Meka VG. Pillai SK. Sakoulas G. et al. Linezolid resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA. J Infect Dis. 2004;190:311–7. doi: 10.1086/421471. [DOI] [PubMed] [Google Scholar]
- 24.Long KS. Vester B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother. 2012;56:603–12. doi: 10.1128/AAC.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bongiorno D. Campanile F. Mongelli G. et al. DNA methylase modifications and other linezolid resistance mutations in coagulase-negative staphylococci in Italy. J Antimicrob Chemother. 2010;65:2336–40. doi: 10.1093/jac/dkq344. [DOI] [PubMed] [Google Scholar]
- 26.Ball AT. Xu Y. Sanchez RJ. et al. Nonadherence to oral linezolid after hospitalization: a retrospective claims analysis of the incidence and consequence of claim reversals. Clin Ther. 2010;32:2246–55. doi: 10.1016/S0149-2918(10)80027-X. [DOI] [PubMed] [Google Scholar]
- 27.Hong T. Li X. Wang J. et al. Sequential linezolid-resistant Staphylococcus epidermidis isolates with G2576T mutation. J Clin Microbiol. 2007;45:3277–80. doi: 10.1128/JCM.02048-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendes RE. Deshpande L. Rodriguez-Noriega E. et al. First report of staphylococcal clinical isolates in Mexico with linezolid resistance caused by cfr: evidence of in vivo cfr mobilization. J Clin Microbiol. 2010;48:3041–3. doi: 10.1128/JCM.00880-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez Garcia M. De la Torre MA. Morales G. et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303:2260–4. doi: 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- 30.Bonilla H. Huband MD. Seidel J. et al. Multicity outbreak of linezolid-resistant Staphylococcus epidermidis associated with clonal spread of a cfr-containing strain. Clin Infect Dis. 2010;51:796–800. doi: 10.1086/656281. [DOI] [PubMed] [Google Scholar]
- 31.Tenover FC. Williams PP. Stocker S. et al. Accuracy of six antimicrobial susceptibility methods for testing linezolid against staphylococci and enterococci. J Clin Microbiol. 2007;45:2917–22. doi: 10.1128/JCM.00913-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 22nd Informational Supplement. M100-S22. Wayne, PA, USA: CLSI; 2012. [Google Scholar]
- 33.Roberts SM. Freeman AF. Harrington SM. et al. Linezolid-resistant Staphylococcus aureus in two pediatric patients receiving low-dose linezolid therapy. Pediatr Infect Dis J. 2006;25:562–4. doi: 10.1097/01.inf.0000219401.70804.1a. [DOI] [PubMed] [Google Scholar]
- 34.Peeters MJ. Sarria JC. Clinical characteristics of linezolid-resistant Staphylococcus aureus infections. Am J Med Sci. 2005;330:102–4. doi: 10.1097/00000441-200508000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Meka VG. Gold HS. Cooke A. et al. Reversion to susceptibility in a linezolid-resistant clinical isolate of Staphylococcus aureus . J Antimicrob Chemother. 2004;54:818–20. doi: 10.1093/jac/dkh423. [DOI] [PubMed] [Google Scholar]
- 36.Gales AC. Sader HS. Andrade SS. et al. Emergence of linezolid-resistant Staphylococcus aureus during treatment of pulmonary infection in a patient with cystic fibrosis. Int J Antimicrob Agents. 2006;27:300–2. doi: 10.1016/j.ijantimicag.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Toh SM. Xiong L. Arias CA. et al. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol Microbiol. 2007;64:1506–14. doi: 10.1111/j.1365-2958.2007.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill RL. Kearns AM. Nash J. et al. Linezolid-resistant ST36 methicillin-resistant Staphylococcus aureus associated with prolonged linezolid treatment in two paediatric cystic fibrosis patients. J Antimicrob Chemother. 2010;65:442–5. doi: 10.1093/jac/dkp494. [DOI] [PubMed] [Google Scholar]
- 39.Wilson P. Andrews JA. Charlesworth R. et al. Linezolid resistance in clinical isolates of Staphylococcus aureus . J Antimicrob Chemother. 2003;51:186–8. doi: 10.1093/jac/dkg104. [DOI] [PubMed] [Google Scholar]
- 40.Hentschke M. Saager B. Horstkotte MA. et al. Emergence of linezolid resistance in a methicillin resistant Staphylococcus aureus strain. Infection. 2008;36:85–7. doi: 10.1007/s15010-007-7220-7. [DOI] [PubMed] [Google Scholar]
- 41.Kelesidis T. Humphries R. Uslan DZ. et al. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin Infect Dis. 2011;52:228–34. doi: 10.1093/cid/ciq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida K. Shoji H. Hanaki H. et al. Linezolid-resistant methicillin-resistant Staphylococcus aureus isolated after long-term, repeated use of linezolid. J Infect Chemother. 2009;15:417–9. doi: 10.1007/s10156-009-0727-3. [DOI] [PubMed] [Google Scholar]